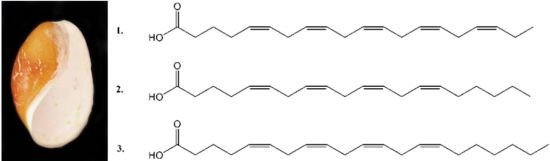
Characterization and Cytotoxicity Studies of the Rare 21:4 n-7 Acid and Other Polyunsaturated Fatty Acids from the Marine Opisthobranch Scaphander lignarius, Isolated Using Bioassay Guided Fractionation
Abstract
:1. Introduction
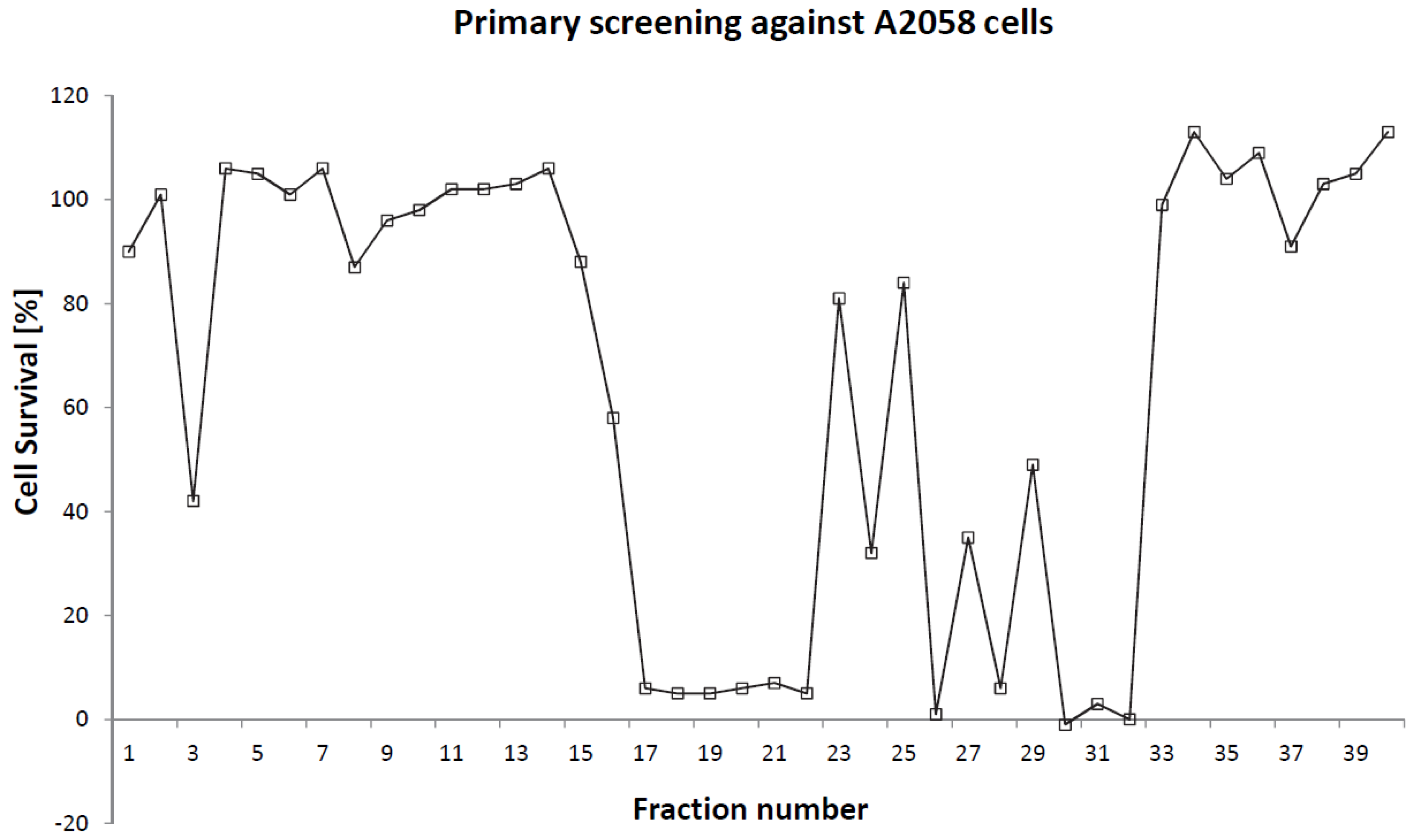
2. Results and Discussion
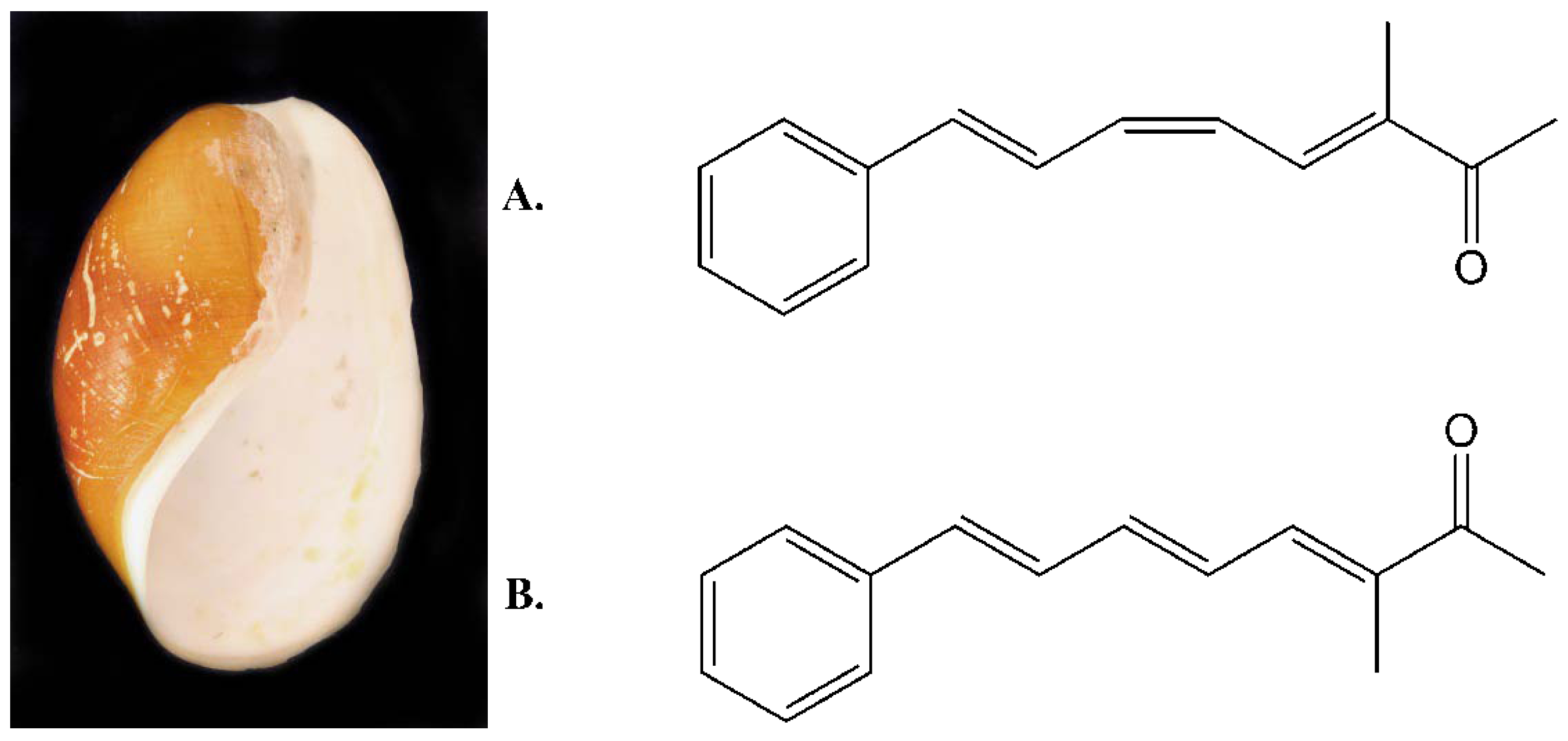
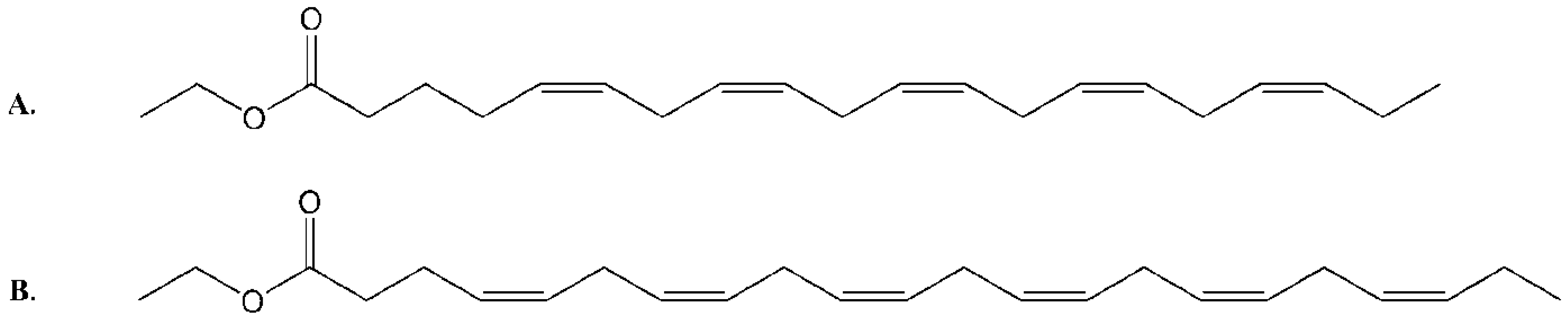
2.1. Isolation and Structural Determination
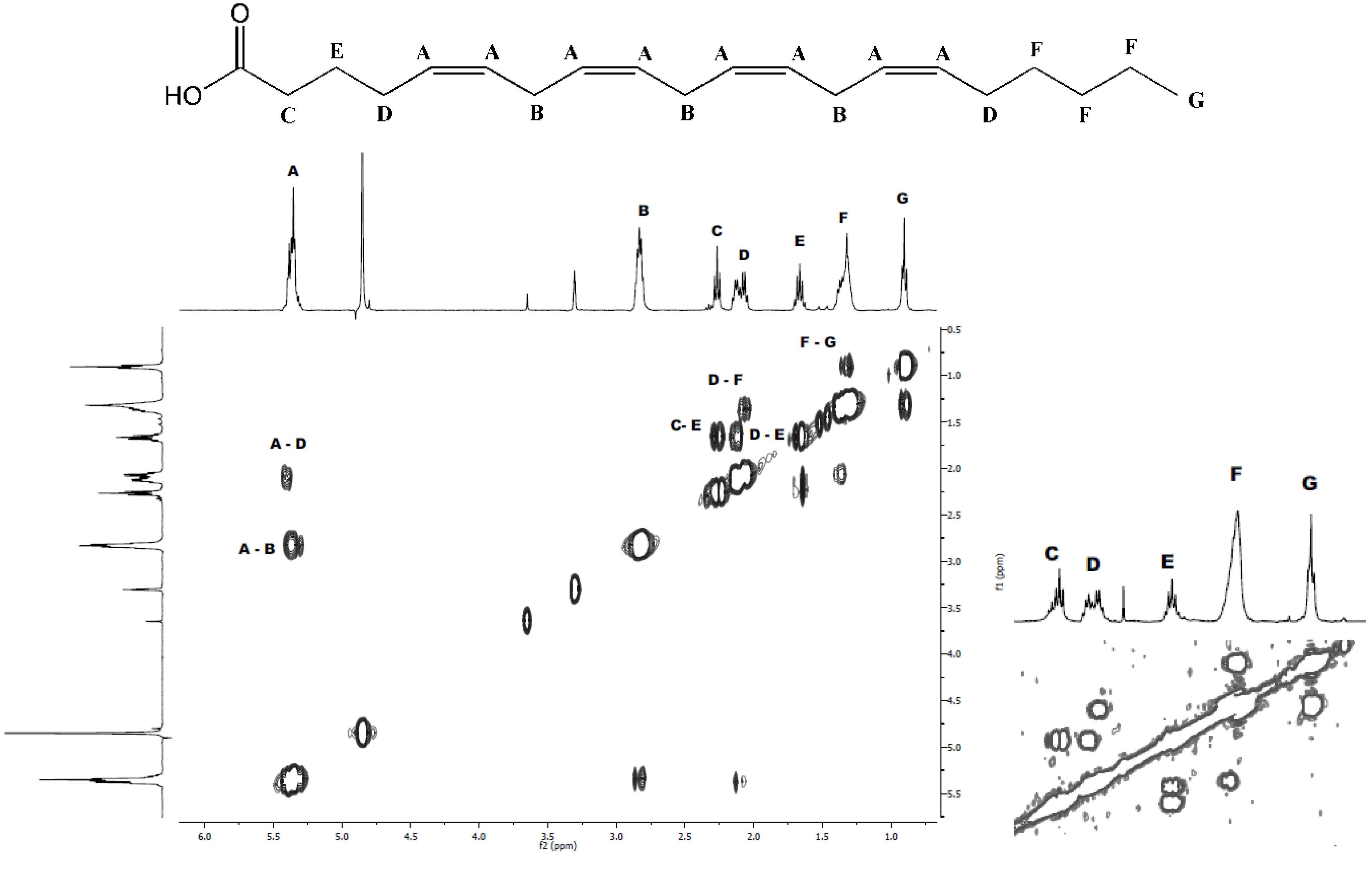

| Fatty acid | Monoisotopic mass | Relative area | % |
|---|---|---|---|
| Monoenonic | |||
| 14:1 | 226.2016 | 137 | 0.03 |
| 16:1 | 254.2328 | 1587 | 0.31 |
| 18:1 | 282.2640 | 1026 | 0.20 |
| 20:1 | 310.2952 | 1182 | 0.23 |
| NMID a | |||
| 18:2 | 280.2484 | 311 | 0.06 |
| 20:2 | 308.2796 | 642 | 0.13 |
| 22:2 | 336.3108 | 210 | 0.04 |
| Polyenonic | |||
| 16:3 | 250.2016 | 883 | 0.17 |
| 18:3 | 278.2328 | 787 | 0.16 |
| 20:3 | 306.2640 | 660 | 0.13 |
| 18:4 | 276.2172 | 1970 | 0.39 |
| 20:4 n-6 | 304.2484 | 88921 | 17.53 |
| 21:4 n-7 | 318.2640 | 46741 | 9.22 |
| 22:4 (2 isomers) | 332.2796 | 6986 + 8824 | 3.12 |
| 20:5 n-3 | 302.2328 | 201409 | 39.72 |
| 22:5 (3 isomers) | 330.2640 | 31588 + 4406 + 4295 | 7.95 |
| 22:6 | 328.2484 | 104560 | 20.61 |
| Sum | 507123 | 100.00 | |

2.2. Cytotoxicity
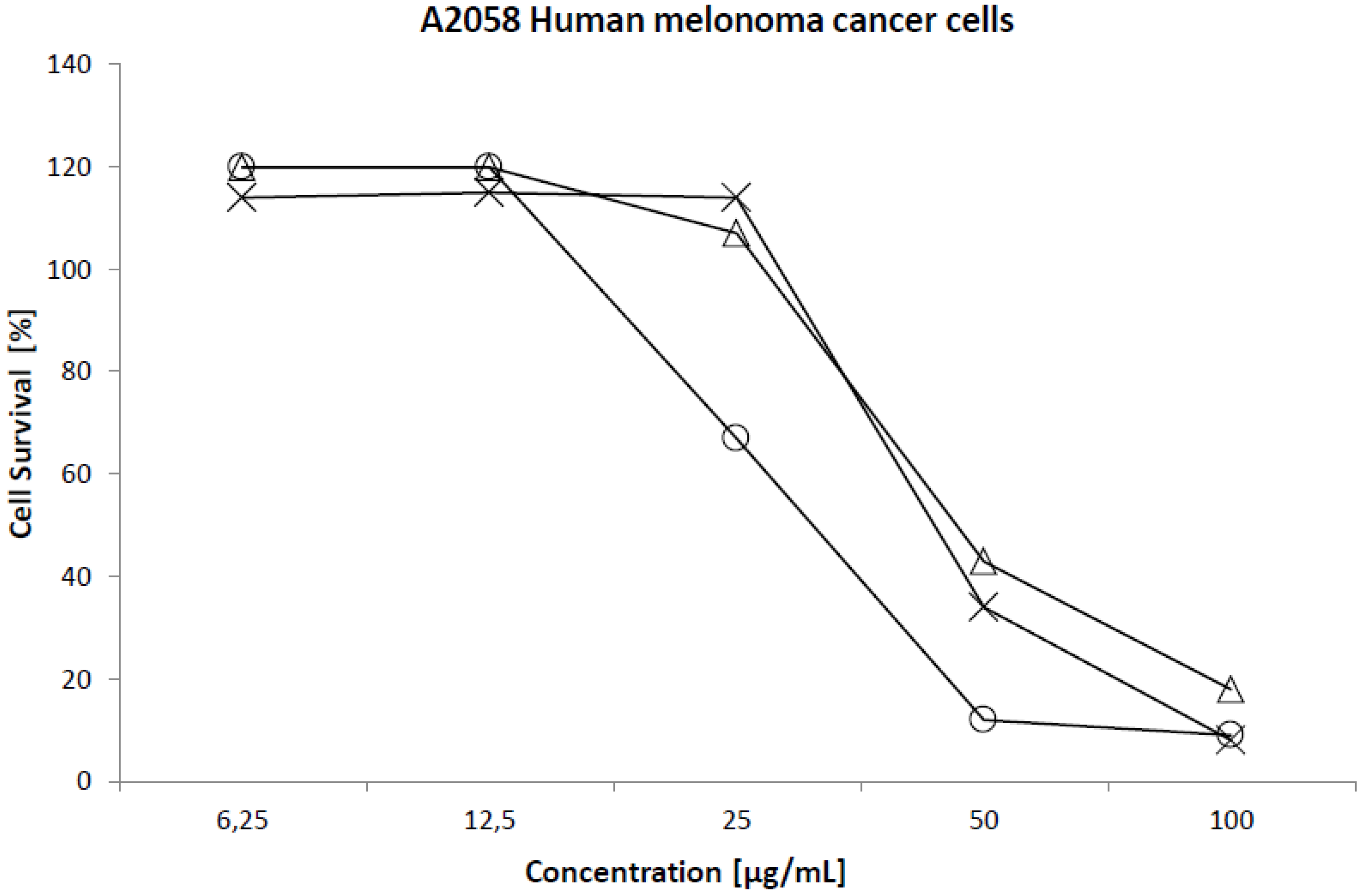
| Cell line | 50% Cytotoxicity (µg/mL) | ||
|---|---|---|---|
| EPA | ARA | HTA | |
| Normal lung fibroblast (MRC5) | 90 | 90 | 42 |
