HPN, a Synthetic Analogue of Bromophenol from Red Alga Rhodomela confervoides: Synthesis and Anti-Diabetic Effects in C57BL/KsJ-db/db Mice
Abstract
:1. Introduction
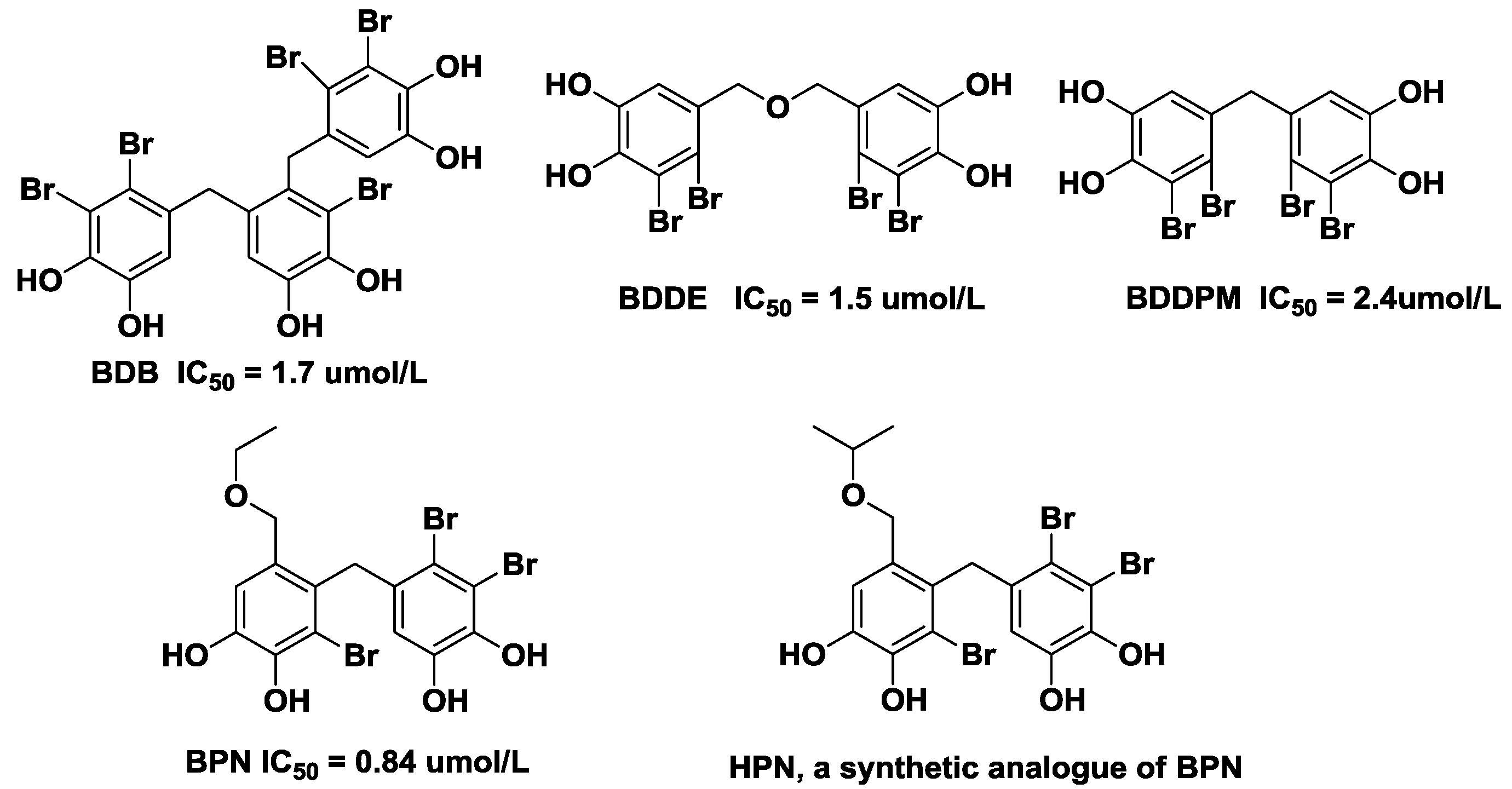
2. Results and Discussion
2.1. Characterization and Identification of HPN
2.2. Enzyme-Based Inhibitory Activities Assays in Vitro
2.3. Effects of HPN on Body Weight, Food and Water Intake
| Group | Body weight (g) a | Food intake (g/day) a | Water intake (mL/day) a | |||
|---|---|---|---|---|---|---|
| initial | final | initial | final | initial | final | |
| Control | 17.7 ± 0.9 | 22.0 ± 2.5 | 3.42 ± 0.03 | 3.26 ± 0.40 | 3.46 ± 0.04 | 3.67 ± 0.19 |
| Model | 36.6 ± 2.1 ** | 53.7 ± 2.6 ** | 5.05 ± 0.03 | 7.67 ± 0.62 | 7.55 ± 0.17 | 13.12 ± 0.95 |
| HPN (40 mg/kg) | 35.9 ± 1.6 | 52.3 ± 3.1 | 4.88 ± 0.07 | 5.46 ± 0.89 | 7.89 ± 0.13 | 9.94 ± 0.41 |
| HPN (20 mg/kg) | 35.7 ± 1.8 | 50.5 ± 4.4 | 4.85 ± 0.02 | 6.54 ± 0.70 | 7.96 ± 0.34 | 12.00 ± 0.39 |
