Iodinin (1,6-Dihydroxyphenazine 5,10-Dioxide) from Streptosporangium sp. Induces Apoptosis Selectively in Myeloid Leukemia Cell Lines and Patient Cells
Abstract
:1. Introduction
2. Results
2.1. Iodinin Shows High Selectivity towards Myeloid Leukemia Cells
| Cell | Reference or ATTC No. | Origin | Disease | Features | EC50 (μM) |
|---|---|---|---|---|---|
| Primary heptaocytes | Rat | Freshly isolated primary hepatocytes in suspension | >5.0 | ||
| Cardiomyoblasts | CRL-1446 | Rat | >5.0 | ||
| NRK | CRL-6509 | Rat | Normal rat kidney fibroblasts | >10 | |
| Jurkat T, Clone E6-1 | TIB-152 | Human | Acute T-cell lymphoblastic leukemia | 0.8 ± 0.2 | |
| SH-SY5Y | CRL-2266 | Human | Neuroblastoma | 2.7 ± 0.2 | |
| HeLa | CCL-2 | Human | Cervical epithelial adenocarcinoma | Low levels of p53 expression | >10 |
| U-87 MG | HTB-14 | Human | Astrocytoma | >10 | |
| NB4 | [13] | Human | Acute promyelocytic leukemia (APL) | t(15;17) (q22;q11-12) translocation, ATRA-induced differentiation | 0.75 ± 0.13 |
| NB4-LR1 | [14] | Human | Acute promyelocytic leukemia (APL) | ATRA and cAMP needed to induce differentiation | 0.70 ± 0.10 |
| IPC-81 | [15] | Rat | APL | Brown Norwegian rat myeloid leukemia model | 0.24 ± 0.15 |
| IPC-81 Bcl-2 | [16] | Rat | APL | Enforced expression of Bcl-2 | 3.15 ± 0.15 |
| Molm13 | [17,18] | Human | Acute myeloid leukemia (AML) | ins(11;9)(q23;p22p23), FLT3 itd | 1.0 ± 0.12 |
| Molm13-SHp53 | Human | AML | Silenced p53 | 1.0 ± 0.09 | |
| MV-4-11 | [18,19] | Human | AML, myelomonocytic | t(4;11) translocation, FLT3 itd | 0.50 ± 0.20 |
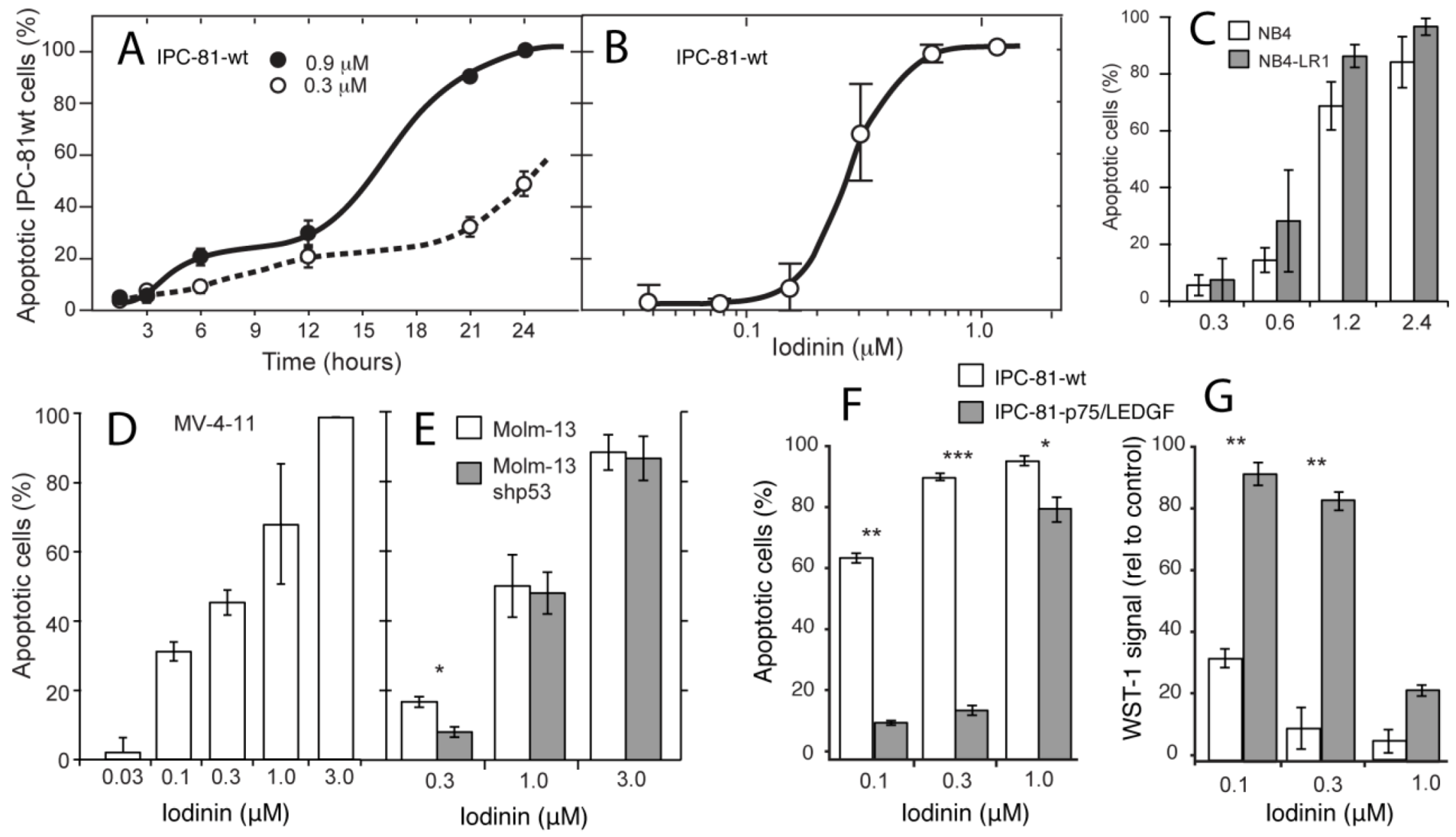
2.2. Iodinin Induces Cell Death with Apoptotic Features, and Shows Structural Similarity to Daunorubicin
