An Ethanol Extract Derived from Bonnemaisonia hamifera Scavenges Ultraviolet B (UVB) Radiation-Induced Reactive Oxygen Species and Attenuates UVB-Induced Cell Damage in Human Keratinocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Scavenging Effect of BHE against Free Radicals

2.2. Effect of BHE against UVB-Induced Apoptosis
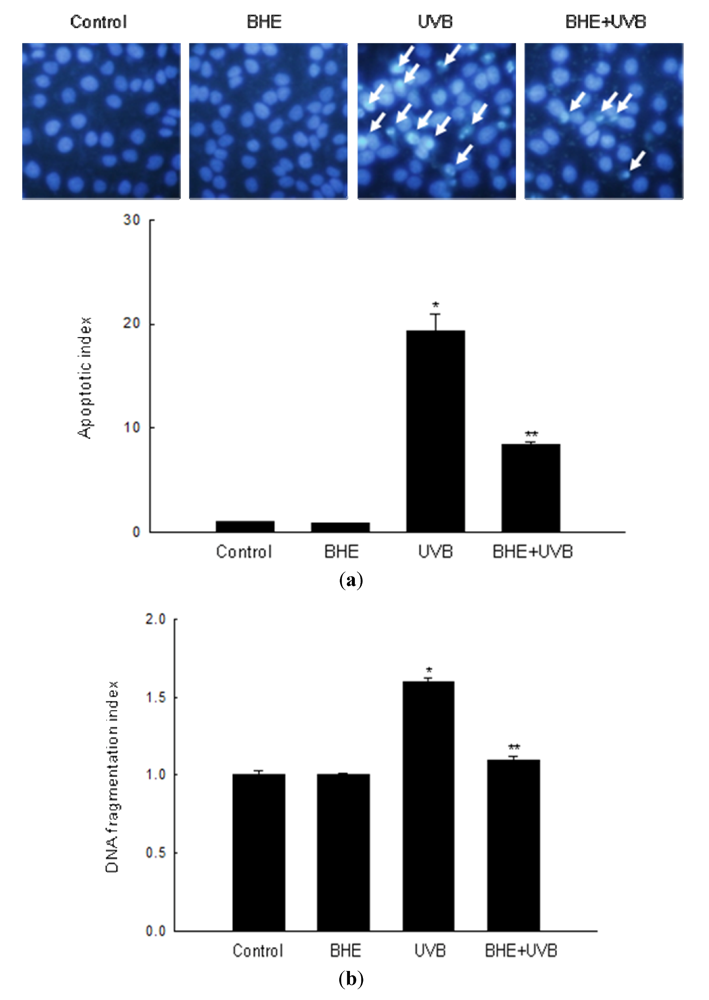
2.3. Effect of BHE against UVB-Induced Oxidative Lipid, Protein and DNA Damage

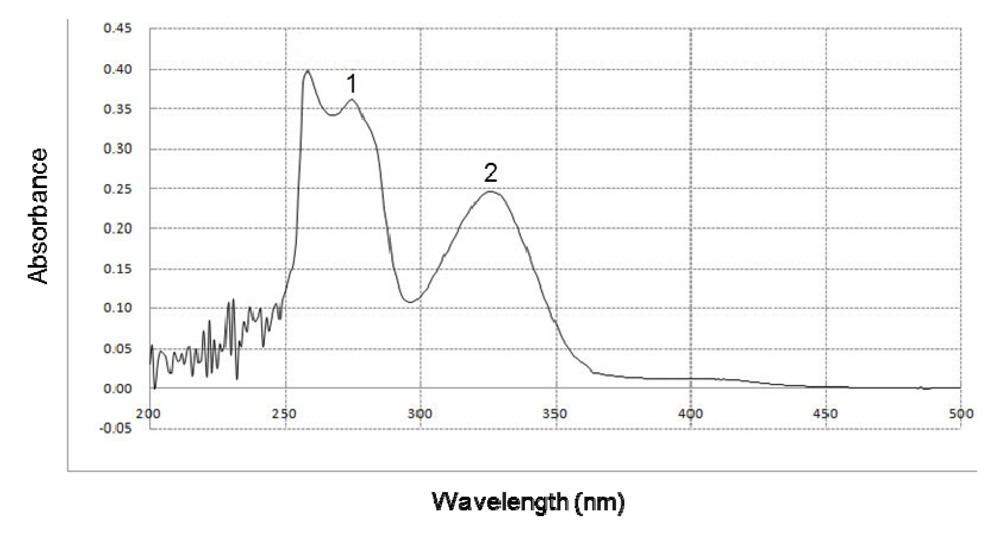
2.4. Effect of BHE on UVB Absorption
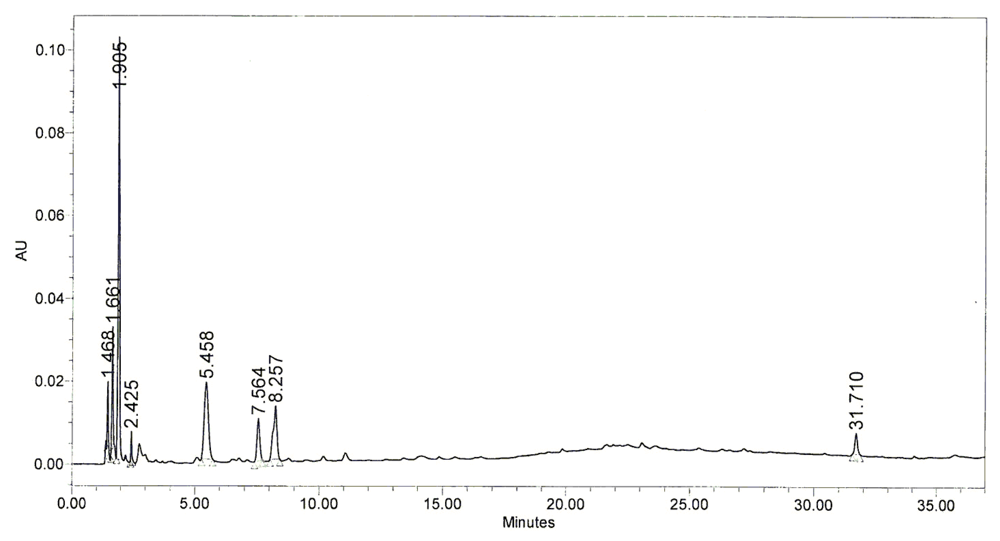
2.5. Chromatography Pattern of BHE
2.6. Discussion
3. Experimental Section
3.1. Preparation of BHE
3.2. Reagents
3.3. Cell Culture
3.4. Detection of the DPPH Radical
3.5. Detection of Intracellular ROS
3.6. Cell Viability Assay
3.7. Detection of the Superoxide Anion
3.8. Detection of the Hydroxyl Radical
3.9. Nuclear Staining with Hoechst 33342
3.10. DNA Fragmentation
3.11. Lipid Peroxidation Assay
3.12. Protein Carbonyl Formation
3.13. Single-Cell Gel Electrophoresis (Comet Assay)
3.14. UV/Visible Light Absorption Analysis
3.15. HPLC Analysis
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Peres, P.S.; Terra, V.A.; Guarnier, F.A.; Cecchini, R.; Cecchini, A.L. Photoaging and chronological aging profile: Understanding oxidation of the skin. J. Photochem. Photobiol. B 2011, 103, 93–97. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Swalwell, H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis 2010, 25, 101–107. [Google Scholar] [CrossRef]
- Barresi, C.; Stremnitzer, C.; Mlitz, V.; Kezic, S.; Kammeyer, A.; Ghannadan, M.; Posa-Markaryan, K.; Selden, C.; Tschachler, E.; Eckhart, L. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J. Invest. Dermatol. 2011, 131, 188–194. [Google Scholar]
- Aitken, G.R.; Henderson, J.R.; Chang, S.C.; McNeil, C.J.; Birch-Machin, M.A. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin. Exp. Dermatol. 2007, 32, 722–727. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Toyokuni, S.; Okamoto, K.; Yodoi, J.; Hiai, H. Persistent oxidative stress in cancer. FEBS Lett. 1995, 358, 1–3. [Google Scholar]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef]
- Lawley, W.; Doherty, A.; Denniss, S.; Chauhan, D.; Pruijn, G.; Van Venrooij, W.J.; Lunec, J.; Herbert, K. Rapid lupus autoantigenrelocalization and reactive oxygen species accumulation following ultraviolet irradiation of human keratinocytes. Rheumatology 2000, 39, 253–261. [Google Scholar] [CrossRef]
- Rafferty, T.S.; Beckett, G.J.; Walker, C.; Bisset, Y.C.; McKenzie, R.C. Selenium protects primary human keratinocytes from apoptosis induced by exposure to ultraviolet radiation. Clin. Exp. Dermatol. 2003, 28, 294–300. [Google Scholar] [CrossRef]