| HPN (10 mg/kg) | 35.7 ± 1.5 | 48.1 ± 3.0 # | 4.79 ± 0.08 | 6.65 ± 0.68 | 7.67 ± 0.04 | 12.92 ± 0.36 |
| Rosiglitazone (20 mg/kg) | 36.0 ± 3.1 | 54.8 ± 4.1 | 5.03 ± 0.06 | 7.09 ± 0.48 | 8.41 ± 0.49 | 11.72 ± 0.34 |
2.4. Effects of HPN on Blood Glucose
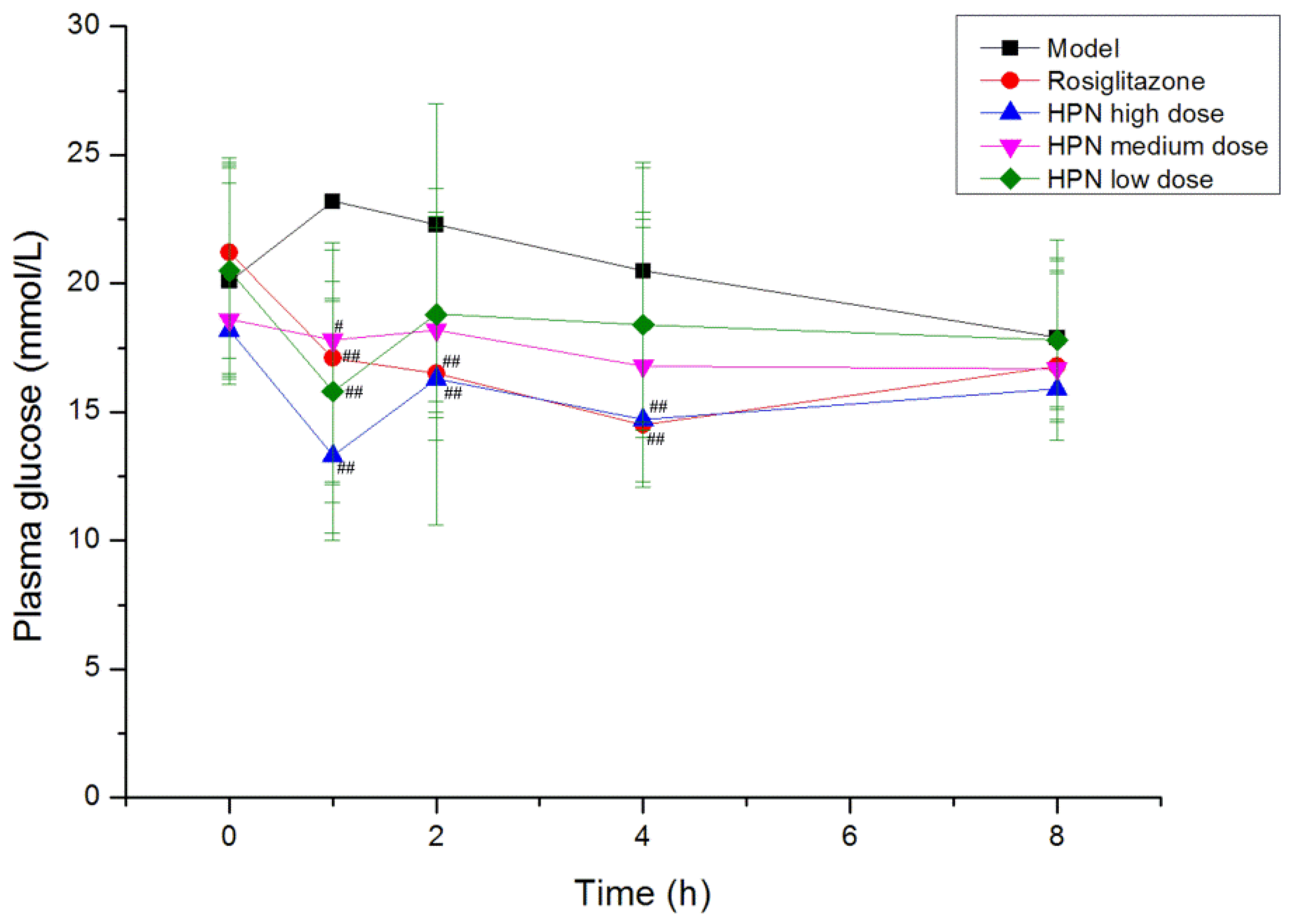
| Group | Dose (mg/kg) | Plasma glucose (mmol/L) a | |||
|---|---|---|---|---|---|
| Baseline | 1 week | 4 weeks | 8 weeks | ||
| Control | 6.0 ± 0.6 | 6.0 ± 0.4 | 6.1 ± 0.3 | 5.8 ± 0.4 | |
| Model | 18.9 ± 4.5 ** | 20.0 ± 3.6 ** | 20.2 ± 4.6 ** | 18.9 ± 4.1 ** | |
| HPN | 40 | 19.8 ± 5.0 | 15.1 ± 3.6 ## | 14.7 ± 3.7 ## | 15.8 ± 2.4 # |
| 20 | 18.6 ± 4.5 | 16.6 ± 3.4 # | 15.4 ± 4.7 # | 15.5 ± 3.3 # | |
| 10 | 19.4 ± 4.9 | 16.0 ± 3.7 # | 17.5 ± 3.9 | 16.5 ± 3.5 | |
| Rosiglitazone | 20 | 18.7 ± 3.8 | 15.5 ± 4.0 # | 15.6 ± 3.1 # | 13.9 ± 2.9 ## |
2.5. Effects of HPN on Serum Lipids
| Group | Dose (mg/kg) | Triglycerides (mmol/L) a | Total cholesterol (mmol/L) a | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4 weeks | 8 weeks | Baseline | 4 weeks | 8 weeks | |||
