Natural Products from Antarctic Colonial Ascidians of the Genera Aplidium and Synoicum: Variability and Defensive Role
Abstract
:1. Introduction
2. Methods and Materials
2.1. Collection of Samples
| Ascidian species name and code number | Latitude | Longitude | Gear | Depth (m) |
|---|---|---|---|---|
| Aplidium falklandicum Millar, 1960 (1) | 70°57.00′ S | 10°33.02′ W | BT | 332.8 |
| Aplidium falklandicum Millar, 1960 (2) | 70°55.92′ S | 10°32.37′ W | AGT | 288 |
| Aplidium falklandicum Millar, 1960 (3) | 70°56.67′ S | 10°32.05′ W | BT | 302.4 |
| Aplidium falklandicum Millar, 1960 (4) | 70°57.11′ S | 10°33.52′ W | BT | 337.2 |
| Aplidium fuegiense Cunningham, 1871 | 71°7′ S | 11°26′ W | AGT | 228.4 |
| Aplidium meridianum (Sluiter, 1906) (1) | 70°56.42′ S | 10°31.61′ W | BT | 284.4 |
| Aplidium meridianum (Sluiter, 1906) (2) | 71°04.30′ S | 01°33.92′ W | BT | 308.8 |
| Aplidium millari Monniot & Monniot, 1994 | 71°04.30′ S | 01°33.92′ W | BT | 308.8 |
| Synoicum adareanum (B&W) (Herdman, 1902) (1) | 70°56′ S | 10°32′ W | BT | 337.2 |
| Synoicum adareanum (B&W) (Herdman, 1902) (2) | 70°55.92′ S | 10°32.37′ W | AGT | 288.0 |
| Synoicum adareanum (B&W) (Herdman, 1902) (3) | 70°56.42′ S | 10°31.61′ W | BT | 284.4 |
| Synoicum adareanum (Br) (Herdman, 1902) | 71°06.44′ S | 11°27.76′ W | AGT | 277.2 |
| Synoicum adareanum (O) (Herdman, 1902) (1 and 3) | 70°55.92′ S | 10°32.37′ W | AGT | 288.0 |
| Synoicum adareanum (O) (Herdman, 1902) (2) | 70°56′ S | 10°32′ W | BT | 337.2 |
2.2. Organic Extractions
| Species name, sample code and body part | [NEE] (mg g−1 DW) | Isolated metabolites |
|---|---|---|
| Aplidium falklandicum 1 | 42.00 | Meridianins (A–G) a + (I–U) b |
| Aplidium falklandicum 2 EXT | 57.23 | Meridianins (A–G) a |
| Aplidium falklandicum 2 INT | 79.3 | Meridianins (A–G) a |
| Aplidium falklandicum 3 EXT | 47.60 | Meridianins (A–G) a |
| Aplidium falklandicum 3 INT | 128.40 | Meridianins (A–G) a |
| Aplidium falklandicum 4 EXT | 23.80 | Meridianins (A–G) a |
| Aplidium falklandicum 4 INT | 19.40 | Meridianins (A–G) a |
| Aplidium fuegiense EXT | 15.12 | Rossinone B |
| Aplidium fuegiense INT | 85.10 | Rossinone B + (derivatives) c |
| Aplidium meridianum 1 | 128.51 | Meridianins (A–G) a |
| Aplidium meridianum 2 | 79.36 | Meridianins (A–G) a |
| Aplidium millari EXT | 39.31 | - |
| Aplidium millari INT | 81.60 | - |
| Synoicum adareanum (B&W) 1 EXT | 20.04 | - |
| Synoicum adareanum (B&W) 1 INT | 33.09 | - |
| Synoicum adareanum (B&W) 2 API | 55.69 | - |
| Synoicum adareanum (B&W) 2 EXT | 18.12 | - |