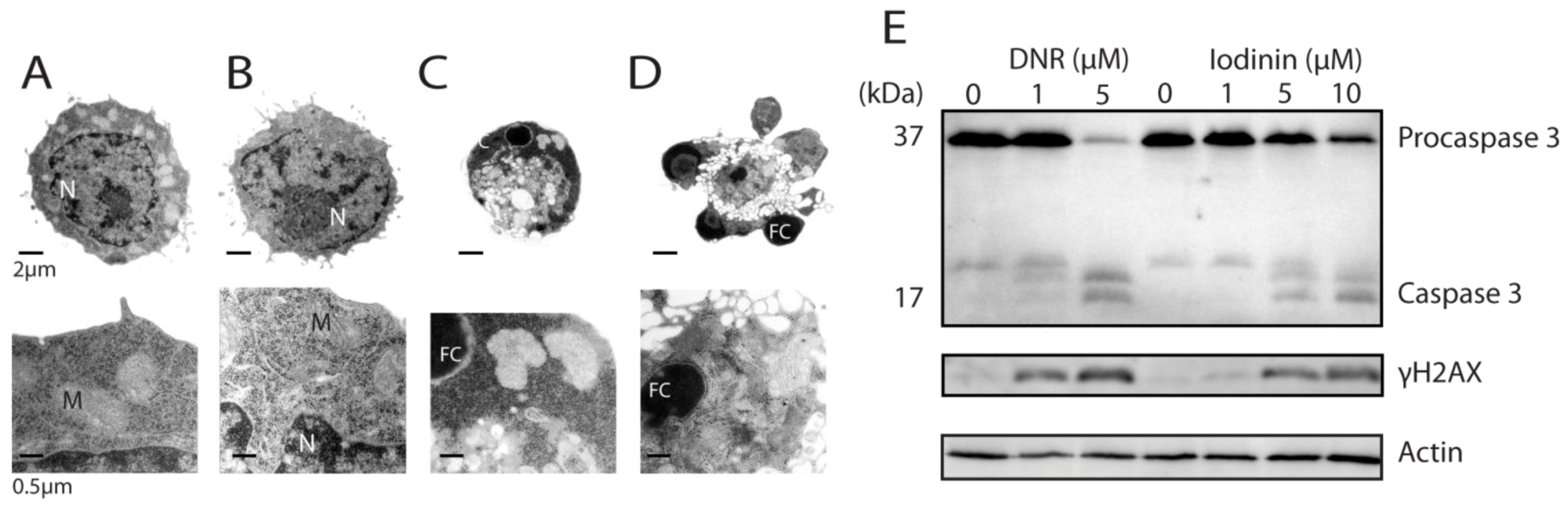

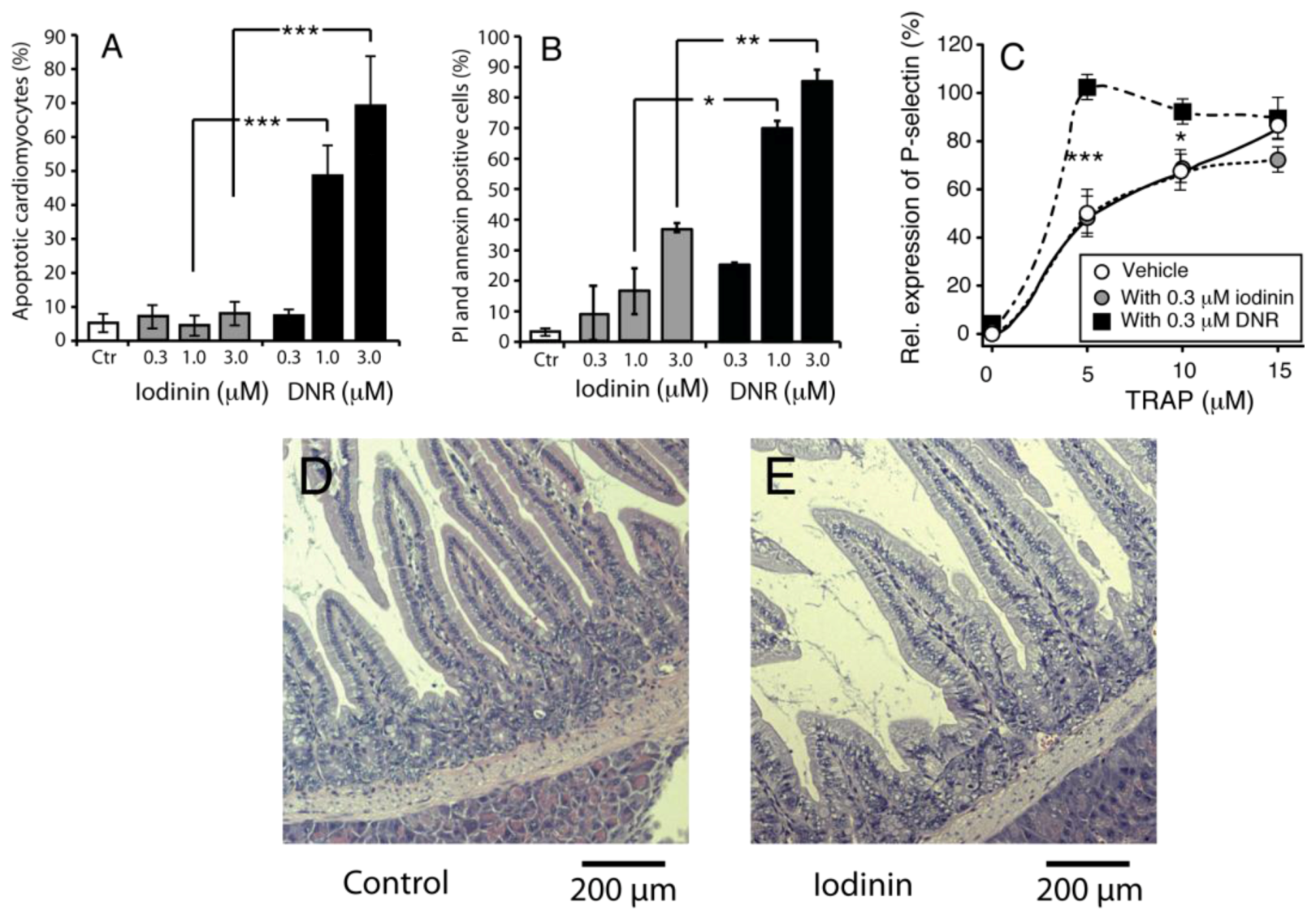
2.3. Iodinin Induces Cell Death in AML-Patient Blasts, but Has Low Toxicity to Cardiomyoblasts, Leukocytes and Platelets
| Patients | Age | Sex | Cytogenetics | FABclassification | FLt3 | NPM1 |
|---|---|---|---|---|---|---|
| AML#1 | 24 | M | Multiple | M2 | wt | wt |
| APL#2 | 39 | M | T(15;17) | M3 | wt | wt |
| AML#3 | 48 | M | Inv(16) | M4 | wt | wt |
| AML#4 | 29 | M | Normal | M4 | ITD | Ins |
| AML#5 | 18 | F | Inv(16) | M4 | wt | wt |
| AML#6 | 29 | F | Normal | M5 | wt | nd |

3. Discussion
4. Experimental Section
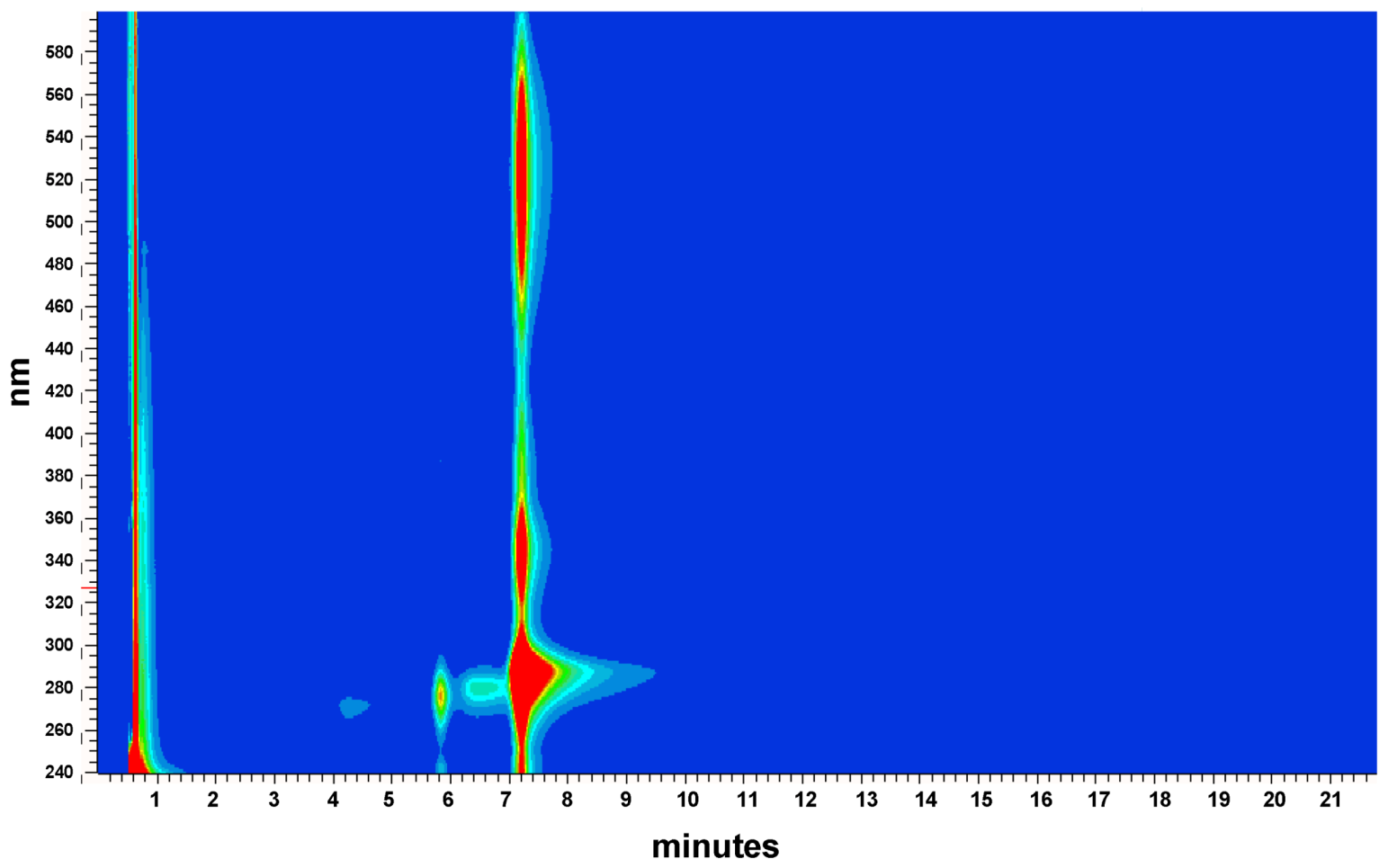
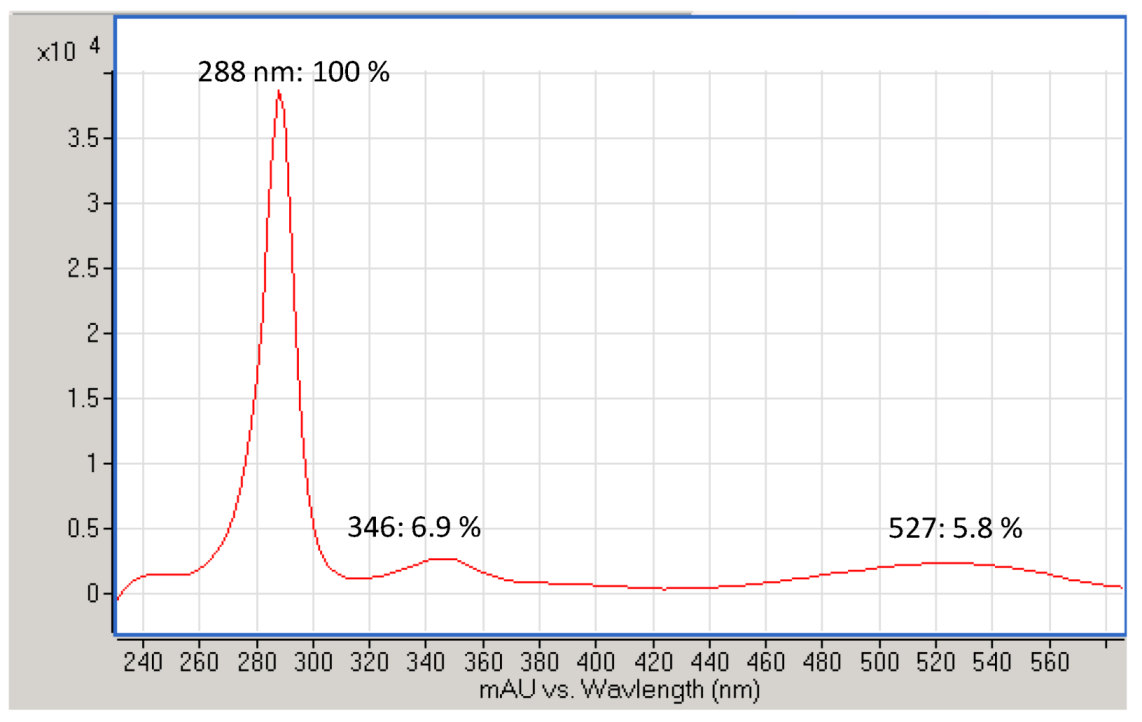
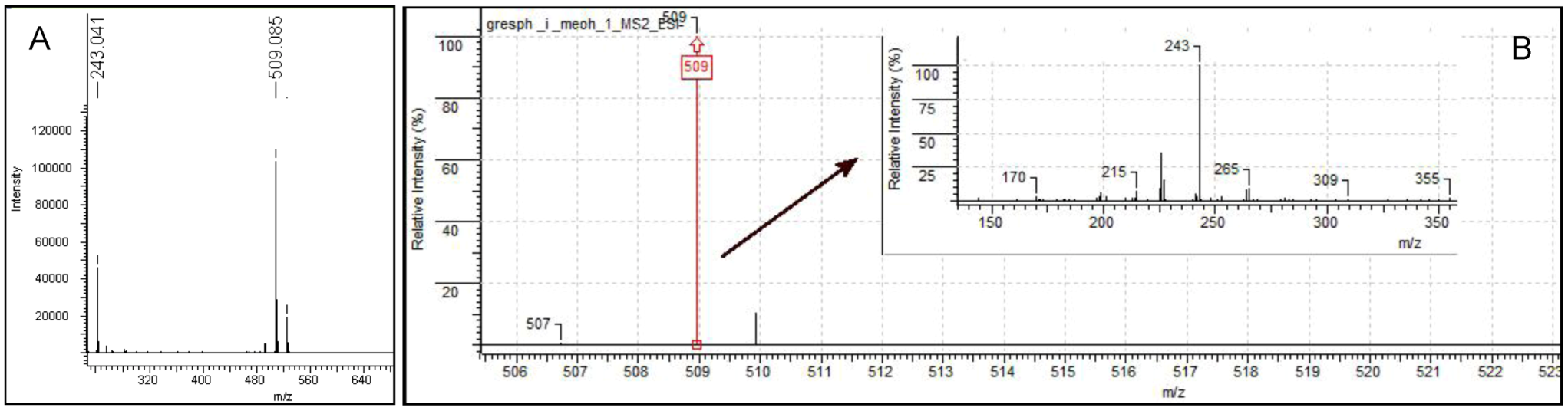
4.1. Purification and Identification of Iodinin from Isolate MP53-27
4.2. Cell Maintenance and Experimental Conditions
4.3. Transmission Electron Microscopy
4.4. Western Blotting
4.5. Molecular Visualisation
4.6. Isolation of Blood Platelets and Measurement of P-Selectin Translocation
4.7. Flow Cytometry of AML Patient Material
4.8. Histopathological Analysis
5. Conclusions
Acknowledgments
Supplementary Information
References
- Fenaux, P.; Chastang, C.; Chevret, S.; Sanz, M.; Dombret, H.; Archimbaud, E.; Fey, M.; Rayon, C.; Huguet, F.; Sotto, J.J.; et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. Blood 1999, 94, 1192–1200. [Google Scholar]
- Mayer, R.J.; Davis, R.B.; Schiffer, C.A.; Berg, D.T.; Powell, B.L.; Schulman, P.; Omura, G.A.; Moore, J.O.; McIntyre, O.R.; Frei, E., III. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N. Engl. J. Med. 1994, 331, 896–903. [Google Scholar] [CrossRef]