- Lee, T.M.; Shiu, C.T. Implications of mycosporine-like amino acid and antioxidant defenses in UV-B radiation tolerance for the algae species Ptercladiella capillacea and Gelidium amansii. Mar. Environ. Res. 2009, 67, 8–16. [Google Scholar] [CrossRef]
- Misonou, T.; Saitoh, J.; Oshiba, S.; Tokitomo, Y.; Maegawa, M.; Inoue, Y.; Hori, H.; Sakurai, T. UV-Absorbing substance in the red alga Porphyra yezoensis (Bangiales, Rhodophyta) block thymine photodimer production. Mar. Biotechnol. 2003, 5, 194–200. [Google Scholar] [CrossRef]
- Nizard, C.; Poggioli, S.; Heusèle, C.; Bulteau, A.L.; Moreau, M.; Saunois, A.; Schnebert, S.; Mahé, C.; Friguet, B. Algae extract protection effect on oxidized protein level in human stratum corneum. Ann. N. Y. Acad. Sci. 2004, 1019, 219–222. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; National University of Ireland: Galway, Irland, 2012. Available online: http://www.algaebase.org (accessed on 6 October 2012).
- Lee, Y.; Kang, S. A Catalogue of the Seaweeds in Korea; Jeju National University Press: Jeju, Korea, 2001; pp. 1–662. [Google Scholar]
- Lee, Y. Marine Algae of JEJU; Academy Publications: Seoul, Korea, 2008; pp. 1–477. [Google Scholar]
- Lee, O.H.; Yoon, K.Y.; Kim, K.J.; You, S.; Lee, B.Y. Seaweed extracts as a potential tool for the attenuation of oxidative damage in obesity-related pathologies. J. Phycol. 2011, 47, 548–556. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, K.W.; Song, C.B.; Ahn, C.B.; Jeon, Y.J. Cytotoxic activities of red algae collected from jeju island against four tumor cell lines. J. Food Sci. Nutr. 2006, 11, 177–183. [Google Scholar] [CrossRef]
- Nylund, G.M.; Cervin, G.; Persson, F.; Hermansson, M.; Steinberg, P.D.; Pavia, H. Seaweed defence against bacteria: A poly-brominated 2-heptanone from the red alga Bonnemaisonia hamifera inhibits bacterial colonisation. Mar. Ecol. Prog. Ser. 2008, 369, 39–50. [Google Scholar]
- Yang, E.J.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Kim, C.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci. B 2010, 11, 315–322. [Google Scholar]
- Heo, S.J.; Cha, S.H.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of red algae from Jeju Island. Algae 2006, 21, 149–156. [Google Scholar]
- Ali, D.; Verma, A.; Mujtaba, F.; Dwivedi, A.; Hans, R.K.; Ray, R.S. UVB-Inducedapoptosis and DNA damaging potential of chrysene via reactive oxygen species in human keratinocytes. Toxicol. Lett. 2011, 204, 199–207. [Google Scholar] [CrossRef]