| Synoicum adareanum (B&W) 2 INT | 27.31 | - |
| Synoicum adareanum (B&W) 3 | 20.88 | - |
| Synoicum adareanum (Br) | 36.83 | - |
| Synoicum adareanum (O) 1 | 20.41 | - |
| Synoicum adareanum (O) 2 EXT | 28.02 | - |
| Synoicum adareanum (O) 2 INT | 26.43 | - |
| Synoicum adareanum (O) 3 EXT | 30.71 | - |
| Synoicum adareanum (O) 3 INT | 66.04 | - |
2.3. Purifications and Chemical Analysis
2.4. Spectral Analysis of the Natural Products
2.5. Feeding Deterrence Assays with Sea Stars
2.6. Feeding Preference Assays with Amphipods
2.7. Antibacterial Tests against a Sympatric Marine Antarctic Bacterium
3. Results
3.1. Ascidian Samples and Organic Extractions
3.2. Chemical Analysis of the Natural Products

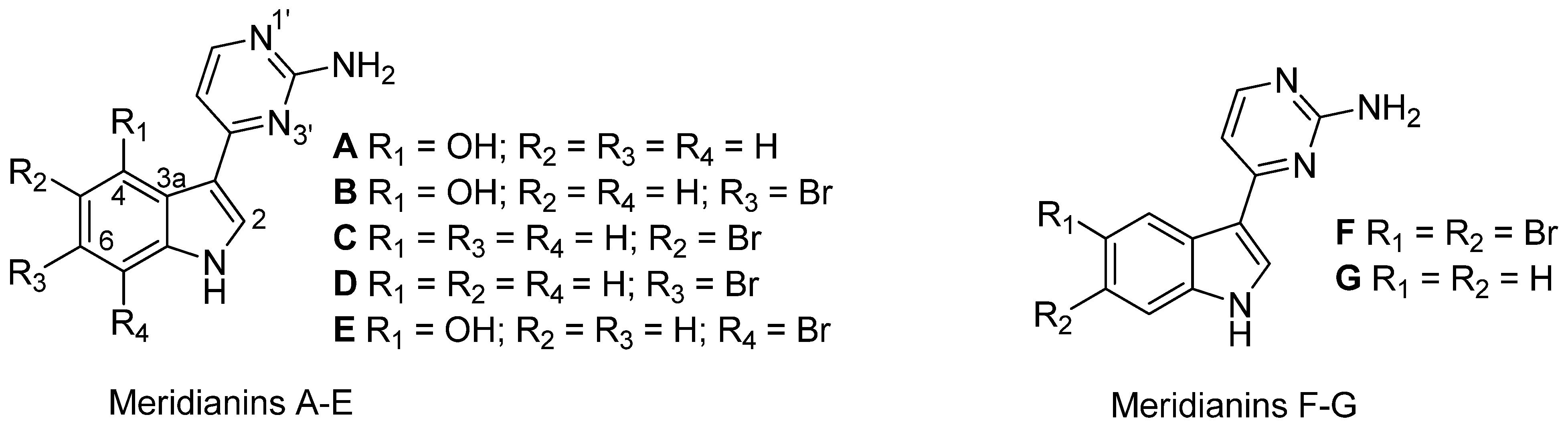
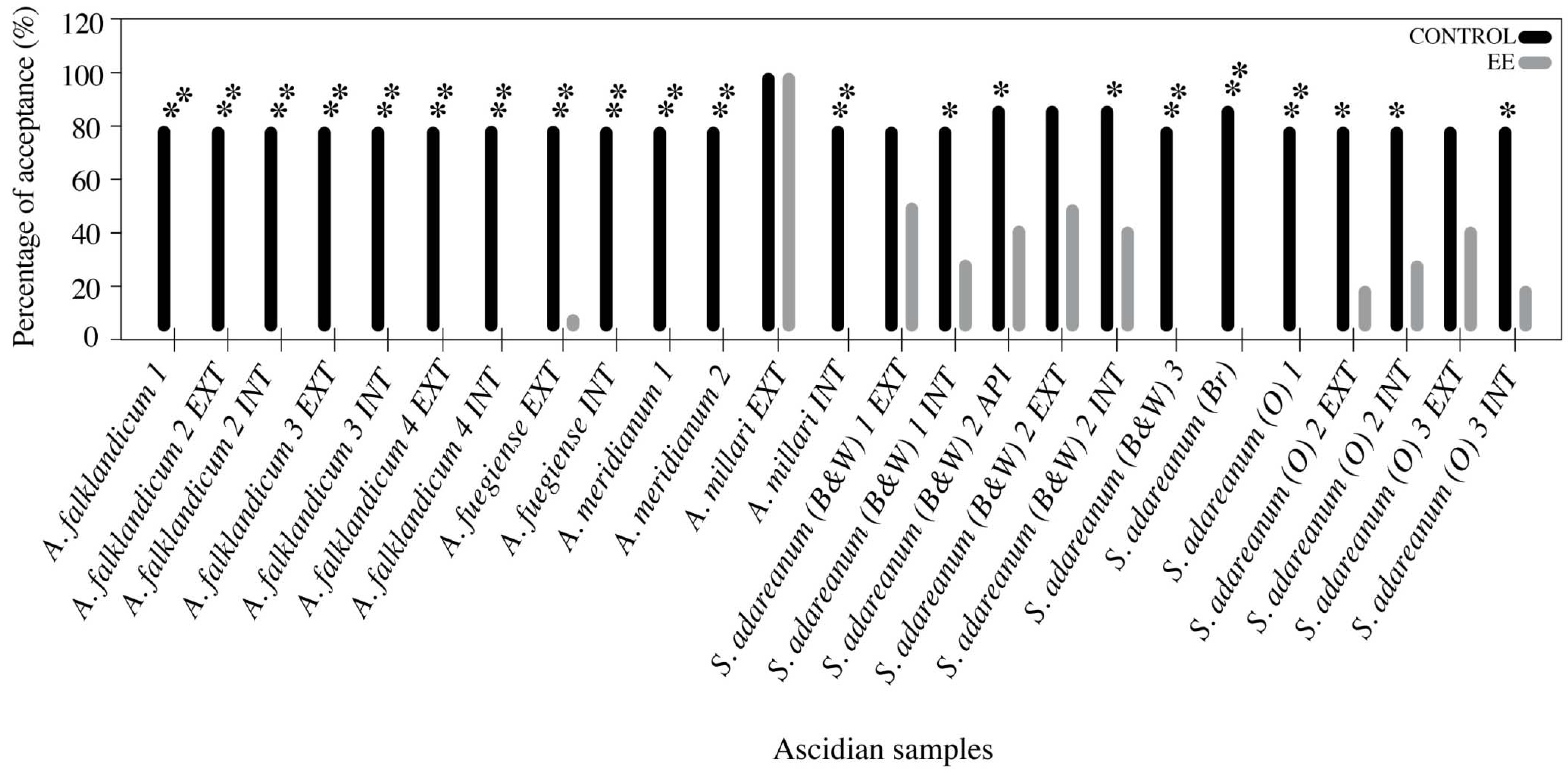
3.3. Feeding Deterrence Assays with Sea Stars
3.4. Feeding Preference Assays with Amphipods

3.5. Antibacterial Tests against a Sympatric Marine Antarctic Bacterium
4. Discussion
4.1. Incidence and Allocation of Chemical Defenses against Predation
4.2. Antibiotic Activity towards Marine Bacteria
4.3. Variability and Origin of Bioactive Natural Products

5. Conclusions
Acknowledgments
References
- Lambert, G. Ecology and natural history of the protochordates. Can. J. Zool. 2005, 83, 34–50. [Google Scholar] [CrossRef]
- Brusca, R.C.; Brusca, G.J. Invertebrates, 2nd ed; Sinauer Associates: Sunderland, MA, USA, 2003; p. 895. [Google Scholar]
- Hirose, E. Ascidian tunic cells: Morphology and functional diversity of free cells outside the epidermis. Invertebr. Biol. 2009, 128, 83–96. [Google Scholar] [CrossRef]
- Tarjuelo, I.; Lopez-Legentil, S.; Codina, M.; Turon, X. Defence mechanisms of adults and larvae of colonial ascidians: patterns of palatability and toxicity. Mar. Ecol. Prog. Ser. 2002, 235, 103–115. [Google Scholar] [CrossRef]
- Koplovitz, G.; McClintock, J.B. An evaluation of chemical and physical defenses against fish predation in a suite of seagrass-associated ascidians. J. Exp. Mar. Biol. Ecol. 2011, 407, 48–53. [Google Scholar] [CrossRef]
- Lambert, G. Early post-metamorphic growth, budding and spicule formation in the compound ascidian Cystodytes lobatus. Biol. Bull. 1979, 157, 464–477. [Google Scholar] [CrossRef]
- Lambert, G.; Lambert, C.C. Extracellular formation of body and tunic spicules in the New Zealand solitary ascidian Pyura pachydermatina (Urochordata, Ascidiacea). Acta Zool. 1997, 78, 51–60. [Google Scholar] [CrossRef]
- López-Legentil, S.; Turón, X.; Schupp, P. Chemical and physical defenses against predators in Cystodytes (Ascidiacea). J. Exp. Mar. Biol. Ecol. 2006, 332, 27–36. [Google Scholar] [CrossRef]