- Mehta, A.; Hoffbrand, V. Haematology at a Glance, 3rd ed; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Burnett, A.; Wetzler, M.; Lowenberg, B. Therapeutic advances in acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 487–494. [Google Scholar] [CrossRef]
- Jemal, A.; Clegg, L.X.; Ward, E.; Ries, L.A.; Wu, X.; Jamison, P.M.; Wingo, P.A.; Howe, H.L.; Anderson, R.N.; Edwards, B.K. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer 2004, 101, 3–27. [Google Scholar] [CrossRef]
- Joel, S.P.; Rohatiner, A. Pharmacology of Antileukemic Drugs. In Leukemia, 7th; Henderson, E.D., Lister, T.A., Greaves, M.F., Eds.; Saunders: Philadelphia, PA, USA, 2002; pp. 394–440. [Google Scholar]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Alster, R. Drug-Induced Thrombocytopenia. In Platelets; Michelson, A., Ed.; Academic Press: New York, NY, USA, 2007; pp. 887–902. [Google Scholar]
- Pierson, L.S., III; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef]
- Laursen, J.B.; Nielsen, J. Phenazine natural products: Biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 2004, 104, 1663–1686. [Google Scholar] [CrossRef]
- McIlwain, H. The anti-streptococcal action of iodinin. Naphthaquinones and anthraquinones as its main natural antagonists. Biochem. J. 1943, 37, 265–271. [Google Scholar]
- Endo, H.; Tada, M.; Katagiri, K. Studies on antitumor activity of phenazine derivatives against S 180 in mice. (V). On iodinin and its isomers. Sci. Rep. Res. Inst. Tohoku Univ. Med. 1967, 14, 169–170. [Google Scholar]
- Lanotte, M.; Martin-Thouvenin, V.; Najman, S.; Balerini, P.; Valensi, F.; Berger, R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991, 77, 1080–1086. [Google Scholar]