- Guerrini, J.S.; Bouvard, V.; Oswald, E.; Alonso, A.; Prétet, J.L.; Tommasino, M.; Mougin, C.; Aubin, F. E6 and E7 proteins from different beta-papillomaviruses types do not interfere in UVB-induced apoptosis of HaCaTkeratinocytes. Exp. Dermatol. 2011, 20, 71–73. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Mantena, S.K.; Meeran, S.M. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Aldini, G.; Granata, P.; Marinello, C.; Beretta, G.; Carini, M.; Facino, R.M. Effects of UVB radiation on 4-hydroxy-2-trans-nonenal metabolism and toxicity in human keratinocytes. Chem. Res. Toxicol. 2007, 20, 416–423. [Google Scholar]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Eiberger, W.; Volkmer, B.; Amouroux, R.; Dhérin, C.; Radicella, J.P.; Epe, B. Oxidative stress impairs the repair of oxidative DNA base modifications in human skin fibroblasts and melanoma cells. DNA Repair 2008, 7, 912–921. [Google Scholar] [CrossRef]
- Pirinccioglu, A.G.; Gökalp, D.; Pirinccioglu, M.; Kizil, G.; Kizil, M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010, 43, 1220–1224. [Google Scholar] [CrossRef]
- Sander, C.S.; Chang, H.; Salzmann, S.; Müller, C.S.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is associated with protein oxidation in human skin in vivo. J. Invest. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar]
- Karol, M.H. How environmental agents influence the aging process. Biomol. Ther. 2009, 17, 113–124. [Google Scholar] [CrossRef]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar]
- Wang, H.; Kochevar, I.E. Involvement of UVB-induced reactive oxygen species in TGF-beta biosynthesis and activation in keratinocytes. Free Radic. Biol. Med. 2005, 38, 890–897. [Google Scholar]
- Masaki, H.; Izutsu, Y.; Yahagi, S.; Okano, Y. Reactive oxygen species in HaCaTkeratinocytes after UVB irradiation are triggered by intracellular Ca2+ levels. J. Investig. Dermatol. Symp. Proc. 2009, 14, 50–52. [Google Scholar] [CrossRef]
- Pallela, R.; Yoon, N.Y.; Kim, S.K. Anti-Photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef]
- McConnell, O.; Fenical, W. Halogen chemistry of the red alga Asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Combaut, G.; Bruneau, Y.; Teste, J.; Codomier, L. Halogen compounds from a red alga, Falkenbergiaru folanosa, tetrasporophyte of Asparagopsis armata. Phytochemistry 1978, 17, 1661–1663. [Google Scholar] [CrossRef]
- Jacobsen, N.; Madsen, J.O. Halogenated metabolites including brominated 2-heptanols and 2-heptyl acetates from tetrasporophytes of the red alga Bonnemaisonia hamifera. Tetrahedron Lett. 1978, 33, 3065–3068. [Google Scholar] [CrossRef]
- Woolard, F.X.; Moore, R.E.; Roller, P.P. Halogenated acetic and acrylic acids from the alga Asparagopsis taxiformis. Phytochemistry 1979, 18, 617–620. [Google Scholar] [CrossRef]