- Stoecker, D. Relationships between chemical defense and ecology in benthic ascidians. Mar. Ecol. Prog. Ser. 1980, 3, 257–265. [Google Scholar] [CrossRef]
- Stoecker, D. Chemical defenses of ascidians against predators. Ecology 1980, 61, 1327–1334. [Google Scholar] [CrossRef]
- Pisut, D.P.; Pawlik, J.R. Anti-predatory chemical defenses of ascidians: Secondary metabolites or inorganic acids? J. Exp. Mar. Biol. Ecol. 2002, 270, 203–214. [Google Scholar] [CrossRef]
- McClintock, J.B.; Amsler, M.O.; Amsler, C.D.; Southworth, K.J.; Petrie, C.; Baker, B.J. Biochemical composition, energy content and chemical antifeedant and antifoulant defenses of the colonial Antarctic ascidian Distaplia cylindrica. Mar. Biol. (Berl.) 2004, 145, 885–894. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Forestieri, R.; Nieto, R.M.; Varela, M.; Nappo, M.; Rodríguez, J.; Jiménez, C.; Castelluccio, F.; Carbone, M.; Ramos-Esplá, A.; et al. Chemical defenses of tunicates of the genus Aplidium from the Weddell Sea (Antarctica). Polar Biol. 2010, 33, 1319–1329. [Google Scholar] [CrossRef]
- Young, C.M.; Bingham, B.L. Chemical defense and aposematic coloration in larvae of the ascidian Ecteinascidia turbinata. Mar. Biol. 1987, 96, 539–544. [Google Scholar] [CrossRef]
- Lindquist, N.; Hay, M.E.; Fenical, W. Defense of ascidians and their conspicuous larvae: Adult vs. Larval chemical defenses. Ecol. Monogr. 1992, 62, 547–568. [Google Scholar] [CrossRef]
- Wahl, M.; Banaigs, B. Marine epibiosis. III. Possible antifouling defense adaptations in Polysyncraton lacazei (Giard) (Didemnidae, Aseidiacea). J. Exp. Mar. Biol. Ecol. 1991, 145, 49–63. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Coates, A.G. Life-cycles and evolution of clonal (modular) animals. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1986, 313, 7–22. [Google Scholar] [CrossRef]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling-some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Bryan, P.J.; McClintock, J.B.; Slattery, M.; Rittschof, D.P. A comparative study of the non-acidic chemically mediated antifoulant properties of three sympatric species of ascidians associated with seagrass habitats. Biofouling 2003, 19, 235–245. [Google Scholar] [CrossRef]
- Davidson, B.S. Ascidians: Producers of amino acid derived metabolites. Chem. Rev. 1993, 93, 1771–1791. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Paul, V.J.; Lindquist, N.; Fenical, W. Chemical defenses of the tropical ascidian Atapozoa sp. and its nudibranch predators Nembrotha spp. Mar. Ecol. Prog. Ser. 1990, 59, 109–118. [Google Scholar] [CrossRef]