| Control | 1.19 ± 0.25 | 1.30 ± 0.26 | 1.19 ± 0.24 | 3.01 ± 0.83 | 3.32 ± 0.80 | 2.99 ± 0.72 | ||
| Model | 1.47 ± 0.30 * | 1.69 ± 0.39 * | 1.74 ± 0.40 ** | 4.20 ± 1.17 * | 7.22 ± 2.91 ** | 7.18 ± 2.18 ** | ||
| HPN | 40 | 1.36 ± 0.35 | 1.32 ± 0.21 # | 1.32 ± 0.34 # | 3.89 ± 0.93 | 4.67 ± 1.55 # | 5.01 ± 1.64 # | |
| 20 | 1.44 ± 0.42 | 1.32 ± 0.27 # | 1.36 ± 0.31 # | 3.86 ± 1.42 | 5.01 ± 1.45 | 5.12 ± 2.31 | ||
| 10 | 1.44 ± 0.28 | 1.45 ± 0.30 | 1.47 ± 0.32 | 4.72 ± 0.91 | 5.54 ± 1.59 | 5.79 ± 1.69 | ||
| Rosiglitazone | 20 | 1.46 ± 0.23 | 1.33 ± 0.27 # | 1.36 ± 0.23 # | 4.19 ± 1.04 | 4.67 ± 1.42 # | 4.44 ± 1.60 ## | |
2.6. Effects of HPN on Glycated Hemoglobin and Glycated Serum Protein
| Group | Dose (mg/kg) | HbA1c a (%) | GSP a (mmol/L) |
|---|---|---|---|
| Control | 1.1 ± 0.5 | 1.3 ± 0.2 | |
| Model | 4.5 ± 1.3 ** | 4.8 ± 1.0 ** | |
| HPN | 40 | 3.2 ± 0.8 # | 3.2 ± 0.9 ## |