| Colon carcinoma (HT29) | 93 | 76 | 41 |
| Melanoma (A2058) | 45 | 43 | 31 |
| Breast carcinoma (MCF7) | 66 | 68 | 44 |
3. Experimental Section
3.1. Collection
3.2. Extraction and Fractionation
3.3. Isolation
| Time (min) | Flow (mL/min) | A (%) | B (%) | |
|---|---|---|---|---|
| Gradient 1 a | 0 | 3 | 90 | 10 |
| 0.5 | 10 | 90 | 10 | |
| 30 | 10 | 40 | 60 | |
| 35 | 10 | 5 | 95 | |
| 45 | 10 | 5 | 95 | |
| Gradient 2 b | 0 | 3 | 50 | 50 |
| 0.5 | 7 | 50 | 50 | |
| 30 | 7 | 10 | 90 | |
| 40 | 7 | 10 | 90 | |
3.4. Structure Determination
3.5. Cytotoxicity Assays
4. Conclusions
Acknowledgments
References
- Paterson, I.; Anderson, E.A. The renaissance of natural products as drug candidates. Science 2005, 310, 451–453. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Svenson, J. MabCent: Arctic marine bioprospecting in Norway. Phytochem. Rev. 2012. [Google Scholar] [CrossRef]
- Berge, J.P.; Barnathan, G. Fatty acids from lipids of marine organisms: Molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. In Marine Biotechnology I; Gal, Y.L., Ulber, R., Eds.; Springer-Verlag: Berlin, Germany, 2005; Volume 96, pp. 49–125. [Google Scholar]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids and their metabolites as modulators of stem cell biology with reference to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2011, 30, 311–324. [Google Scholar] [CrossRef]
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef]
