Single and Competitive Adsorption of 17α-Ethinylestradiol and Bisphenol A with Estrone, β-Estradiol, and Estriol onto Sediment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Differences in Adsorption Capacity of EE2 and BPA in the Binary System
| EDCs | Parameters | Conditions | ||||
| Single | Coexisted with E1 | Coexisted with E2 | Coexisted with EE2 | Coexisted with E3 | ||
| BPA | R2 | 0.9931 | 0.9689 | 0.9658 | 0.9619 | 0.9424 |
| k | 0.0072 | 0.0128 | 0.0112 | 0.0083 | 0.0078 | |
| tan θi | - | 0.0057 | 0.0040 | 0.0011 | 0.0006 | |
| EDCs | Parameters | Conditions | ||||
| Single | Coexisted with E1 | Coexisted with E2 | Coexisted with E3 | Coexisted with BPA | ||
| EE2 | R2 | 0.9192 | 0.9687 | 0.9699 | 0.9210 | 0.9340 |
| k | 0.03223 | 0.0254 | 0.02705 | 0.03381 | 0.03149 | |
| tan θi | - | −0.0068 | −0.0052 | 0.0016 | −0.0007 | |
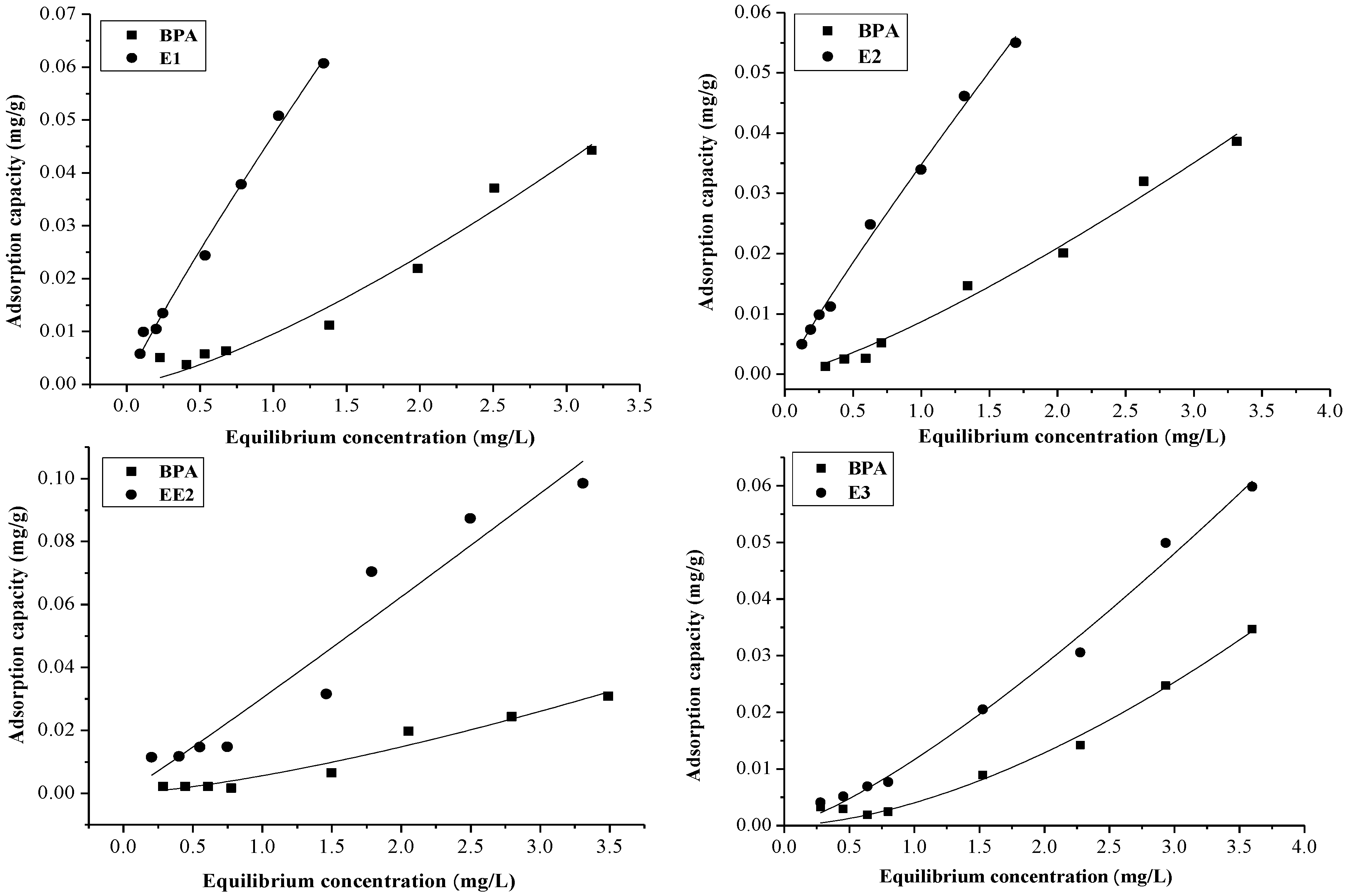

2.2. The Differences of Adsorption Capacity of Multiple EDCs
