Identification of the Major ACE-Inhibitory Peptides Produced by Enzymatic Hydrolysis of a Protein Concentrate from Cuttlefish Wastewater
Abstract
:1. Introduction
2. Results and Discussion
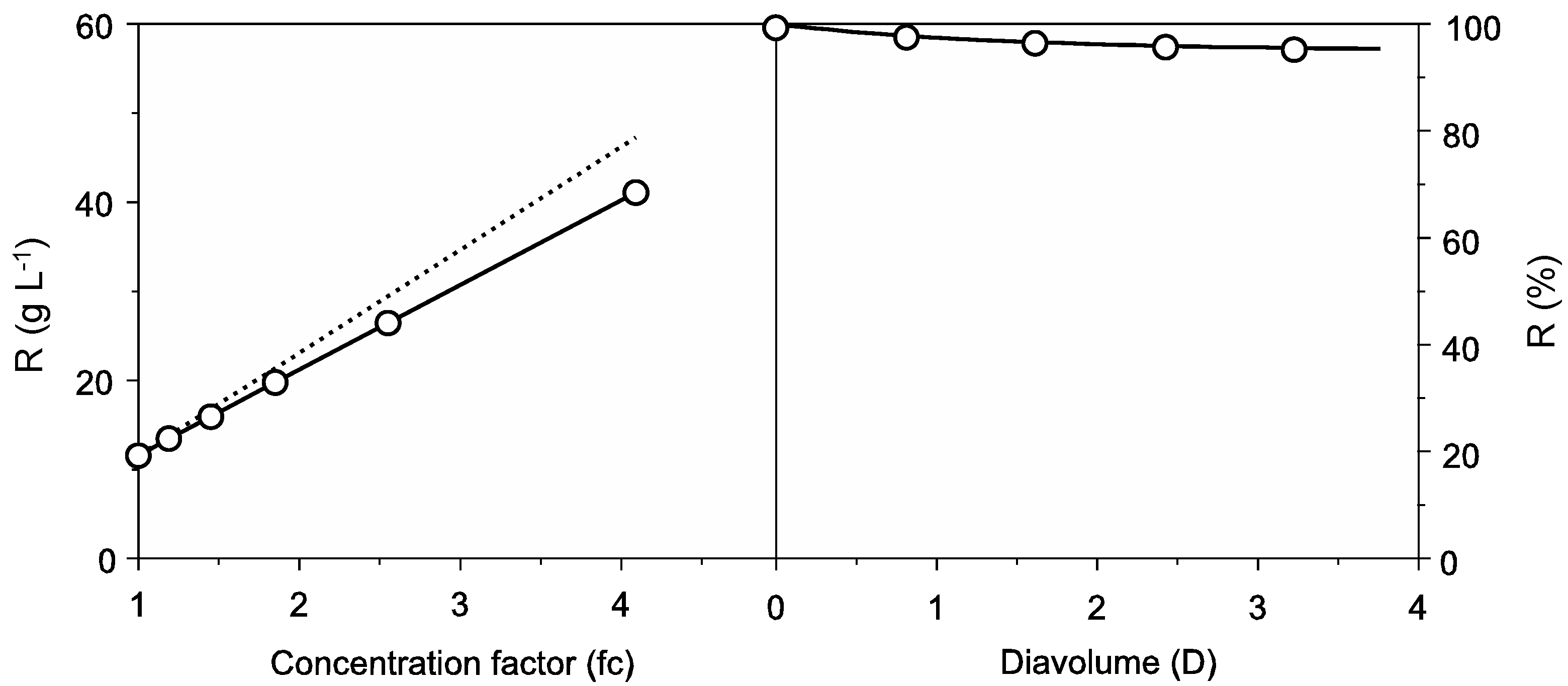
2.1. Protein Recovery by Ultrafiltration-Diafiltration (UF-DF)
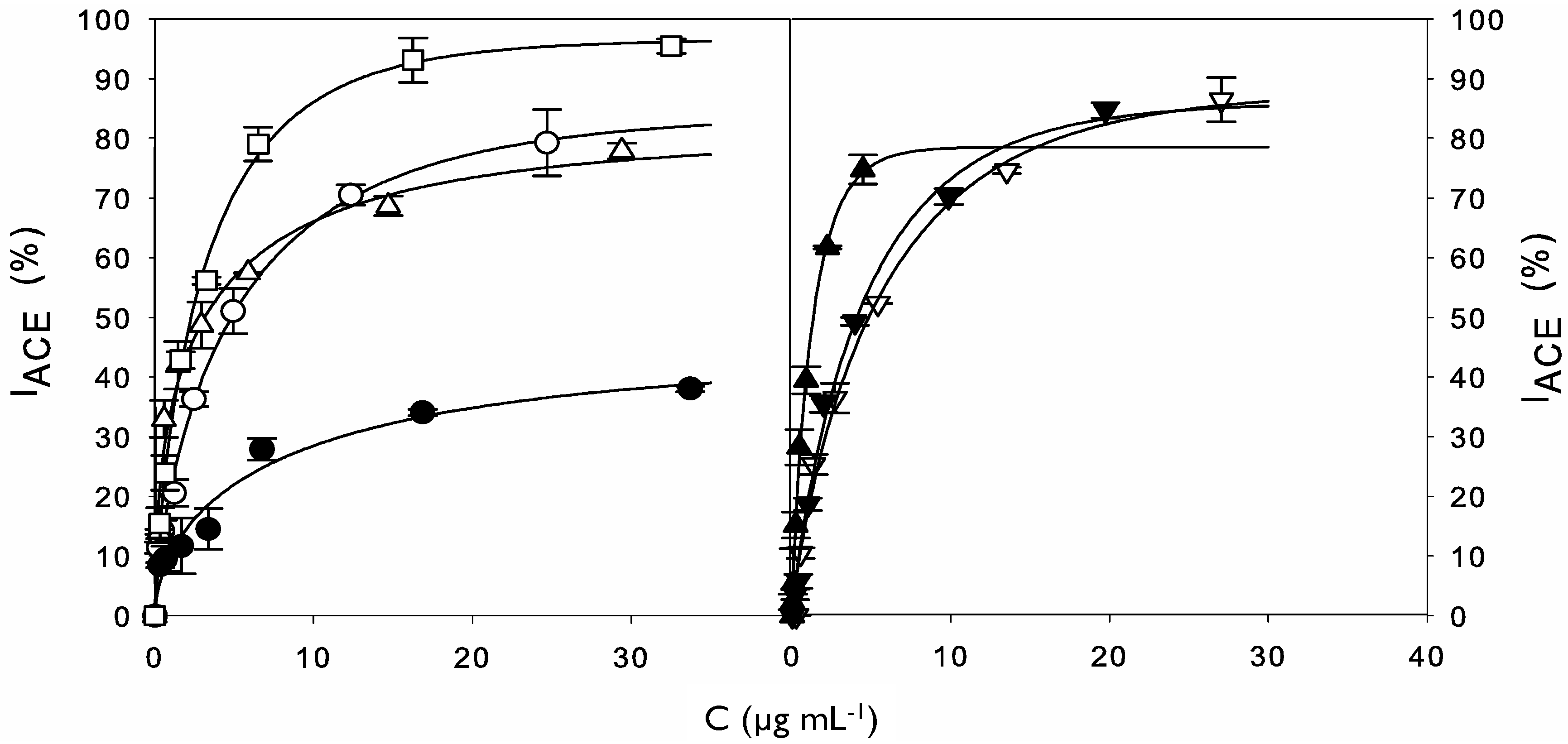
2.2. ACE Inhibitory Activity of Cuttlefish Hydrolysate and Their UF Fractions
| Values of DH, IACE (%) and IC50 for Hydrolysis and UF Processes | ||||
|---|---|---|---|---|
| DH (%) | IACE (%) | IC50 (μg mL−1) | ||
| – | 37.8 ± 0.4 | 292.5 ± 20.3 | ||
| Hydrolysis | 0.5 h | 17.5 ± 0.2 | 76.2 ± 3.2 | 214.4 ± 27.3 |