- Harris, W.S. N-3 Fatty acids and serum lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 645–654. [Google Scholar]
- Rivellese, A.A.; Maffettone, A.; Iovine, C.; DiMarino, L.; Annuzzi, G.; Mancini, M.; Riccardi, G. Long-Term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care 1996, 19, 1207–1213. [Google Scholar]
- Menendez, J.A.; Lupu, R.; Colomer, R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6 n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur. J. Cancer Prev. 2005, 14, 263–270. [Google Scholar] [CrossRef]
- Calviello, G.; Serini, S.; Piccioni, E.; Pessina, G. Antineoplastic effects of N-3 polyunsaturated fatty acids in combination with drugs and radiotherapy: Preventive and therapeutic strategies. Nutr. Cancer 2009, 61, 287–301. [Google Scholar] [CrossRef]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef]
- Rezanka, T.; Sigler, K. Odd-Numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. Prog. Lipid Res. 2009, 48, 206–238. [Google Scholar] [CrossRef]
- Bouchet, P.; Rocroi, J.P.; Fryda, J.; Hausdorf, B.; Ponder, W.; Valdes, A.; Waren, A. Classification and nomenclator of gastropod families. Malacologia 2005, 47, 1–368. [Google Scholar]
- Cutignano, A.; Avila, C.; Domenech-Coll, A.; d’Ippolito, G.; Cimino, G.; Fontana, A. First biosynthetic evidence on the phenyl-containing polyketides of the marine mollusc Scaphander lignarius. Org. Lett. 2008, 10, 2963–2966. [Google Scholar]