- Marshall, R.A.; Harper, D.B.; McRoberts, W.C.; Dring, M.J. Volatile bromocarbons produced by Falkenbergia stages of Asparagopsis spp. (Rhodophyta). Limnol. Oceanogr. 1999, 44, 1348–1352. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids: Their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef]
- Lim, C.; Lee, J.; Cho, Y. Structures and some properties of the antimicrobial compounds in the red alga Symphyocladia latiuscula. Han’guk Susan Hakhoechi 2000, 33, 280–287. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Afolayan, A.F.; Mann, M.G.; Lategan, C.A.; Smith, P.J.; Bolton, J.J.; Beukes, D.R. Antiplasmodial halogenated monoterpenes from the marine red alga Plocamium cornutum. Phytochemistry 2009, 70, 597–600. [Google Scholar] [CrossRef]
- De la Mare, J.A.; Lawson, J.C.; Chiwakata, M.T.; Beukes, D.R.; Edkins, A.L.; Blatch, G.L. Quinones and halogenated monoterpenes of algal origin show anti-proliferative effects against breast cancer cells in vitro. Invest. New Drugs 2012, 30, 2187–2200. [Google Scholar] [CrossRef]
- Antunes, E.M.; Afolayan, A.F.; Chiwakata, M.T.; Fakee, J.; Knott, M.G.; Whibley, C.E.; Hendricks, D.T.; Bolton, J.J.; Beukes, D.R. Identification and in vitro anti-esophageal cancer activity of a series of halogenatedmonoterpenes isolated from the South African seaweeds Plocamium suhrii and Plocamium cornutum. Phytochemistry 2011, 72, 769–772. [Google Scholar] [CrossRef]
- Tsujino, I.; Saito, T. Studies on the compounds specific for each group of marine algae, I: Presence of characteristic ultraviolet absorbing material in Rhodophyacaeae. Bull. Fac. Fish. Hokkaido Univ. 1961, 12, 49–58. [Google Scholar]
- Ravanat, J.L.; Douki, T.; Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B 2001, 63, 88–102. [Google Scholar] [CrossRef]
- Davies, M.J.; Truscott, R.J. Photo-Oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol. B 2001, 63, 114–125. [Google Scholar] [CrossRef]
- Girotti, A.W. Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B 2001, 63, 103–113. [Google Scholar] [CrossRef]
- Mack, J.A.; Anand, S.; Maytin, E.V. Proliferation and cornification during development of the mammalian epidermis. Birth Defects Res. C Embryo Today 2005, 75, 314–329. [Google Scholar]
- Kulms, D.; Schwarz, T. Molecularmechanisms of UV-inducedapoptosis. Photodermatol. Photoimmunol. Photomed. 2000, 16, 195–201. [Google Scholar]
- Pustisek, N.; Situm, M. UV-Radiation, apoptosis and skin. Coll. Antropol. 2011, 35, 339–341. [Google Scholar]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Ueno, I.; Kohno, M.; Yoshihira, K.; Hirono, I. Quantitative determination of the superoxide radicals in the xanthineoxidase reaction by measurement of the electron spin resonance signal of the superoxide radical spin adduct of 5,5-dimethyl-1-pyrroline-1-oxide. J. Pharmacobiodyn. 1984, 7, 563–569. [Google Scholar] [CrossRef]