- Ruchaud, S.; Duprez, E.; Gendron, M.C.; Houge, G.; Genieser, H.G.; Jastorff, B.; Doskeland, S.O.; Lanotte, M. Two distinctly regulated events, priming and triggering, during retinoid-induced maturation and resistance of NB4 promyelocytic leukemia cell line. Proc. Natl. Acad. Sci. USA 1994, 91, 8428–8432. [Google Scholar]
- Lacaze, N.; Gombaud-Saintonge, G.; Lanotte, M. Conditions controlling long-term proliferation of Brown Norway rat promyelocytic leukemia in vitro: Primary growth stimulation by microenvironment and establishment of an autonomous Brown Norway “leukemic stem cell line”. Leuk. Res. 1983, 7, 145–154. [Google Scholar] [CrossRef]
- Seite, P.; Ruchaud, S.; Hillion, J.; Gendron, M.C.; Bruland, O.; Segal-Bendirdjian, E.; Døskeland, S.O.; Lillehaug, J.R.; Lanotte, M. Ectopic expression of Bcl-2 switches over nuclear signalling for cAMP-induced apoptosis to granulocytic differentiation. Cell Death Differ. 2000, 7, 1081–1089. [Google Scholar] [CrossRef]
- Matsuo, Y.; MacLeod, R.A.; Uphoff, C.C.; Drexler, H.G.; Nishizaki, C.; Katayama, Y.; Kimura, G.; Fujii, N.; Omoto, E.; Harada, M.; et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia 1997, 11, 1469–1477. [Google Scholar]
- Quentmeier, H.; Reinhardt, J.; Zaborski, M.; Drexler, H.G. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia 2003, 17, 120–124. [Google Scholar] [CrossRef]
- Lange, B.; Valtieri, M.; Santoli, D.; Caracciolo, D.; Mavilio, F.; Gemperlein, I.; Griffin, C.; Emanuel, B.; Finan, J.; Nowell, P.; et al. Growth factor requirements of childhood acute leukemia: Establishment of GM-CSF-dependent cell lines. Blood 1987, 70, 192–199. [Google Scholar]
- Motyckova, G.; Stone, R.M. Treatment of elderly acute myeloid leukemia patients. Curr. Treat. Options Oncol. 2011, 12, 341–353. [Google Scholar] [CrossRef]