2.2.1. The Adsorption of BPA and EE2 in a Ternary EDCs System
| EDCs | Parameters | Conditions | |||
| Single | Coexisted with E1 and E2 | Coexisted with E2 and E3 | Coexisted with E1 and E3 | ||
| BPA | R2 | 0.9955 | 0.9868 | 0.9876 | 0.9818 |
| k | 0.0072 | 0.0109 | 0.0114 | 0.0122 | |
| tan θi | - | 0.0037 | 0.0042 | 0.0050 | |
| Parameters | Conditions | ||||
| Single | Coexisted with E2 and EE2 | Coexisted with E1 and EE2 | Coexisted with EE2 and E3 | ||
| R2 | 0.9955 | 0.9909 | 0.9349 | 0.9947 | |
| k | 0.0072 | 0.0105 | 0.0109 | 0.0094 | |
| tan θi | - | 0.0033 | 0.0037 | 0.0022 | |
| EDCs | Parameters | Conditions | |||
| Single | Coexisted with E1 and E2 | Coexisted with E1 and E3 | Coexisted with E2 and E3 | ||
| EE2 | R2 | 0.9236 | 0.9641 | 0.9922 | 0.9856 |
| k | 0.0322 | 0.0241 | 0.0408 | 0.0373 | |
| tan θi | - | −0.0081 | 0.0086 | 0.0051 | |
| Parameters | Conditions | ||||
| Single | Coexisted with E1 and BPA | Coexisted with E2 and BPA | Coexisted with E3 and BPA | ||