- Kohno, M.; Mizuta, Y.; Kusai, M.; Masumizu, T.; Makino, K. Measurements of superoxide anion radical and superoxide anion scavenging activity by electron spin resonance spectroscopy coupled with DMPO spin trapping. Bull. Chem. Soc. Jpn. 1994, 67, 1085–1090. [Google Scholar] [CrossRef]
- Li, L.; Abe, Y.; Mashino, T.; Mochizuki, M.; Miyata, N. Signal enhancement in ESR spin-trapping for hydroxyl radicals. Anal. Sci. 2003, 19, 1083–1084. [Google Scholar] [CrossRef]
- Li, L.; Abe, Y.; Kanagawa, K.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. Distinguishing the 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-OH radical quenching effect from the hydroxyl radical scavenging effect in the ESR spin-trapping method. Anal. Chim. Acta 2004, 512, 121–124. [Google Scholar] [CrossRef]
- Beauchamp, M.C.; Letendre, E.; Renier, G. Macrophage lipoprotein lipase expression is increased in patients with heterozygous familial hypercholesterolemia. J. Lipid Res. 2002, 43, 215–222. [Google Scholar]
- Singh, N.P. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat. Res. 2000, 455, 111–127. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Ranjan, S.K.; Nair, C.K. Effect of vinblastine sulfate on gamma-radiation-induced DNA single-strand breaks in murine tissues. Mutat. Res. 2003, 536, 15–25. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Piao, M.J.; Hyun, Y.J.; Cho, S.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Ko, M.H.; Hyun, J.W. An Ethanol Extract Derived from Bonnemaisonia hamifera Scavenges Ultraviolet B (UVB) Radiation-Induced Reactive Oxygen Species and Attenuates UVB-Induced Cell Damage in Human Keratinocytes. Mar. Drugs 2012, 10, 2826-2845. https://doi.org/10.3390/md10122826
Piao MJ, Hyun YJ, Cho SJ, Kang HK, Yoo ES, Koh YS, Lee NH, Ko MH, Hyun JW. An Ethanol Extract Derived from Bonnemaisonia hamifera Scavenges Ultraviolet B (UVB) Radiation-Induced Reactive Oxygen Species and Attenuates UVB-Induced Cell Damage in Human Keratinocytes. Marine Drugs. 2012; 10(12):2826-2845. https://doi.org/10.3390/md10122826
Chicago/Turabian StylePiao, Mei Jing, Yu Jae Hyun, Suk Ju Cho, Hee Kyoung Kang, Eun Sook Yoo, Young Sang Koh, Nam Ho Lee, Mi Hee Ko, and Jin Won Hyun. 2012. "An Ethanol Extract Derived from Bonnemaisonia hamifera Scavenges Ultraviolet B (UVB) Radiation-Induced Reactive Oxygen Species and Attenuates UVB-Induced Cell Damage in Human Keratinocytes" Marine Drugs 10, no. 12: 2826-2845. https://doi.org/10.3390/md10122826