- Huang, T.S.; Myklebust, L.M.; Kjarland, E.; Gjertsen, B.T.; Pendino, F.; Bruserud, O.; Doskeland, S.O.; Lillehaug, J.R. LEDGF/p75 has increased expression in blasts from chemotherapy-resistant human acute myelogenic leukemia patients and protects leukemia cells from apoptosis in vitro. Mol. Cancer 2007, 6, 31. [Google Scholar]
- Riedl, S.J.; Salvesen, G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell. Biol. 2007, 8, 405–413. [Google Scholar]
- Takahashi, A.; Ohnishi, T. Does gammaH2AX foci formation depend on the presence of DNA double strand breaks? Cancer Lett. 2005, 229, 171–179. [Google Scholar] [CrossRef]
- Walters, D.K.; Wu, X.; Tschumper, R.C.; Arendt, B.K.; Huddleston, P.M.; Henderson, K.J.; Dispenzieri, A.; Jelinek, D.F. Evidence for ongoing DNA damage in multiple myeloma cells as revealed by constitutive phosphorylation of H2AX. Leukemia 2011, 25, 1344–1353. [Google Scholar] [CrossRef]
- Durrieu, F.; Belloc, F.; Lacoste, L.; Dumain, P.; Chabrol, J.; Dachary-Prigent, J.; Morjani, H.; Boisseau, M.R.; Reiffers, J.; Bernard, P.; et al. Caspase activation is an early event in anthracycline-induced apoptosis and allows detection of apoptotic cells before they are ingested by phagocytes. Exp. Cell. Res. 1998, 240, 165–175. [Google Scholar] [CrossRef]
- Dai, J.; Punchihewa, C.; Mistry, P.; Ooi, A.T.; Yang, D. Novel DNA bis-intercalation by MLN944, a potent clinical bisphenazine anticancer drug. J. Biol. Chem. 2004, 279, 46096–46103. [Google Scholar] [CrossRef]
- Robinson, H.; Priebe, W.; Chaires, J.B.; Wang, A.H. Binding of two novel bisdaunorubicins to DNA studied by NMR spectroscopy. Biochemistry 1997, 36, 8663–8670. [Google Scholar] [CrossRef]