| R2 | 0.9236 | 0.9514 | 0.9969 | 0.9884 | |
| k | 0.0322 | 0.0352 | 0.9029 | 0.0307 | |
| tan θi | - | 0.0030 | 0.8461 | −0.0015 | |
2.2.2. The Adsorption of BPA and EE2 in a Quaternary EDCs System
| EDCs | Parameters | Conditions | ||||
| Single | With E1, E2, and EE2 | With E1, E2, and E3 | With E1, EE2, and E3 | With E2, EE2, and E3 | ||
| BPA | R2 | 0.9955 | 0.9931 | 0.9952 | 0.9693 | 0.9899 |
| k | 0.00715 | 0.0098 | 0.0396 | 0.0058 | 0.0130 | |
| tan θi | - | 0.0026 | 0.0324 | −0.0013 | 0.0058 | |
| EDCs | Parameters | Conditions | ||||
| Single | With E1, E2, and E3 | With E1, E2, and BPA | With E1, E3, and BPA | With E2, E3, and BPA | ||
| EE2 | R2 | 0.9236 | 0.9794 | 0.9899 | 0.9965 | 0.9329 |
| k | 0.0322 | 0.0396 | 0.0333 | 0.0290 | 0.0255 | |
| tan θi | - | 0.0074 | 0.0011 | −0.0032 | −0.0067 | |
2.2.3. The Adsorption of BPA and EE2 in a Quinary EDCs System
| EDCs | BPA | ||
| Parameters | R2 | k | tan θi |
| Single | 0.9955 | 0.0072 | - |