- Cimino, G.; Spinella, A.; Sodano, G. Potential alarm pheromones from the mediterranean opisthobranch Scaphander lignarius. Tetrahedron Lett. 1989, 30, 5003–5004. [Google Scholar] [CrossRef]
- Cutignano, A.; Avila, C.; Rosica, A.; Romano, G.; Laratta, B.; Domenech-Coll, A.; Cimino, G.; Mollo, E.; Fontana, A. Biosynthesis and cellular localization of functional polyketides in the gastropod mollusc Scaphander lignarius. Chembiochem 2012, 13, 1759–1766. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Paterson, I.; Findlay, A.D. Recent advances in the total synthesis of polyketide natural products as promising anticancer agents. Aust. J. Chem. 2009, 62, 624–638. [Google Scholar] [CrossRef]
- Johns, R.B.; Nichols, P.D.; Perry, G.J. Fatty-Acid components of 9 species of mollusks of the littoral-zone from Australian waters. Comp. Biochem. Phys. B 1980, 65, 207–214. [Google Scholar] [CrossRef]
- Saito, H. Unusual novel n-4 polyunsaturated fatty acids in cold-seep mussels (Bathymodiolus japonicus and Bathymodiolus platifrons), originating from symbiotic methanotrophic bacteria. J. Chromatogr. A 2008, 1200, 242–254. [Google Scholar]
- Barnathan, G. Non-Methylene-Interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar]
- Nagao, K.; Yanagita, T. Conjugated fatty acids in food and their health benefits. J. Biosci. Bioeng. 2005, 100, 152–157. [Google Scholar] [CrossRef]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Das, U.N. Gamma-linolenic acid, arachidonic-acid, and eicosapentaenoic acid as potential anticancer drugs. Nutrition 1990, 6, 429–434. [Google Scholar]