- Kurkjian, C.D.; Hozer, H. Leukopenia Anemia and Thrombocytopenia. In Cancer, Principles and Practice of Oncology, 8th; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Wolters Kluwer: London, UK, 2008; pp. 2617–2631. [Google Scholar]
- De Koning, B.A.; Lindenbergh-Kortleve, D.J.; Pieters, R.; Buller, H.A.; Renes, I.B.; Einerhand, A.W. Alterations in epithelial and mesenchymal intestinal gene expression during doxorubicin-induced mucositis in mice. Dig. Dis. Sci. 2007, 52, 1814–1825. [Google Scholar] [CrossRef]
- Estey, E. High cytogenetic or molecular genetic risk acute myeloid leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2010, 2010, 474–480. [Google Scholar] [CrossRef]
- Han, L.N.; Zhou, J.; Schuringa, J.J.; Vellenga, E. Treatment strategies in acute myeloid leukemia. Chin. Med. J. 2011, 124, 1409–1421. [Google Scholar]
- Foss, B.; Bruserud, O. Platelet functions and clinical effects in acute myelogenous leukemia. Thromb. Haemost. 2008, 99, 27–37. [Google Scholar]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2010. Molecules 2011, 16, 5629–5646. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.X.; Ouyang, L.; Cheng, Y.; Liu, B. Plant natural compounds: Targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012, 45, 466–476. [Google Scholar] [CrossRef]
- Fulda, S. Modulation of apoptosis by natural products for cancer therapy. Planta Medica 2010, 76, 1075–1079. [Google Scholar] [CrossRef]
- Nagle, A.; Hur, W.; Gray, N.S. Antimitotic agents of natural origin. Curr. Drug Targets 2006, 7, 305–326. [Google Scholar] [CrossRef]
- Chen, G.; Wang, F.; Trachootham, D.; Huang, P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion 2010, 10, 614–625. [Google Scholar] [CrossRef]