| 20 | 3.5 ± 0.9 # | 3.3 ± 1.4 # | |
| 10 | 3.7 ± 1.2 | 3.7 ± 1.3 # | |
| Rosiglitazone | 20 | 2.8 ± 0.9 ## | 3.1 ± 1.0 ## |
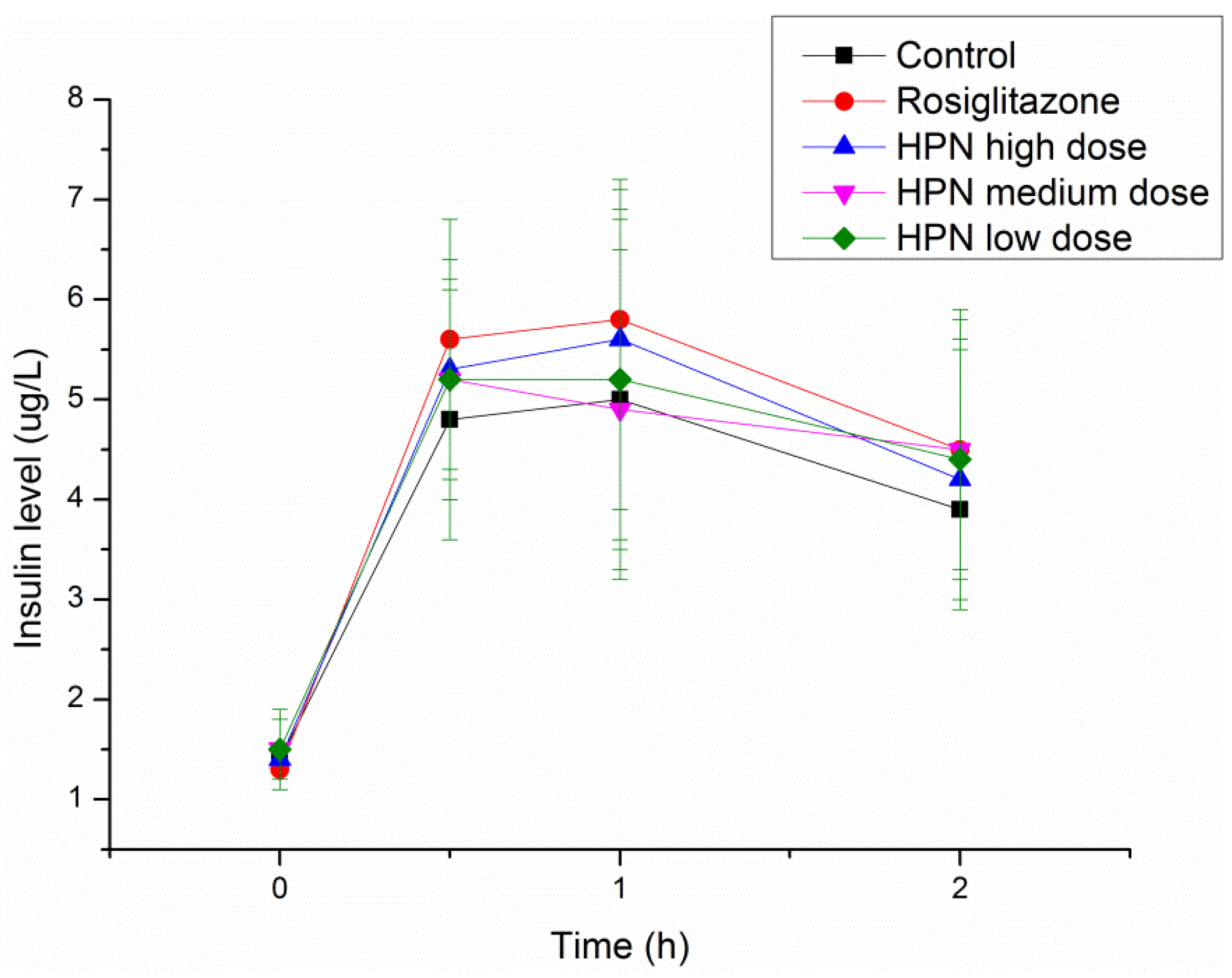
2.7. Effects of HPN on Plasma Insulin Level
| Group | Dose (mg/kg) | Insulin (ng/mL) a | |
|---|---|---|---|
| 4 weeks | 8 weeks | ||
| Control | 1.18 ± 0.37 | 1.36 ± 0.42 | |
| Model | 22.56 ± 6.61 ** | 27.94 ± 4.78 ** | |
| 40 | 15.30 ± 6.26 # | 20.80 ± 8.31 # | |
| HPN | 20 | 15.94 ± 7.02 | 22.22 ± 6.10 # |
| 10 | 15.55 ± 3.79 # | 23.40 ± 6.50 | |
| Rosiglitazone | 20 | 10.60 ± 3.71 ## | 10.80 ± 3.43 ## |
2.8. PTP1B Expression in Pancreatic Tissue (Western Blotting)

2.9. Intraperitoneal Glucose Tolerance Test in Sprague–Dawley Rats
| Group | Dose (mg/kg) | Plasma glucose (mmol/L) a | |||
|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 2 h | ||
| Control | 4.0 ± 0.4 | 10.7 ± 3.5 | 10.7 ± 2.8 | 7.3 ± 1.4 | |
| HPN | 40 | 4.0 ± 0.8 | 10.4 ± 2.2 | 10.3 ± 2.1 | 7.4 ± 0.9 |