| 2 h | 23.8 ± 1.8 | 80.4 ± 2.3 | 122.5 ± 13.1 | |
| 8 h | 36.4 ± 0.9 | 92.1 ± 0.6 | 76.8 ± 15.2 | |
| Ultrafiltration | 10 kDa retentate | – | 83.3 ± 0.6 | 273.9 ± 30.1 |
| 1 kDa retentate | – | 94.6 ± 2.9 | 235.6 ± 20.2 | |
| 1 kDa permeate | – | 68.0 ± 1.4 | 58.4 ± 4.6 | |
2.3. Identification of Major ACE Inhibitory Peptides
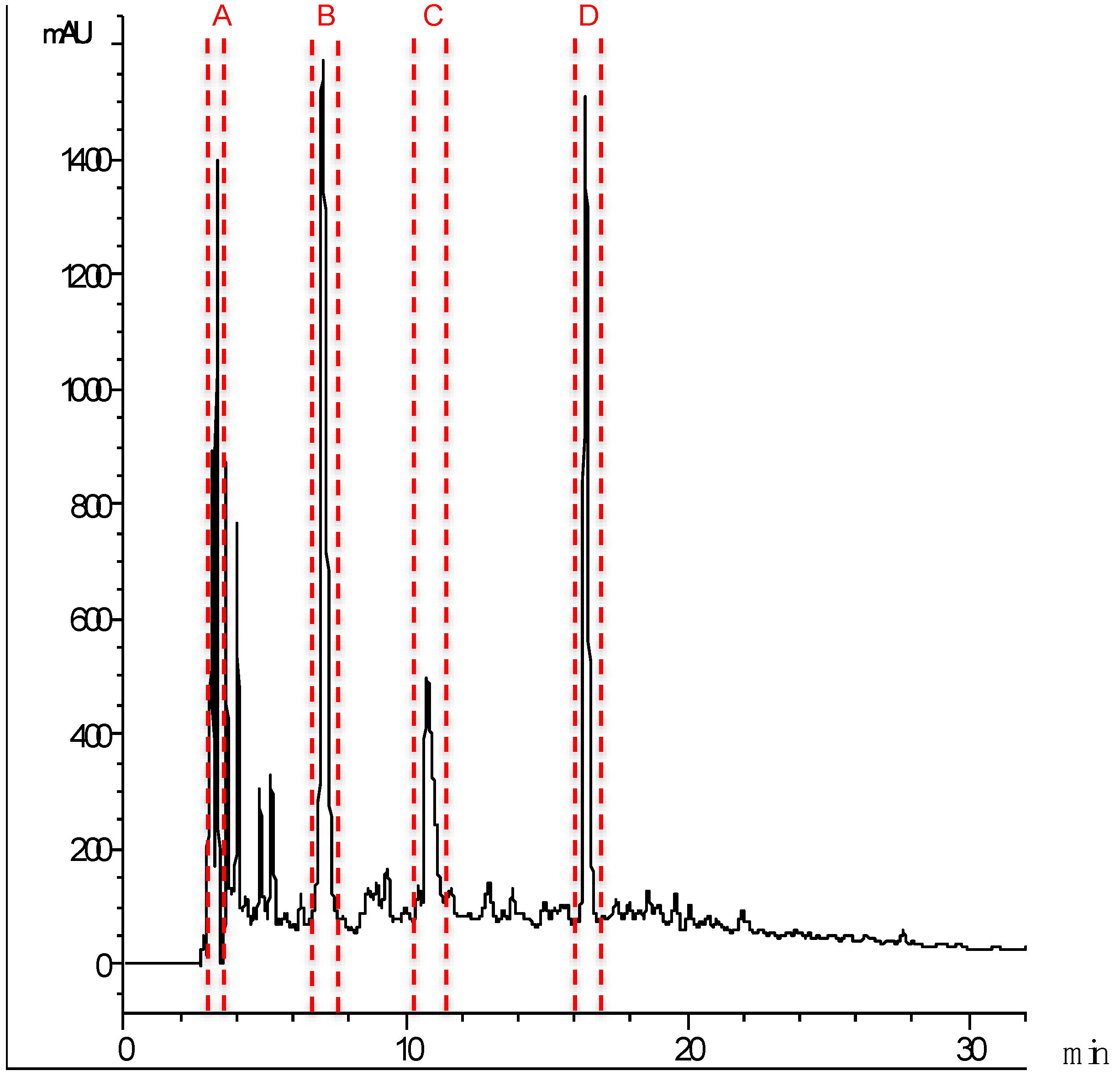
| Fraction | Peptides | Protein Source | Mass (Da) | IC50 (μg mL−1) |
|---|---|---|---|---|
| A | GNALVFLLP | 943.57 | 1.92 ± 0.40 | |
| FALASSLVN | 921.53 | |||
| SPSIAPAL | 755.40 | |||
| GLFAAHRK | 899.54 | |||