- Wheate, N.J.; Brodie, C.R.; Collins, J.G.; Kemp, S.; Aldrich-Wright, J.R. DNA intercalators in cancer therapy: Organic and inorganic drugs and their spectroscopic tools of analysis. Mini Rev. Med. Chem. 2007, 7, 627–648. [Google Scholar] [CrossRef]
- Jones, G.P.; Lewis, D.G.; Tate, M.E.; Snow, M.R.; Tiekink, E.R. Structure of the pseudomonad fungal antibiotic phenazine-1-carboxylic acid. Acta Crystallogr. C 1988, 44, 2220–2222. [Google Scholar] [CrossRef]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Disc. Today 2011, 16, 354–360. [Google Scholar] [CrossRef]
- Jorgensen, H.; Fjaervik, E.; Hakvag, S.; Bruheim, P.; Bredholt, H.; Klinkenberg, G.; Ellingsen, T.E.; Zotchev, S.B. Candicidin biosynthesis gene cluster is widely distributed among Streptomyces spp. isolated from the sediments and the neuston layer of the Trondheim Fjord, Norway. Appl. Environ. Microbiol. 2009, 75, 3296–3303. [Google Scholar] [CrossRef]
- Oftedal, L.; Selheim, F.; Wahlsten, M.; Sivonen, K.; Doskeland, S.O.; Herfindal, L. Marine benthic cyanobacteria contain apoptosis-inducing activity synergizing with daunorubicin to kill leukemia cells, but not cardiomyocytes. Mar. Drugs 2010, 8, 2659–2672. [Google Scholar] [CrossRef]
- Bøe, R.; Gjertsen, B.T.; Vintermyr, O.K.; Houge, G.; Lanotte, M.; Døskeland, S.O. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp. Cell Res. 1991, 195, 237–246. [Google Scholar] [CrossRef]
- Herrmann, M.; Lorenz, H.M.; Voll, R.; Grunke, M.; Woith, W.; Kalden, J.R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucl. Acids Res. 1994, 22, 5506–5507. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Mac, version 19.0; IBM Corp.: Armonk, NY, USA, 2010.