- Paul, V.J. Ecological Roles of Marine Natural Products; Cornell Universiry Press: New York, NY, USA, 1992. [Google Scholar]
- Davis, A.R.; Bremner, J.B. Potencial Antifouling Natural Products from Ascidians: A Review. In Marine Biotechnology; FIngerman, M., Nagabhushanam, R., Thompson, M.-F., Eds.; Science Publishers: Washington, DC, USA, 1999; Volume 3. [Google Scholar]
- Hernández Franco, L.; Bal de Kier Joffé, E.; Puricelli, L.; Tatián, M.; Seldes, A.M.; Palermo, J.A. Indole alkaloids from the Tunicate Aplidium meridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef]
- Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Palmerolide A, a cytotoxic macrolide from the Antarctic tunicate Synoicum adareanum. J. Am. Chem. Soc. 2006, 128, 5630–5631. [Google Scholar]
- Miyata, Y.; Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Valeriote, F.A.; Baker, B.J. Ecdysteroids from the antarctic tunicate Synoicum adareanum. J. Nat. Prod. 2007, 70, 1859–1864. [Google Scholar] [CrossRef]
- Seldes, A.M.; Rodríguez Brasco, M.F.; Hernández Franco, L.; Palermo, J.A. Identification of two meridianins from the crude extract of the tunicate Aplidium meridianun by tandem mass spectometry. Nat. Prod. Res. 2007, 21, 555–563. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar]
- Lindquist, N.; Fenical, W. New tambjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators. Origin of the tambjamines in Atapozoa. Experientia 1991, 47, 504–506. [Google Scholar] [CrossRef]
- Rottmayr, E.M.; Steffan, B.; Wanner, G. Pigmentation and tunic cells in Cystodytes dellechiajei (Urochordata, Ascidiacea). Zoomorphology 2001, 120, 159–170. [Google Scholar] [CrossRef]
- Salomon, C.E.; Faulkner, D.J. Localization studies of bioactive cyclic peptides in the ascidian Lissoclinum patella. J. Nat. Prod. 2002, 65, 689–692. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Nelson, J.T.; Rasko, D.A.; Sudek, S.; Eisen, J.A.; Haygood, M.G.; Ravel, J. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc. Natl. Acad. Sci. USA 2005, 102, 7315–7320. [Google Scholar]
- Sings, H.L.; Rinehart, K.L. Compounds produced from potential tunicate-blue-green algal symbiosis: A review. J. Ind. Microbiol. Biotechnol. 1996, 17, 385–396. [Google Scholar] [CrossRef]
- Hildebrand, M.; Waggoner, L.E.; Lim, G.E.; Sharp, K.H.; Ridley, C.P.; Haygood, M.G. Approaches to identify, clone, and express symbiont bioactive metabolite genes. Nat. Prod. Rep. 2004, 21, 122–142. [Google Scholar] [CrossRef]