- Wendel, M.; Heller, A.R. Anticancer actions of omega-3 fatty acids—Current state and future perspectives. Anti-Cancer Agents Med. Chem. 2009, 9, 457–470. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids enhance free radical generation and lipid peroxidation to induce apoptosis of tumor cells. Clin. Lipid. 2011, 6, 463–489. [Google Scholar] [CrossRef]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef]
- Dunbar, L.M.; Bailey, J.M. Enzyme deletions and essential fatty-acid metabolism in cultured-cells. J. Biol. Chem. 1975, 250, 1152–1153. [Google Scholar]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms—production by leukocytes of superoxide a potential bactericidal agent. J. Clin. Invest. 1973, 52, 741–744. [Google Scholar] [CrossRef]
- Das, U.N.; Begin, M.E.; Ells, G.; Huang, Y.S.; Horrobin, D.F. Polyunstaurated fatty-acids augment free-radical generation in tumor-cells in vitro. Biochem. Biophys. Res. Commun. 1987, 145, 15–24. [Google Scholar]
- Huang, P.; Feng, L.; Oldham, E.A.; Keating, M.J.; Plunkett, W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 2000, 407, 390–395. [Google Scholar] [CrossRef]
- Das, U.N. Tumoricidal action of cis-unsaturated fatty-acids and their relationship to free-radicals and lipid-peroxidation. Cancer Lett. 1991, 56, 235–243. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Santalova, E.A.; Gorshkova, I.A.; Dmitrenok, A.S.; Guzii, A.G.; Gorbach, V.I.; Svetashev, V.I.; Stonik, V.A. A new cytotoxic fatty acid (5Z,9Z)-22-methyl-5,9-tetracosadienoic acid and the sterols from the far eastern sponge Geodinella robusta. Lipids 2002, 37, 75–80. [Google Scholar] [CrossRef]