- Krakstad, C.; Herfindal, L.; Gjertsen, B.T.; Bøe, R.; Vintermyr, O.K.; Fladmark, K.E.; Døskeland, S.O. CaM-kinaseII-dependent commitment to microcystin-induced apoptosis is coupled to cell budding, but not to shrinkage or chromatin hypercondensation. Cell Death Differ. 2006, 13, 1191–1202. [Google Scholar] [CrossRef]
- Dixon, S.L.; Smondyrev, A.M.; Rao, S.N. PHASE: A novel approach to pharmacophore modeling and 3D database searching. Chem. Biol. Drug Des. 2006, 67, 370–372. [Google Scholar] [CrossRef]
- Maestro, version 9.2; Schrödinger, LLC: New York, NY, USA, 2011.
- 49. Discovery Studio Modeling Environment, release 3.5, Accelrys Software Inc. San Diego, CA, USA, 2012.
- Tysnes, O.B.; Verhoeven, A.J.; Holmsen, H. Rates of production and consumption of phosphatidic acid upon thrombin stimulation of human platelets. Eur. J. Biochem. 1988, 174, 75–79. [Google Scholar] [CrossRef]
- Selheim, F.; Holmsen, H.; Vassbotn, F.S. PI 3-kinase signalling in platelets: The significance of synergistic, autocrine stimulation. Platelets 2000, 11, 69–82. [Google Scholar] [CrossRef]
- Shattil, S.J.; Cunningham, M.; Hoxie, J.A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood 1987, 70, 307–315. [Google Scholar]
- Selheim, F.; Holmsen, H.; Vassbotn, F.S. Platelet-derived growth factor inhibits platelet activation in heparinized whole blood. Thromb. Res. 1999, 95, 185–196. [Google Scholar] [CrossRef]
- Gavasso, S.; Gjertsen, B.; Anderssen, E.; Myhr, K.; Vedeler, C. Immunogenic effects of recombinant interferon-β therapy disrupt the JAK/STAT pathway in primary immune cells from patients with multiple sclerosis. Mult. Scler. 2012, 18, 1116–1124. [Google Scholar] [CrossRef]
- Bruserud, O.; Gjertsen, B.T.; Foss, B.; Huang, T.S. New strategies in the treatment of acute myelogenous leukemia (AML): In vitro culture of aml cells—The present use in experimental studies and the possible importance for future therapeutic approaches. Stem Cells 2001, 19, 1–11. [Google Scholar] [CrossRef]
- Xu, H.; Lv, M.; Tian, X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003-2007. Curr. Med. Chem. 2009, 16, 327–349. [Google Scholar] [CrossRef]
- Cholewinski, G.; Dzierzbicka, K.; Kolodziejczyk, A.M. Natural and synthetic acridines/acridones as antitumor agents: Their biological activities and methods of synthesis. Pharmacol. Rep. 2011, 63, 305–336. [Google Scholar]
- Meng, C.K.; Fenn, J.B. Formation of charged clusters during electrospray ionization of organic solute species. Org. Mass Spectrom. 1991, 26, 542–549. [Google Scholar] [CrossRef]
- Baures, P.W.; Wiznycia, A.; Beatty, A.M. Hydrogen bonding isosteres: Bimolecular carboxylic acid and amine-N-oxide interactions mediated via CH...O hydrogen bonds. Bioorg. Med. Chem. 2000, 8, 1599–1605. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Smedsgaard, J. Fungal metabolite screening: Database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A 2003, 1002, 111–136. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Myhren, L.E.; Nygaard, G.; Gausdal, G.; Sletta, H.; Teigen, K.; Degnes, K.F.; Zahlsen, K.; Brunsvik, A.; Bruserud, Ø.; Døskeland, S.O.; et al. Iodinin (1,6-Dihydroxyphenazine 5,10-Dioxide) from Streptosporangium sp. Induces Apoptosis Selectively in Myeloid Leukemia Cell Lines and Patient Cells. Mar. Drugs 2013, 11, 332-349. https://doi.org/10.3390/md11020332
Myhren LE, Nygaard G, Gausdal G, Sletta H, Teigen K, Degnes KF, Zahlsen K, Brunsvik A, Bruserud Ø, Døskeland SO, et al. Iodinin (1,6-Dihydroxyphenazine 5,10-Dioxide) from Streptosporangium sp. Induces Apoptosis Selectively in Myeloid Leukemia Cell Lines and Patient Cells. Marine Drugs. 2013; 11(2):332-349. https://doi.org/10.3390/md11020332
Chicago/Turabian StyleMyhren, Lene E., Gyrid Nygaard, Gro Gausdal, Håvard Sletta, Knut Teigen, Kristin F. Degnes, Kolbjørn Zahlsen, Anders Brunsvik, Øystein Bruserud, Stein Ove Døskeland, and et al. 2013. "Iodinin (1,6-Dihydroxyphenazine 5,10-Dioxide) from Streptosporangium sp. Induces Apoptosis Selectively in Myeloid Leukemia Cell Lines and Patient Cells" Marine Drugs 11, no. 2: 332-349. https://doi.org/10.3390/md11020332