| FTPESLAI | 877.50 | |||
| DAAIKTLTK | 960.60 | |||
| MMQLRKA | 877.50 | |||
| RALKIPAM | 899.54 | |||
| FTVKPVSR | 933.59 | |||
| SSSKAKKKP | 960.60 | |||
| KAVPIAIL | 824.58 | |||
| TRAASCP | 705.30 | |||
| INKAVTGLK | 943.57 | |||
| PEGSIRP | 755.40 | |||
| B | VEDAEVGKK | 974.52 | 4.00 ± 0.19 | |
| FAGDDAPRA | 919.43 | |||
| AGSVNKSK | 790.44 | |||
| KAGSELGL | 774.44 | |||
| QSLEVSK | 790.43 | |||
| KEAEVSK | 790.43 | |||
| GAEVTVSK | 790.43 | |||
| QEVSLSK | 790.43 | |||
| AGEVSLSK | 790.43 | |||
| NFGCSVK | 754.35 | |||
| SVCGGFGK | 754.35 | |||
| AGDDPAR | 701.32 | |||
| C | ADQSEGALQK | Myosin HC | 1045.5 | 8.63 ± 0.86 |
| ISEQEASQR | Tropmyosin | 1046.5 | ||
| LGEGGRSTHE | Myosin HC | 1041.5 | ||
| RLKEAENR | Actin | 981.5 | ||
| TDQLGEGGRS | Myosin HC | 1018.5 | ||
| D | DEDATGVIR | Tn C | 974.47 | 7.18 ± 2.44 |
| GTDPEDALRN | Myosin RLC | 1086.49 | ||
| GVNLEDAKRS | Myosin HC | 1087.56 | ||
| LTEAPLNPK | Actin | 981.55 | ||
| REDIDGNIK | Myosin LCK | 1058.54 |
3. Experimental Section
3.1. Cuttlefish Processing Wastewater
3.2. Total Protein Analysis
3.3. Ultrafiltration-Diafiltration Process
3.4. Preparation of Enzymatic Hydrolysates
3.5. Degree of Hydrolysis Determination
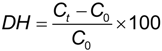