| 20 | 4.0 ± 0.4 | 10.7 ± 3.1 | 10.3 ± 2.1 | 7.8 ± 1.0 | |
| 10 | 4.2 ± 0.5 | 10.9 ± 2.9 | 10.8 ± 2.7 | 8.3 ± 1.1 | |
| Rosiglitazone | 20 | 3.9 ± 0.3 | 9.7 ± 1.7 | 10.4 ± 2.5 | 7.4 ± 1.1 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Preparation of HPN

3.3. Enzymatic Activity Assay in Vitro
3.4. Animals
3.5. Experiment Design
- Model group: db/db mice given 0.5% CMC-Na and 2% Tween-80.
- Positive drug group: db/db mice given rosiglitazone (20 mg/kg).
- HPN high-dose group: db/db mice given HPN (40 mg/kg).
- HPN medium-dose group: db/db mice given HPN (20 mg/kg).
- HPN low-dose group: db/db mice given HPN (10 mg/kg).
3.6. Western Blotting Assay
3.7. Intraperitoneal Glucose Tolerance Test (IPGTT) in SD Rats
- Control group: SD rats given 0.5% CMC-Na.
- Positive drug group: SD rats given rosiglitazone (20 mg/kg).
- HPN high-dose group: SD rats given HPN (40 mg/kg).
- HPN medium-dose group: SD rats given HPN (20 mg/kg).
- HPN low-dose group: SD rats given HPN (10 mg/kg).