- Turon, X.; Lopez-Legentil, S.; Banaigs, B. Cell types, microsymbionts, and pyridoacridine distribution in the tunic of three color morphs of the genus Cystodytes (Ascidiacea, Polycitorida). Invertebr. Biol. 2005, 124, 355–369. [Google Scholar] [CrossRef]
- Taylor, M. W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef]
- López-Legentil, S.; Dieckmann, R.; Bontemps-Subielos, N.; Turon, X.; Banaigs, B. Qualitative variation of alkaloids in color morphs of Cystodytes (Ascidiacea). Biochem. Syst. Ecol. 2005, 33, 1107–1119. [Google Scholar] [CrossRef]
- Seleghim, M.H.R.; de Lira, S.P.; Campana, P.T.; Berlinck, R.G.S.; Custodio, M.R. Localization of granulatimide alkaloids in the tissues of the ascidian Didemnum granulatum. Mar. Biol. 2007, 150, 967–975. [Google Scholar] [CrossRef]
- Carbone, M.; Núñez-Pons, L.; Castelluccio, F.; Avila, C.; Gavagnin, M. New meroterpenoids from the Antarctic ascidian Aplidium fuegiense. Tetrahedron 2012, 68, 3541–3544. [Google Scholar] [CrossRef]
- Rhoades, D.F.; Gates, R.G. Toward a general theory of plant antiherbivore chemistry. Recent Adv. Phytochem. 1976, 10, 168–213. [Google Scholar]
- Furrow, F.B.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Surface sequestration of chemical feeding deterrents in the Antarctic sponge Latrunculia apicalis as an optimal defense against sea star spongivory. Mar. Biol. 2003, 143, 443–449. [Google Scholar] [CrossRef]
- Harvell, C.D.; Fenical, W. Chemical and structural defenses of Caribbean gorgonians (Pseudopterogorgia spp.)-intracolony localization of defense. Limnol. Oceanogr. 1989, 34, 382–389. [Google Scholar] [CrossRef]
- Goodbody, I. The physiology of ascidians. Adv. Mar. Biol. 1975, 12, 1–149. [Google Scholar] [CrossRef]
- Dayton, P.K.; Robillia, G.A.; Paine, R.T.; Dayton, L.B. Biological accommodation in benthic community at McMurdo Sound Antarctica. Ecol. Monogr. 1974, 44, 105–128. [Google Scholar] [CrossRef]
- McClintock, J.B. Trophic biology of Antarctic echinoderms. Mar. Ecol. Prog. Ser. 1994, 111, 191–202. [Google Scholar] [CrossRef]
- De Broyer, C.; Lowry, J.K.; Jazdzewski, K.; Robert, H. Catalogue of the Gammaridean and Corophiidean Amphipoda of the Southern Ocean, with Distribution and Ecological Data. In Census of Antarctic Marine Life: Synopsis of the Amphipoda of the Southern Ocean; de Broyer, C., Ed.; Bulletin de l’Institut Royal des Sciences Naturelles de Belgique: Bruxelles, Belgium, 2007; Volume 1, pp. 1–325, Part 1. [Google Scholar]
- Sloan, N.A. Aspects of the feeding biology of asteroids. Oceanogr. Mar. Biol. Ann. Rev. 1980, 18, 57–124. [Google Scholar]