3.6. Angiotensin I-Converting Enzyme (ACE) Inhibition Assay
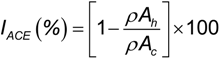
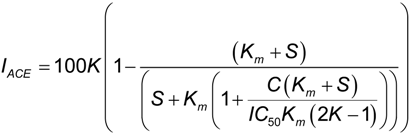
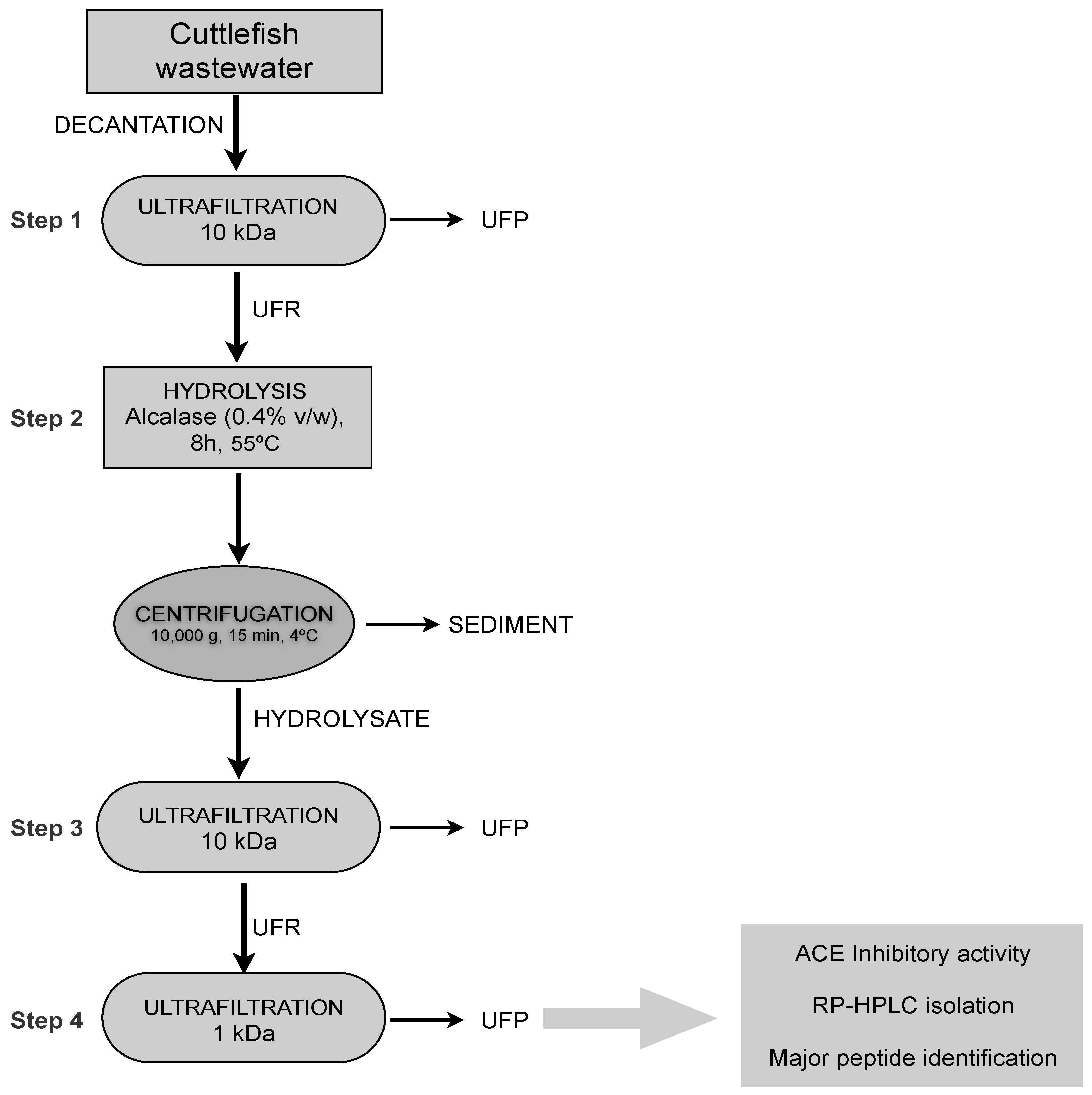
3.7. Isolation of Major ACE-Inhibitory Peptides
3.8. Mass Spectrometry Analysis of ACE-Inhibitory Peptides
3.9. Mass Spectrometry Data Processing
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- World Health Organization. Causes of Death 2008: Data Sources and Methods; World Health Organization: Geneva, Switzerland, 2011. Available online: http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods.pdf (accessed on 20 October 2013).