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Tahrani, A.A.; Bailey, C.J.; Del Prato, S.; Barnett, A.H. Management of type 2 diabetes: New and future developments in treatment. Lancet 2011, 378, 182–197. [Google Scholar]
- Popov, D. Novel protein tyrosine phosphatase 1B inhibitors: Interaction requirements for improved intracellular efficacy in type 2 diabetes mellitus and obesity control. Biochem. Biophys. Res. Commun. 2011, 410, 377–381. [Google Scholar] [CrossRef]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.C.; et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef]
- Hooft van Huijsduijnen, R.; Sauer, W.H.; Bombrun, A.; Swinnen, D. Prospects for inhibitors of protein tyrosine phosphatase 1B as antidiabetic drugs. J. Med. Chem. 2004, 47, 4142–4146. [Google Scholar] [CrossRef]
- Bialy, L.; Waldmann, H. Inhibitors of protein tyrosine phosphatases: Next-generation drugs? Angew. Chem. Int. Ed. 2005, 44, 3814–3839. [Google Scholar] [CrossRef]
- Heneberg, P. Use of protein tyrosine phosphatase inhibitors as promising targeted therapeutic drugs. Curr. Med. Chem. 2009, 16, 706–733. [Google Scholar] [CrossRef]
- Patel, D.; Jain, M.; Shah, S.R.; Bahekar, R.; Jadav, P.; Joharapurkar, A.; Dhanesha, N.; Shaikh, M.; Sairam, K.V.; Kapadnis, P. Discovery of potent, selective and orally bioavailable triaryl-sulfonamide based PTP1B inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1111–1117. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.T.; Zhao, J.; Shi, X.; Hu, S.C.; Gao, K. Norditerpenoids from Agathis macrophylla. Food Chem. 2012, 131, 972–976. [Google Scholar] [CrossRef]
- Sun, L.P.; Shen, Q.; Piao, H.H.; Ma, W.P.; Gao, L.X.; Zhang, W.; Nan, F.J.; Li, J.; Piao, H.R. Synthesis and biological evaluation of (+/−)-3-(2-(2-fluorobenzyloxy) naphthalen-6-yl)-2-aminopropanoic acid derivatives as novel PTP1B inhibitors. Eur. J. Med. Chem. 2011, 46, 3630–3638. [Google Scholar] [CrossRef]
- Song, Z.; He, X.P.; Li, C.; Gao, L.X.; Wang, Z.X.; Tang, Y.; Xie, J.; Li, J.; Chen, G.R. Preparation of triazole-linked glycosylated α-ketocarboxylic acid derivatives as new PTP1B inhibitors. Carbohydr. Res. 2011, 346, 140–145. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Dao, T.T.; Kim, J.; Phong, D.T.; Ndinteh, D.T.; Mbafor, J.T.; Oh, W.K. New 5-deoxyflavonoids and their inhibitory effects on protein tyrosine phosphatase 1B (PTP1B) activity. Bioorg. Med. Chem. 2011, 19, 3378–3383. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Xing, S.; Ho, W.T.; Fu, X.; Zhao, Z.J. Tea contains potent inhibitors of tyrosine phosphatase PTP1B. Biochem. Biophys. Res. Commun. 2011, 407, 98–102. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, W.; Kim, S.N.; Yoon, G.; Cheon, S.H. Design, synthesis, and evaluation of bromo-retrochalcone derivatives as protein tyrosine phosphatase 1B inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3755–3758. [Google Scholar] [CrossRef]
- Li, C.; He, X.P.; Zhang, Y.J.; Li, Z.; Gao, L.X.; Shi, X.X.; Xie, J.; Li, J.; Chen, G.R.; Tang, Y. Click to a focused library of benzyl 6-triazolo(hydroxy)benzoic glucosides: Novel construction of PTP1B inhibitors on a sugar scaffold. Eur. J. Med. Chem. 2011, 46, 4212–4218. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Q.; Lu, J.C.; Li, J.Y.; Liu, W.Y.; Yang, J.J.; Li, J.; Xiao, K. Phenolic compounds from the leaves of Cyclocarya paliurus (Batal.) Ijinskaja and their inhibitory activity against PTP1B. Food Chem. 2010, 119, 1491–1496. [Google Scholar] [CrossRef]