- Ramos-Esplá, A.A.; Cárcel, J.A.; Varela, M. Zoogeographical relationships of the littoral ascidiofauna around the Antarctic Peninsula, in the Scotia Arc and in the Magellan region. Sci. Mar. 2005, 69, 215–223. [Google Scholar]
- Primo, C.; Vazquez, E. Antarctic ascidians: An isolated and homogeneous fauna. Polar Res. 2009, 28, 403–414. [Google Scholar] [CrossRef]
- Varela, M. Contribución al conocimiento de las ascidias coloniales (Chordata: Tunicata) de la Antártida Occidental y Región Magallánica. Ph.D. Thesis, University of Alicante, Alicante, Spain, April 2007. [Google Scholar]
- Avila, C.; Taboada, S.; Núñez-Pons, L. Marine Antarctic chemical ecology: What is next? Mar. Ecol. 2008, 29, 1–70. [Google Scholar]
- Avila, C.; Iken, K.; Fontana, A.; Gimino, G. Chemical ecology of the Antarctic nudibranch Bathydoris hodgsoni Eliot, 1907: Defensive role and origin of its natural products. J. Exp. Mar. Biol. Ecol. 2000, 252, 27–44. [Google Scholar] [CrossRef]
- Iken, K.; Avila, C.; Fontana, A.; Gavagnin, M. Chemical ecology and origin of defensive compounds in the Antarctic nudibranch Austrodoris kerguelenensis (Opisthobranchia: Gastropoda). Mar. Biol. 2002, 141, 101–109. [Google Scholar] [CrossRef]
- Atwater, W.O.; Benedict, F.G. Experiments on the Metabolism of Matter and Energy in the Human Body, 1898-1900; Government Printing Office: Washington, DC, USA, 1902. [Google Scholar]
- Koplovitz, G.; McClintock, J.B.; Amsler, C.D.; Baker, B.J. Palatability and chemical anti-predatory defenses in common ascidians from the Antarctic Peninsula. Aquat. Biol. 2009, 7, 81–92. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Stadistics in Biological Research, 3rd ed; W. H. Freeman & Co.: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Núñez-Pons, L.; Rodríguez-Arias, M.; Gómez-Garreta, A.; Ribera-Siguán, A.; Avila, C. Feeding deterrency in Antarctic marine organisms: Bioassays with an omnivorous lyssianasid amphipod. Mar. Ecol. Prog. Ser. 2012, in press. [Google Scholar]
- Peterson, C.H.; Renaud, P.E. Analysis of feeding preference experiments. Oecologia 1989, 80, 82–86. [Google Scholar] [CrossRef]
- Mahon, A.R.; Amsler, C.D.; McClintock, J.B.; Amsler, M.O.; Baker, B.J. Tissue-specific palatability and chemical defenses against macropredators and pathogens in the common articulate brachiopod Liothyrella uva from the Antarctic Peninsula. J. Exp. Mar. Biol. Ecol. 2003, 290, 197–210. [Google Scholar] [CrossRef]
- Rodríguez, J. Unpublished work, University of La Coruña: La Coruña, Spain, 2012.