| With E1, E2, EE2, and E3 | 0.9961 | 0.0030 | −0.0042 (Antagonistic effect) |
| EDCs | EE2 | ||
| Parameters | R2 | k | tan θi |
| Single | 0.9236 | 0.0322 | - |
| With E1, E2, E3, and BPA | 0.9848 | 0.0212 | −0.0110 (Antagonistic effect) |
2.3. Selectivity of Sediment for EDCs
2.3.1. Selectivity of Sediment for EDCs in a Binary System
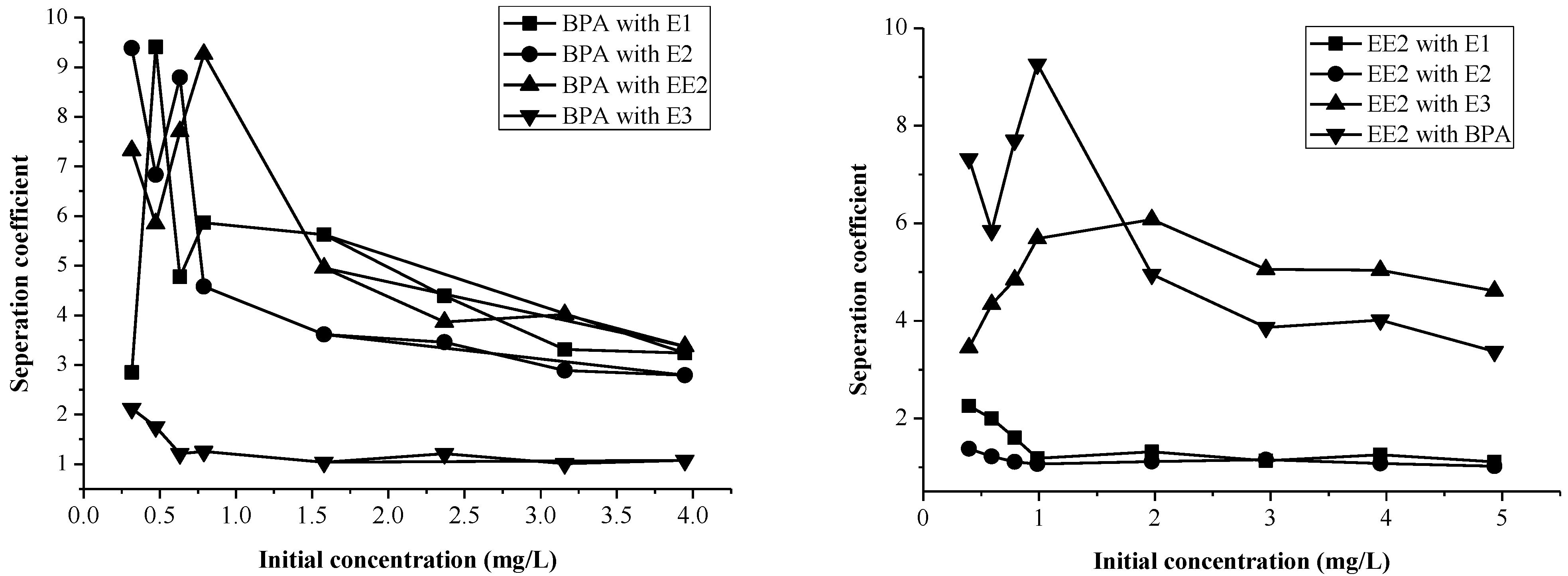
2.3.2. The Selectivity of Sediment for EDCs in a Ternary System

2.3.3. The Selectivity of Sediment for EDCs in a Quaternary and Quinary System

3. Experimental and Methods
3.1. Physicochemical Properties of Sediment
3.2. Materials and Chemicals
3.3. Sorption Isotherms of EDCs
3.4. Analysis of EDCs
3.5. Variation of Adsorption Capacity
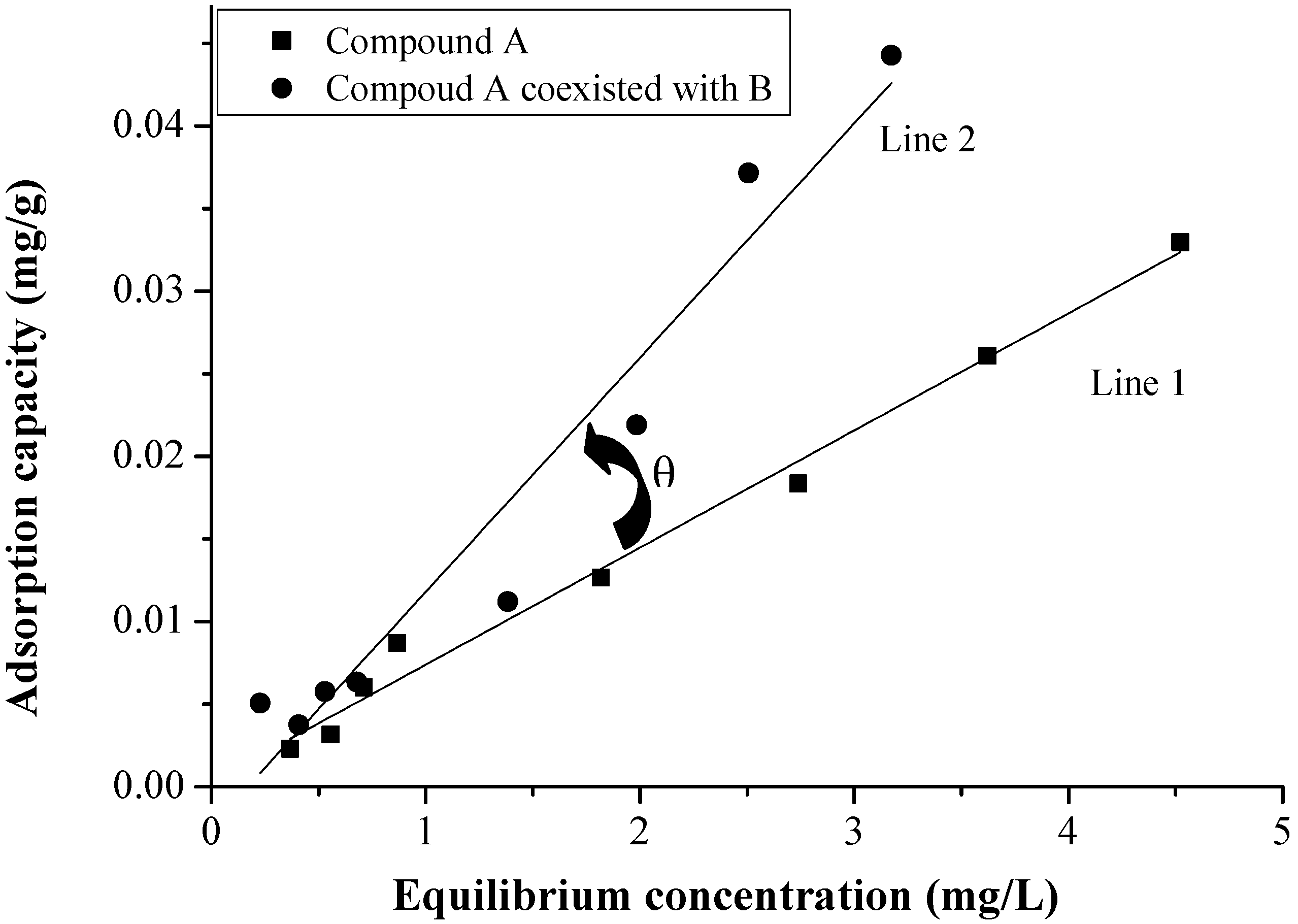
3.6. The Selectivity of Sediment for EDCs
4. Conclusions
- (1)
- The order of selectivity is accordance with the adsorption capacity (E1 > EE2 > E2 > E3 > BPA) showed the adsorption of EDCs in sediment is dominated in physical adsorption.