- World Health Organization. A Global Brief on Hypertension. Silent Killer, Global Public Health Crisis; World Health Organization: Geneva, Switzerland, 2013. Available online: http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf (accessed on 14 December 2013).
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Al Mazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Fang, H.; Luo, M.; Sheng, Y.; Li, Z.; Wu, Y.; Liu, C. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef]
- Israili, Z.H.; Hall, W.D. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann. Int. Med. 1992, 117, 234–242. [Google Scholar] [CrossRef]
- Estévez, N.; Fuciños, P.; Sobrosa, A.C.; Pastrana, L.; Pérez, N.; Rúa, M.L. Modeling the angiotensin-converting enzyme inhibitory activity of peptide mixtures obtained from cheese whey hydrolysates using concentration-response curves. Biotechnol. Prog. 2012, 28, 1197–1206. [Google Scholar] [CrossRef]
- Alemán, A.; Pérez-Santín, E.; Bordenave-Juchereau, S.; Arnaudin, I.; Gómez-Guillén, M.C.; Montero, P. Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res. Int. 2011, 44, 1044–1051. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- He, H.; Chen, X.; Sun, C.; Zhang, Y.; Gao, P. Preparation and functional evaluation of oligopeptide-enriched hydrolysate from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Bioresour. Technol. 2006, 97, 385–390. [Google Scholar] [CrossRef]
- Jung, W.-K.; Mendis, E.; Je, J.-Y.; Park, P.-J.; Son, B.W.; Kim, H.C.; Choi, Y.K.; Kim, S.-K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
- Balti, R.; Nedjar-Arroume, N.; Bougatef, A.; Guillochon, D.; Nasri, M. Three novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) using digestive proteases. Food Res. Int. 2010, 43, 1136–1143. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; González, M.P.; Murado, M.A. Production of antihypertensive and antioxidant activities by enzymatic hydrolysis of protein concentrates recovered by ultrafiltration from cuttlefish processing wastewaters. Biochem. Eng. J. 2013, 76, 43–54. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J. Agric. Food Chem. 2000, 48, 657–666. [Google Scholar] [CrossRef]
- Pérez-Santín, E.; Calvo, M.M.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Compositional properties and bioactive potential of waste material from shrimp cooking juice. LWT-Food Sci. Technol. 2013, 54, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Murado, M.A.; Fraguas, J.; Montemayor, M.I.; Vázquez, J.A.; González, P. Preparation of highly purified chondroitin sulphate from skate (Raja clavata) cartilage by-products. Process optimization including a new procedure of alkaline hydroalcoholic hydrolysis. Biochem. Eng. J. 2010, 49, 126–132. [Google Scholar] [CrossRef]
- Ferjani, E.; Ellouze, E.; Ben Amar, R. Treatment of seafood processing wastewaters by ultrafiltration-nanofiltration cellulose acetate membranes. Desalination 2005, 177, 43–49. [Google Scholar] [CrossRef]
- Ellouze, E.; Ben Amar, R.; Ben Salah, A.H. Cross-flow microfiltration using ceramic membranes applied to the cuttlefish effluents treatment: Effect of operating parameters and the addition of pre or post-treatment. Desalination 2005, 177, 229–240. [Google Scholar] [CrossRef]
- Balti, R.; Nedjar-Arroume, N.; Adjé, E.Y.; Guillochon, D.; Nasri, M. Analysis of novel angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysates of cuttlefish (Sepia officinalis) muscle proteins. J. Agric. Food Chem. 2010, 58, 3840–3846. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, N.; Kim, E.-A.; Kang, M.C.; Lee, S.-H.; Kang, S.-M.; Lee, J.-B.; Jeon, B.-T.; Kim, S.-K.; Park, S.-J.; et al. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Jeon, Y.-J.; Kim, Y.-T.; Je, J.-Y. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by alcalase hydrolysis. Process Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Wijesekara, I.; Qian, Z.-J.; Ryu, B.; Ngo, D.-H.; Kim, S.-K. Purification and identification of antihypertensive peptides from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Res. Int. 2011, 44, 703–707. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Dong, S.; Liu, Z.; Zhao, X.; Wang, J.; Zeng, M. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides 2009, 30, 1028–1033. [Google Scholar] [CrossRef]