- Teo, S.L.M.; Ryland, J.S. Toxicity and palatability of some British ascidians. Mar. Biol. 1994, 120, 297–303. [Google Scholar] [CrossRef]
- Koplovitz, G.; McClintock, J.B.; Amsler, C.D.; Baker, B.J. A comprehensive evaluation of the potential chemical defenses of Antarctic ascidians against sympatric fouling microorganisms. Mar. Biol. 2011, 158, 2661–2671. [Google Scholar] [CrossRef]
- Lebar, M.D.; Luttenton, L.; McClintock, B.; Amsler, C.D.; Baker, B. Accumulation of vanadium, manganese, and nickel in Antarctic tunicates. Polar Biol. 2011, 34, 587–590. [Google Scholar] [CrossRef]
- Hirose, E. Acid containers and cellular networks in the ascidian tunic with special remarks on ascidian phylogeny. Zool. Sci. 2001, 18, 723–731. [Google Scholar] [CrossRef]
- McClintock, J.B.; Amsler, C.D.; Baker, B. Overview of the chemical ecology of benthic marine invertebrates along the western Antarctic Peninsula. Integr. Comp. Biol. 2010, 50, 967–980. [Google Scholar] [CrossRef]
- Peters, K.J.; Amsler, C.D.; McClintock, J.B.; van Soest, R.W.M.; Baker, B.J. Palatability and chemical defenses of sponges from the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2009, 385, 77–85. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Paris, D.; Melck, D.; Ríos, P.; Cristobo, J.; Castelluccio, F.; Gavagnin, M.; Avila, C. Chemo-ecological studies on hexactinellid sponges from the Southern Ocean. Naturwissenschaften 2012, 99, 353–368. [Google Scholar] [CrossRef]
- McClintock, J.B.; Heine, J.; Slattery, M.; Weston, J. Biochemical and energetic composition, population biology, and chemical defense of the Antarctic ascidian Cnemidocarpa verrucosa Lesson. J. Exp. Mar. Biol. Ecol. 1991, 147, 163–175. [Google Scholar] [CrossRef]
- Hirose, E.; Taneda, Y.; Ishii, T. Two modes of tunic cuticle formation in a colonial ascidian Aplidium yamazii, responding to wounding. Dev. Comp. Immunol. 1997, 21, 25–34. [Google Scholar] [CrossRef]
- Núñez-Pons, L. Unpublished work, University of Barcelona: Barcelona, Spain, 2012.
- Teo, S.L.M.; Ryland, J.S. Potential antifouling mechanisms using toxic-chemicals in some British ascidians. J. Exp. Mar. Biol. Ecol. 1995, 188, 49–62. [Google Scholar] [CrossRef]
- Lindsay, B.S.; Barrows, L.R.; Copp, B.R. Structural requirements for biological activity of the marine alkaloid ascididemin. Bioorg. Med. Chem. Lett. 1995, 5, 739–742. [Google Scholar] [CrossRef]
- Davis, A.R.; Wright, A.E. Inhibition of larval settlement by natural products from the ascidian, Eudistoma olivaceum (Van Name). J. Chem. Ecol. 1990, 16, 1349–1357. [Google Scholar] [CrossRef]
- Davis, A.R. Alkaloids and ascidian chemical defense: Evidence for the ecological role of natural products from Eudistoma olivaceum. Mar. Biol. 1991, 111, 375–379. [Google Scholar] [CrossRef]
- Slattery, M.; McClintock, J.B.; Heine, J.N. Chemical defenses in Antarctic soft corals: Evidence for antifouling compounds. J. Exp. Mar. Biol. Ecol. 1995, 190, 61–77. [Google Scholar] [CrossRef]
- Amsler, C.D.; Moeller, C.B.; McClintock, J.B.; Iken, K.B.; Baker, B.J. Chemical defenses against diatom fouling in Antarctic marine sponges. Biofouling 2000, 16, 29–45. [Google Scholar] [CrossRef]
- Peters, K.J.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Potential chemical defenses of Antarctic sponges against sympatric microorganisms. Polar Biol. 2010, 33, 649–658. [Google Scholar] [CrossRef]
- Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 2008, 25, 1131–1166. [Google Scholar] [CrossRef]