- Xu, J.Q.; Shen, Q.; Li, J.; Hu, L.H. Dammaranes from Gynostemma pentaphyllum and synthesis of their derivatives as inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. 2010, 18, 3934–3939. [Google Scholar] [CrossRef]
- Huang, X.C.; Li, J.; Li, Z.Y.; Shi, L.; Guo, Y.W. Sesquiterpenes from the Hainan sponge Dysidea septosa. J. Nat. Prod. 2008, 71, 1399–1403. [Google Scholar] [CrossRef]
- Mao, S.C.; Guo, Y.W.; Shen, X. Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg. Med. Chem. Lett. 2006, 16, 2947–2950. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. Isolation, characterization, and antioxidant activity of bromophenols of the marine red alga Rhodomela confervoides. J. Agric. Food. Chem. 2011, 59, 9916–9921. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar]
- Sun, X.; Xu, N.; Guo, J.; Yan, X. Antitumor effects and the mechanism of two kinds of bromophenols from marine algae. China J. Chin. Mater. Med. 2010, 35, 1173–1176. [Google Scholar]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef]
- Shi, D.Y.; Xu, F.; He, J.; Li, J.; Fan, X.; Han, L.J. Inhibition of bromophenols against PTP1B and anti-hyperglycemic effect of Rhodomela confervoides extract in diabetic rats. Chin. Sci. Bull. 2008, 53, 2476–2479. [Google Scholar] [CrossRef]
- Shi, D.; Li, J.; Jiang, B.; Guo, S.; Su, H.; Wang, T. Bromophenols as inhibitors of protein tyrosine phosphatase 1B with antidiabetic properties. Bioorg. Med. Chem. Lett. 2012, 22, 2827–2832. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, D.; Cui, Y.; Guo, S. Design, synthesis, and biological evaluation of bromophenol derivatives as protein tyrosine phosphatase 1B inhibitors. Arch. Pharm. (Weinheim) 2012, 345, 444–453. [Google Scholar] [CrossRef]
- Guo, S.J.; Li, J.; Li, T.; Shi, D.Y.; Han, L.J. Synthesis of three bromophenols from red algae as PTP1B inhibitors. Chin. J. Oceanol. Limn. 2011, 29, 68–74. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.Y.; Hu, L.H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta 2006, 1760, 1505–1512. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, D.; Guo, S.; Jiang, B.; Guo, C.; Wang, T.; Zhang, L.; Li, J. HPN, a Synthetic Analogue of Bromophenol from Red Alga Rhodomela confervoides: Synthesis and Anti-Diabetic Effects in C57BL/KsJ-db/db Mice. Mar. Drugs 2013, 11, 350-362. https://doi.org/10.3390/md11020350
Shi D, Guo S, Jiang B, Guo C, Wang T, Zhang L, Li J. HPN, a Synthetic Analogue of Bromophenol from Red Alga Rhodomela confervoides: Synthesis and Anti-Diabetic Effects in C57BL/KsJ-db/db Mice. Marine Drugs. 2013; 11(2):350-362. https://doi.org/10.3390/md11020350
Chicago/Turabian StyleShi, Dayong, Shuju Guo, Bo Jiang, Chao Guo, Tao Wang, Luyong Zhang, and Jingya Li. 2013. "HPN, a Synthetic Analogue of Bromophenol from Red Alga Rhodomela confervoides: Synthesis and Anti-Diabetic Effects in C57BL/KsJ-db/db Mice" Marine Drugs 11, no. 2: 350-362. https://doi.org/10.3390/md11020350
APA StyleShi, D., Guo, S., Jiang, B., Guo, C., Wang, T., Zhang, L., & Li, J. (2013). HPN, a Synthetic Analogue of Bromophenol from Red Alga Rhodomela confervoides: Synthesis and Anti-Diabetic Effects in C57BL/KsJ-db/db Mice. Marine Drugs, 11(2), 350-362. https://doi.org/10.3390/md11020350




