The Medicinal Chemistry of Imidazotetrazine Prodrugs
Abstract
:| 1. Introduction | 798 | |
| 2. Mechanism of Action | 799 | |
| 3. Elucidation of Prodrug Activation | 800 | |
| 3.1. The Chemistry of MTZ and ETZ | 801 | |
| 4. Kinetic Considerations | 805 | |
| 4.1 Prodrug Activation Kinetics | 805 | |
| 4.2. Ultimate Electrophile Lifetime | 807 | |
| 5. Temozolomide Co-crystals | 807 | |
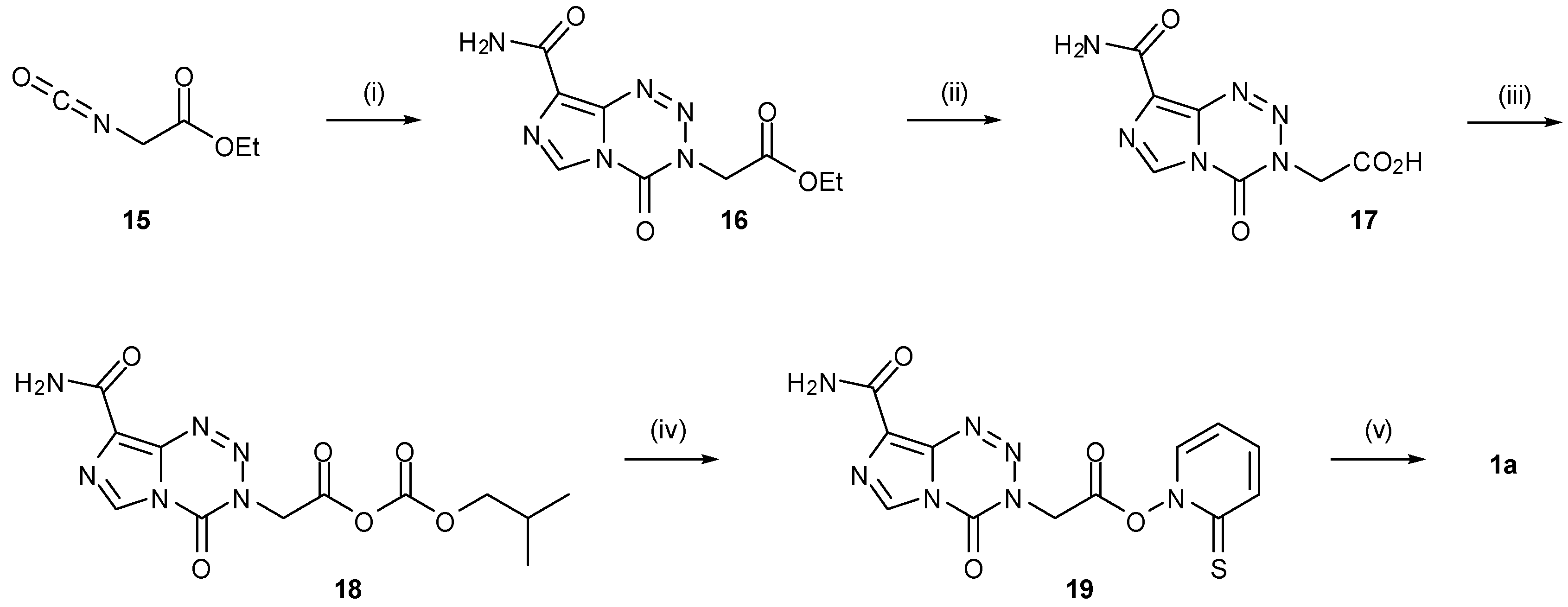
| 6. Synthesis of Temozolomide and the Imidazotetrazine Core | 810 | |
| 7. Synthesis of Structural Analogues | 817 | |
| 7.1. Alternative Cores | 817 | |