- (2)
- The EDCs in ternary and quinary system shows a high selectivity of sediment.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Snyder, S.A.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, personal care products, and endocrine disruptors in water: Implications for the water industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Citulski, J.A.; Farahbakhsh, K. Fate of endocrine-active compounds during municipal biosolids treatment: A review. Environ. Sci. Technol. 2010, 44, 8367–8376. [Google Scholar] [CrossRef]
- Shore, L.S.; Shemesh, M. Naturally produced steroid hormones and their release into the environment. Pure Appl. Chem. 2003, 75, 1859–1871. [Google Scholar] [CrossRef]
- Pan, B.; Lin, D.H.; Mshayekhi, H.; Xing, B.S. Adsorption and hysteresis of bisphenol A and 17α-ethinyl estradiol on carbon nanomaterials. Environ. Sci. Technol. 2008, 42, 5480–5485. [Google Scholar] [CrossRef]
- Segner, H.; Navas, J.M.; Schafers, C.; Wenzel, A. Potencies of estrogenic compounds in in vitro screening assays and in life cycle tests with zebrafish in vivo. Ecotoxicol. Environ. Saf. 2003, 54, 315–322. [Google Scholar] [CrossRef]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef]
- Cao, X.L.; Dufresne, G.; Belisle, S.; Clement, G.; Falicki, M.; Beraldin, F.; Rulibikiye, A. Levels of bisphenol A in canned liquid infant formula products in Canada and dietary intake estimates. J. Agric. Food Chem. 2008, 56, 7919–7924. [Google Scholar] [CrossRef]
- Khanal, S.K.; Xie, B.; Thompson, M.L.; Sung, S.W.; Ong, S.K.; van Leeuwen, J. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ. Sci. Technol. 2006, 40, 6537–6546. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Environ. Sci. 2007, 104, 8897–8901. [Google Scholar]
- Kinney, C.A.; Furlong, E.T.; Werner, S.L.; Cahill, J.D. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ. Toxicol. Chem. 2006, 25, 317–326. [Google Scholar] [CrossRef]
- Combalbert, S.; Bellet, V.; Dabert, P.; Bernet, N.; Balaguer, P.; Hernandez-Raquet, G. Fate of steroid hormones and endocrine activities in swine manure disposal and treatment facilities. Water Res. 2012, 46, 895–906. [Google Scholar] [CrossRef]
- Pojana, G.; Gomiero, A.; Jonkers, N.; Marcomini, A. Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ. Int. 2007, 33, 929–936. [Google Scholar] [CrossRef]
- Mortazavi, S.; Bakhtiari, A.R.; Sari, A.E.; Bahramifar, N.; Rahbarizade, F. Phenolic endocrine disrupting chemicals (EDCs) in Anzali Wetland, Iran: Elevated concentrations of 4-nonylphenol, octhylphenol and bisphenol A. Mar. Pollut. Bull. 2012, 64, 1067–1073. [Google Scholar] [CrossRef]
- Fei, Y.H.; Li, X.D.; Li, X.Y. Organic diagenesis in sediment and its impact on the adsorption of bisphenol A and nonylphenol onto marine sediment. Mar. Pollut. Bull. 2011, 63, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ying, G.G.; Kong, L.X.; Wang, L.; Zhao, J.L.; Zhou, L.J.; Zhang, L.J. Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ. Pollut. 2011, 159, 1490–1498. [Google Scholar] [CrossRef]
- Sun, K.; Jin, J.; Gao, B.; Zhang, Z.; Wang, Z.; Pan, Z.; Xu, D.; Zhao, Y. Sorption of 17α-ehinyl estradiol, bisphenol A and phenanthrene to different size fractions of soil and sediment. Chemosphere 2012, 88, 577–583. [Google Scholar] [CrossRef]
- Card, M.L.; Chin, Y.P.; Lee, L.S.; Khan, B. Prediction and experimental evaluation of soil sorption by natural hormones and hormone mimics. J. Agric. Food Chem. 2012, 60, 1480–1487. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Liu, X.; Lv, J. Adsorption and aerobic biodegradation of four selected endocrine disrupting chemicals in soil-water system. Int. Biodeterior. Biodegrad. 2013, 76, 3–7. [Google Scholar] [CrossRef]
- Liu, Z.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment-physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Zhang, C.; Li, S.; Zhou, C.; Li, X. Single and Competitive Adsorption of 17α-Ethinylestradiol and Bisphenol A with Estrone, β-Estradiol, and Estriol onto Sediment. Mar. Drugs 2014, 12, 1349-1360. https://doi.org/10.3390/md12031349
Li Y, Zhang C, Li S, Zhou C, Li X. Single and Competitive Adsorption of 17α-Ethinylestradiol and Bisphenol A with Estrone, β-Estradiol, and Estriol onto Sediment. Marine Drugs. 2014; 12(3):1349-1360. https://doi.org/10.3390/md12031349
Chicago/Turabian StyleLi, Yu, Chen Zhang, Shanshan Li, Changzhi Zhou, and Xiaopeng Li. 2014. "Single and Competitive Adsorption of 17α-Ethinylestradiol and Bisphenol A with Estrone, β-Estradiol, and Estriol onto Sediment" Marine Drugs 12, no. 3: 1349-1360. https://doi.org/10.3390/md12031349