- Wu, S.; Sun, J.; Tong, Z; Lan, X.; Zhao, Z.; Liao, D. Optimization of hydrolysis conditions for the production of angiotensin-I converting enzyme-inhibitory peptides and isolation of a novel peptide from lizard fish (Saurida elongata) muscle protein hydrolysate. Mar. Drugs 2012, 10, 1066–1080. [Google Scholar] [CrossRef]
- Hyun, C.-K.; Shin, H.-K. Utilization of bovine blood plasma proteins for the production of angiotensin I converting enzyme inhibitory peptides. Process Biochem. 2000, 36, 65–71. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kwon, J.Y.; Kim, S.-K. A novel Angiotensin I converting enzyme inhibitory peptide from Alaska Pollack (Theragra chalcogramma) frame protein hydrolysate. J. Agric. Food Chem. 2004, 52, 7842–7845. [Google Scholar] [CrossRef]
- Alemán, A.; Gómez-Guillén, M.C.; Montero, P. Identification of ACE-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Res. Int. 2013, 54, 790–795. [Google Scholar] [CrossRef]
- Gordon, A.; Barbut, S. Effect of chloride salts on protein extraction and interfacial protein film formation in meat batters. J. Sci. Food Agric. 1992, 58, 227–238. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Ma, H.; Zhao, W.; Qu, W.; Zhao, J.; Luo, L.; Zhu, W. Modeling the QSAR of ACE-inhibitory peptides with ANN and its applied illustration. Int. J. Pept. 2012, 2012, 1–9. [Google Scholar]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Cushman, D.W. Enzymes of the renin-angiotensin system and their inhibitors. Ann. Rev. Biochem. 2012, 51, 283–308. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Identification of a novel angiotensin-I-converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine β-lactoglobulin. FEBS Lett. 1997, 402, 99–101. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Meisel, H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br. J. Nutr. 2000, 84, S33–S37. [Google Scholar]
- Haque, E.; Chand, R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur. Food Res. Technol. 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Cheung, H.-S.; Wang, F.-L.; Ondetti, M.; Sabo, E.; Cushman, D. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme: Importance of the COOH-terminal dipeptide sequences. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar]
- Barker, T.W.; Worgan, J.T. The utilisation of palm oil processing effluents as substrates for microbial protein production by the fungus Aspergillus oryzae. Appl. Microbiol. Biotechnol. 1981, 11, 234–240. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Zakora, M.; Otte, J. Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates. J. Dairy Res. 2006, 73, 178–186. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amado, I.R.; Vázquez, J.A.; González, P.; Esteban-Fernández, D.; Carrera, M.; Piñeiro, C. Identification of the Major ACE-Inhibitory Peptides Produced by Enzymatic Hydrolysis of a Protein Concentrate from Cuttlefish Wastewater. Mar. Drugs 2014, 12, 1390-1405. https://doi.org/10.3390/md12031390
Amado IR, Vázquez JA, González P, Esteban-Fernández D, Carrera M, Piñeiro C. Identification of the Major ACE-Inhibitory Peptides Produced by Enzymatic Hydrolysis of a Protein Concentrate from Cuttlefish Wastewater. Marine Drugs. 2014; 12(3):1390-1405. https://doi.org/10.3390/md12031390
Chicago/Turabian StyleAmado, Isabel Rodríguez, José Antonio Vázquez, Pilar González, Diego Esteban-Fernández, Mónica Carrera, and Carmen Piñeiro. 2014. "Identification of the Major ACE-Inhibitory Peptides Produced by Enzymatic Hydrolysis of a Protein Concentrate from Cuttlefish Wastewater" Marine Drugs 12, no. 3: 1390-1405. https://doi.org/10.3390/md12031390