| 7.2. 6- and 8-Analogues | 819 | |
| 7.3. 3-Analogues | 822 | |
| 8. Design of MGMT/MMR-Independent Anti-Cancer Agents | 825 | |
| 8.1. Introduction to NGP Analogues | 826 | |
| 8.2. Synthesis of Novel N-Linked Imidazotetrazine Dimers | 828 | |
| 8.3. Properties and Activity of N-Linked Compounds | 829 | |
| 9. Conclusions | 931 | |
1. Introduction
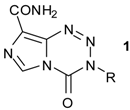 | |||
| R | Dose mg/kg/day | T/C b % | |
| 1a | CH3 (TMZ) | 160 | 151 |
| 80 a | 154 | ||
| 40 a | 181 | ||
| 1b | (CH2)2Cl (MTZ) | 40 | 458 |
| 16 a | 302 | ||
| 1c | CH2CH3 (ETZ) | 640 | 123 |
| 80 a | 111 | ||
| 1d | (CH2)2Br | 160 | 137 |
| 1e | (CH2)2CH3 | 320 | 103 |
| 1f | (CH2)2OCH3 | 320 | 98 |
| 1g | (CH2)3Cl | 320 | 108 |
| 1h | CH2CHClCH2Cl | 320 | 103 |
| 1j | CH2CH=CH2 | 320 | 97 |
| 1k | CH(CH3)CH2CH3 | 320 | 83 |
| 1m | (CH2)5CH3 | 320 | 103 |
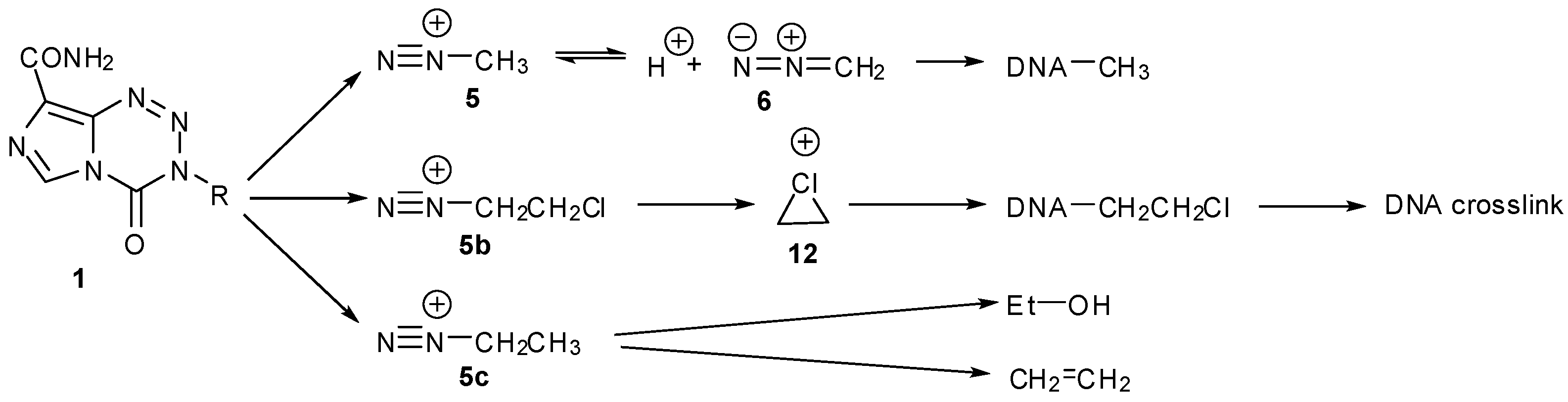