- Das, U.N.; Prasad, V.; Reddy, D.R. Local application of gamma-linolenic acid in the treatment of human gliomas. Cancer Lett. 1995, 94, 147–155. [Google Scholar] [CrossRef]
- Saito, H. Characteristics of fatty acid composition of the deep-sea vent crab, Shinkaia crosnieri Baba and Williams. Lipids 2011, 46, 723–740. [Google Scholar] [CrossRef]
- Nechev, J.; Christie, W.W.; Robaina, R.; De Diego, F.; Popov, S.; Stefanov, K. Chemical composition of the sponge Hymeniacidon sanguinea from the Canary Islands. Comp. Biochem. Phys. A 2004, 137, 365–374. [Google Scholar] [CrossRef]
- Chang, K.J.L.; Dunstan, G.A.; Abell, G.C.J.; Clementson, L.A.; Blackburn, S.I.; Nichols, P.D.; Koutoulis, A. Biodiscovery of new Australian thraustochytrids for production of biodiesel and long-chain omega-3 oils. Appl. Microbiol. Biotechnol. 2012, 93, 2215–2231. [Google Scholar] [CrossRef]
- Gao, P.; Hirano, T.; Chen, Z.; Yasuhara, T.; Nakata, Y.; Sugimoto, A. Isolation and identification of C-19 fatty acids with anti-tumor activity from the spores of Ganoderma lucidum (reishi mushroom). Fitoterapia 2012, 83, 490–499. [Google Scholar] [CrossRef]