- Riesenfeld, C.S.; Murray, A.E.; Baker, B.J. Characterization of the microbial community and polyketide biosynthetic potential in the palmerolide-producing tunicate Synoicum adareanum. J. Nat. Prod. 2008, 71, 1812–1818. [Google Scholar] [CrossRef]
- Zubía, E.; Ortega, M.J.; Salvá, J. Natural products chemistry in marine ascidians of the genus Aplidium. Mini Rev. Org. Chem. 2005, 2, 389–399. [Google Scholar] [CrossRef]
- Wang, W.; Namikoshi, M. Bioactive nitrogenous metabolites from ascidians. Heterocycles 2007, 74, 53–88. [Google Scholar] [CrossRef]
- Riguera, R. Isolating bioactive compounds from marine organisms. J. Mar. Biotechnol. 1997, 5, 187–193. [Google Scholar]
- Zhang, Z.; Chen, J.; Yang, Z.; Tang, Y. Rapid Biomimetic Total Synthesis of (+/−)-Rossinone B. Org. Lett. 2010, 12, 5554–5557. [Google Scholar] [CrossRef]
- Kelecom, A. Secondary metabolites from marine microorganisms. An. Acad. Bras. Cienc. 2002, 74, 151–170. [Google Scholar] [CrossRef]
- Franks, A.; Haywood, P.; Holmstrom, C.; Egan, S.; Kjelleberg, S.; Kumar, N. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules 2005, 10, 1286–1291. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. The nature and role of pigments of marine invertebrates. Nat. Prod. Rep. 2006, 23, 223–255. [Google Scholar] [CrossRef]
- Ivanova, V.; Kolarova, M.; Aleksieva, K.; Graffe, U.; Dahse, H.M.; Laatsch, H. Microbiaeratin, a new natural indole alkaloid from a Microbispora aerata strain, isolated from Livingston Island, Antarctia. Prep. Biochem. Biotechnol. 2007, 37, 161–168. [Google Scholar] [CrossRef]
- Gompel, M.; Leost, M.; Bal de Kier Joffé, E.; Puricelli, L.; Hernández Franco, L.; Palermo, J.A.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar]
- Walker, S.R.; Carter, E.J.; Huff, B.C.; Morris, J.C. Variolins and related alkaloids. Chem. Rev. 2009, 109, 3080–3098. [Google Scholar] [CrossRef]
- Lebar, M.D.; Baker, B.J. Synthesis and structure reassessment of Psammopemmin A. Aust. J. Chem. 2010, 63, 862–866. [Google Scholar] [CrossRef]
- Tatián, M. Personal communication with the authors, Department of Biodiversity and Experimental Biology, University of Córdoba: Córdoba, Argentina, 2010.
- Butler, M.S.; Capon, R.J.; Lu, C.C. Psammopemmins (A-C), Novel Brominated 4-Hydroxyindole Alkaloids from an Antarctic Sponge, Psammopemma sp. Aust. J. Chem. 1992, 45, 1871–1877. [Google Scholar]
- Pauletti, P.M.; Cintra, L.S.; Braguine, C.G.; da Silva, A.A.; Silva, M.; Cunha, W.R.; Januario, A.H. Halogenated Indole Alkaloids from Marine Invertebrates. Mar. Drugs 2010, 8, 1526–1549. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Núñez-Pons, L.; Carbone, M.; Vázquez, J.; Rodríguez, J.; Nieto, R.M.; Varela, M.M.; Gavagnin, M.; Avila, C. Natural Products from Antarctic Colonial Ascidians of the Genera Aplidium and Synoicum: Variability and Defensive Role. Mar. Drugs 2012, 10, 1741-1764. https://doi.org/10.3390/md10081741
Núñez-Pons L, Carbone M, Vázquez J, Rodríguez J, Nieto RM, Varela MM, Gavagnin M, Avila C. Natural Products from Antarctic Colonial Ascidians of the Genera Aplidium and Synoicum: Variability and Defensive Role. Marine Drugs. 2012; 10(8):1741-1764. https://doi.org/10.3390/md10081741
Chicago/Turabian StyleNúñez-Pons, Laura, Marianna Carbone, Jennifer Vázquez, Jaime Rodríguez, Rosa María Nieto, María Mercedes Varela, Margherita Gavagnin, and Conxita Avila. 2012. "Natural Products from Antarctic Colonial Ascidians of the Genera Aplidium and Synoicum: Variability and Defensive Role" Marine Drugs 10, no. 8: 1741-1764. https://doi.org/10.3390/md10081741