- Chang, K.J.L.; Mansour, M.P.; Dunstan, G.A.; Blackburn, S.I.; Koutoulis, A.; Nichols, P.D. Odd-Chain polyunsaturated fatty acids in thraustochytrids. Phytochemistry 2011, 72, 1460–1465. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vasskog, T.; Andersen, J.H.; Hansen, E.; Svenson, J. Characterization and Cytotoxicity Studies of the Rare 21:4 n-7 Acid and Other Polyunsaturated Fatty Acids from the Marine Opisthobranch Scaphander lignarius, Isolated Using Bioassay Guided Fractionation. Mar. Drugs 2012, 10, 2676-2690. https://doi.org/10.3390/md10122676
Vasskog T, Andersen JH, Hansen E, Svenson J. Characterization and Cytotoxicity Studies of the Rare 21:4 n-7 Acid and Other Polyunsaturated Fatty Acids from the Marine Opisthobranch Scaphander lignarius, Isolated Using Bioassay Guided Fractionation. Marine Drugs. 2012; 10(12):2676-2690. https://doi.org/10.3390/md10122676
Chicago/Turabian StyleVasskog, Terje, Jeanette H. Andersen, Espen Hansen, and Johan Svenson. 2012. "Characterization and Cytotoxicity Studies of the Rare 21:4 n-7 Acid and Other Polyunsaturated Fatty Acids from the Marine Opisthobranch Scaphander lignarius, Isolated Using Bioassay Guided Fractionation" Marine Drugs 10, no. 12: 2676-2690. https://doi.org/10.3390/md10122676
APA StyleVasskog, T., Andersen, J. H., Hansen, E., & Svenson, J. (2012). Characterization and Cytotoxicity Studies of the Rare 21:4 n-7 Acid and Other Polyunsaturated Fatty Acids from the Marine Opisthobranch Scaphander lignarius, Isolated Using Bioassay Guided Fractionation. Marine Drugs, 10(12), 2676-2690. https://doi.org/10.3390/md10122676