2. Mechanism of Action
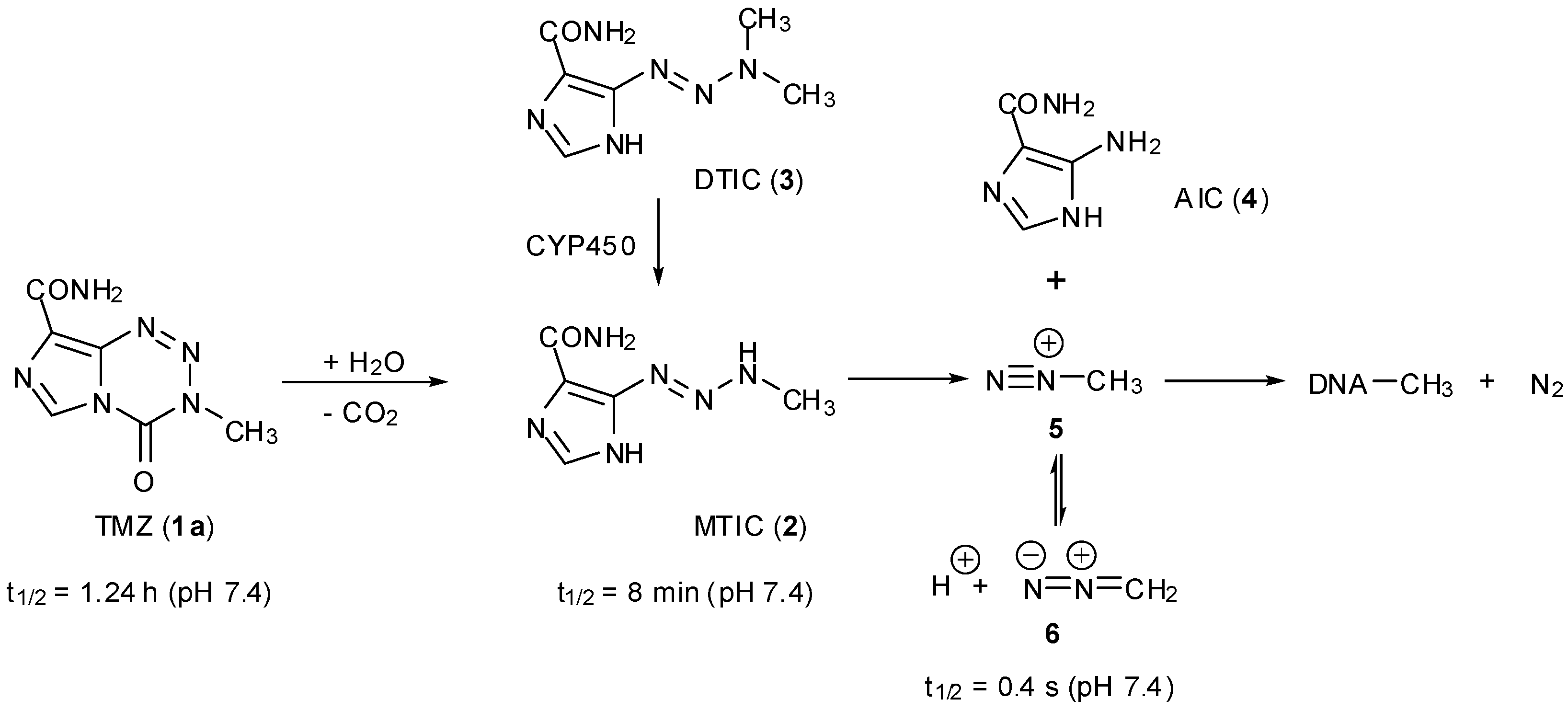
3. Elucidation of Prodrug Activation
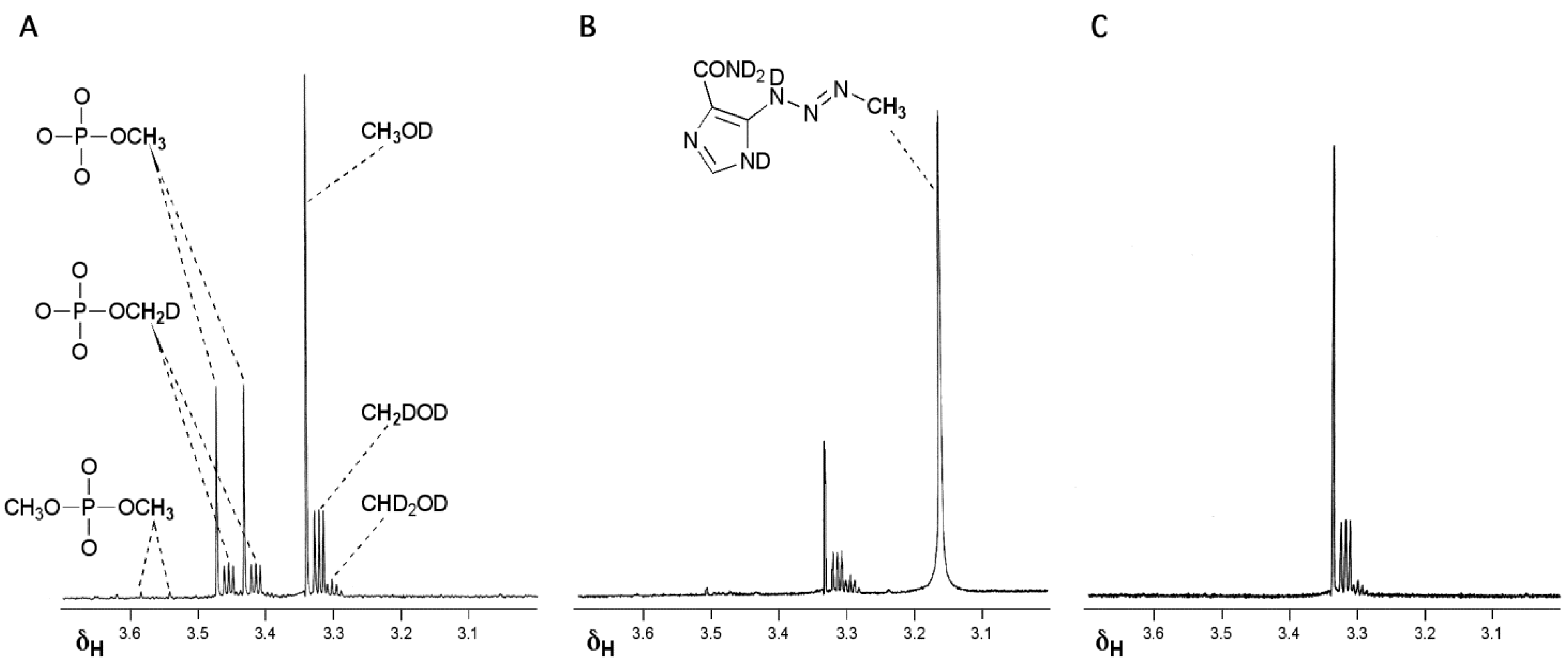
3.1. The Chemistry of MTZ and ETZ

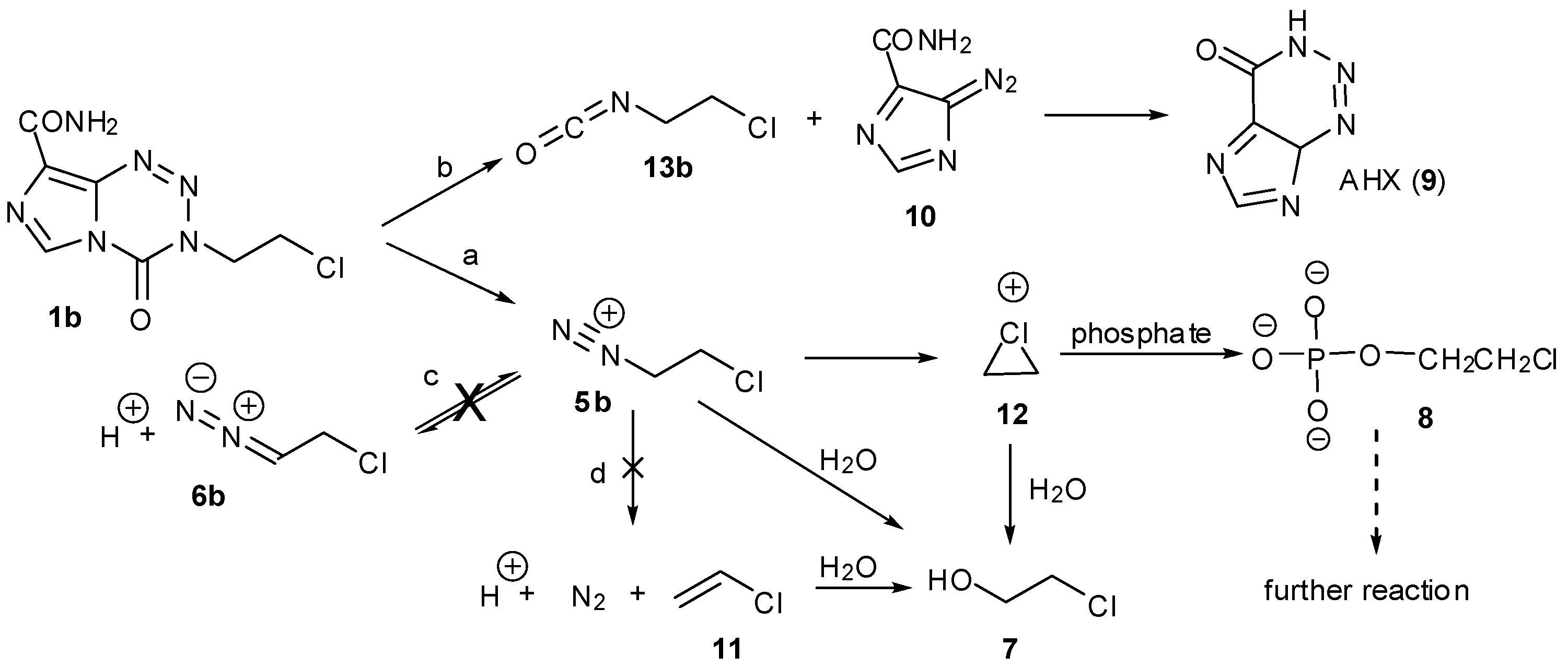
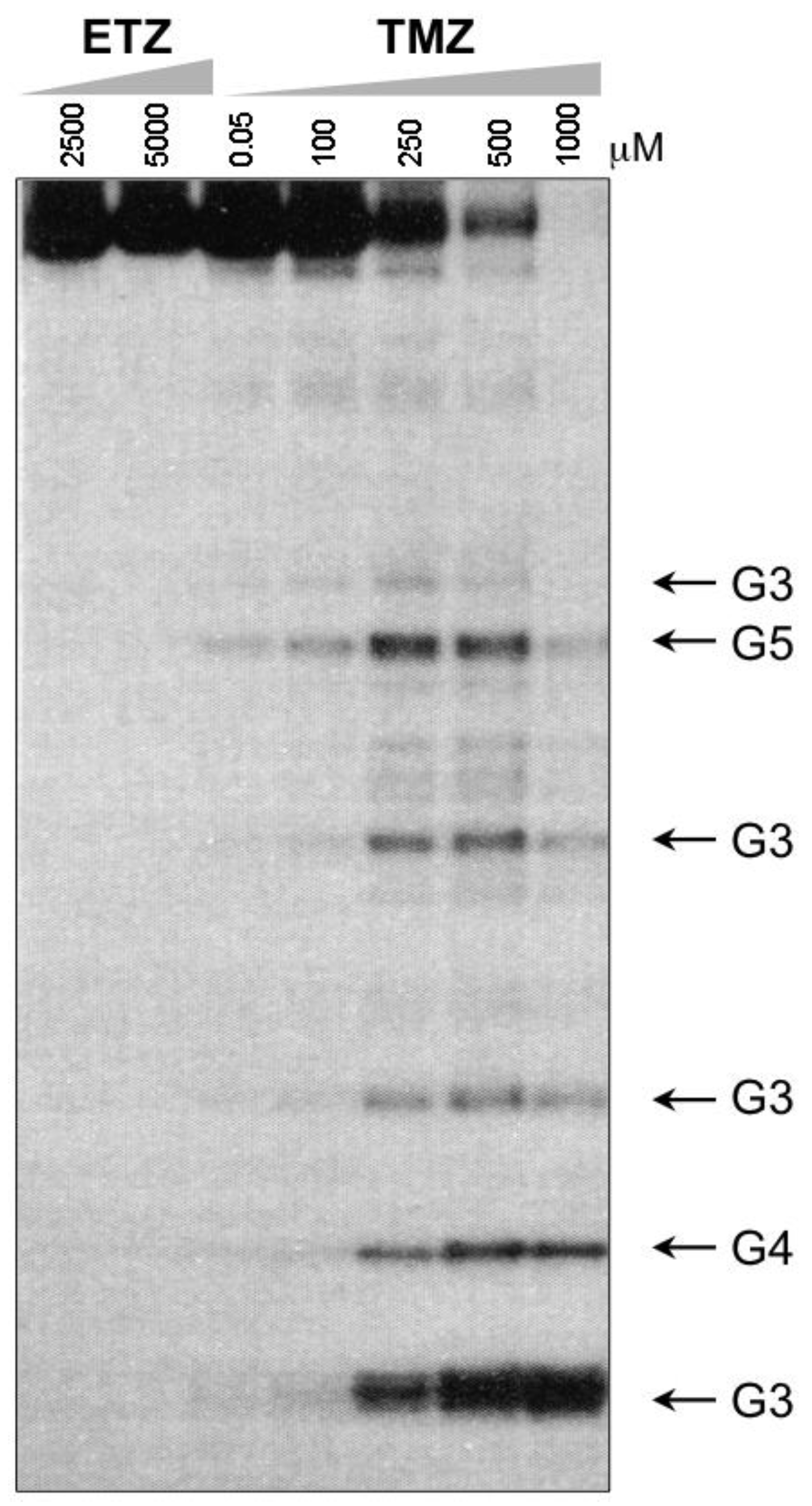
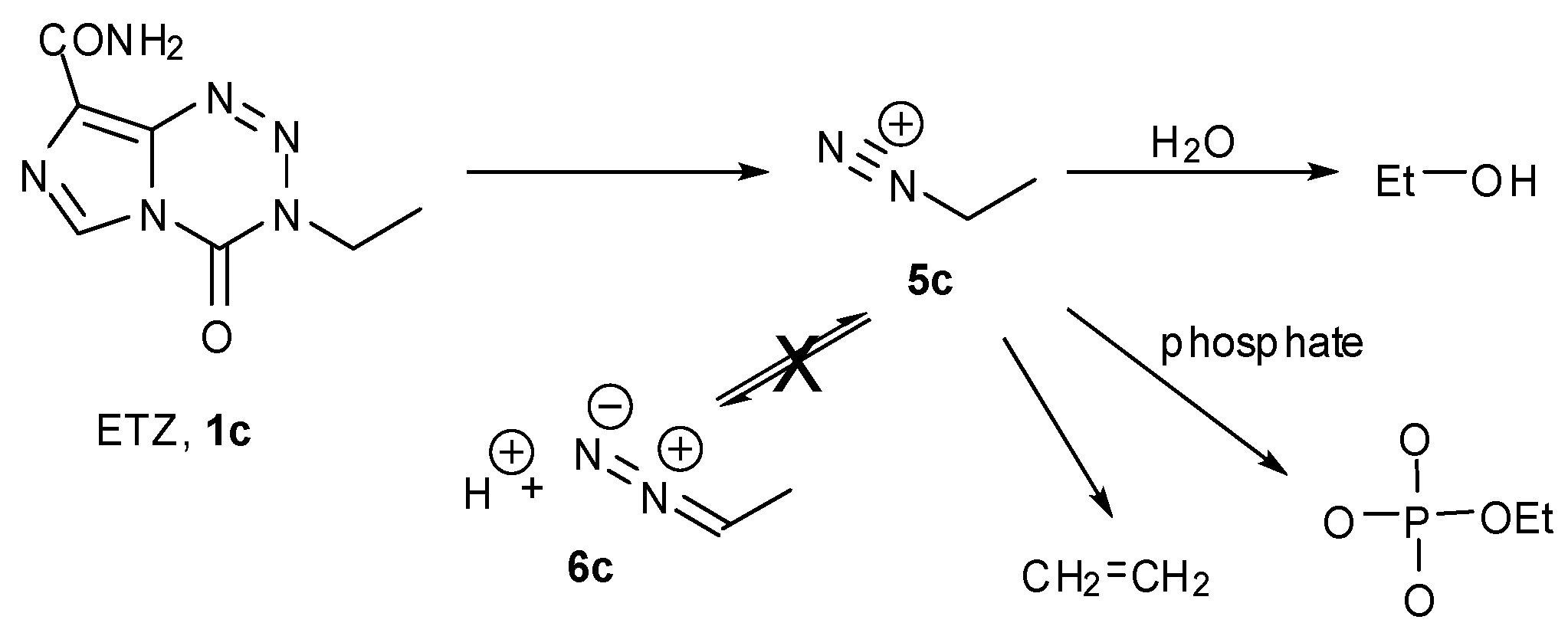
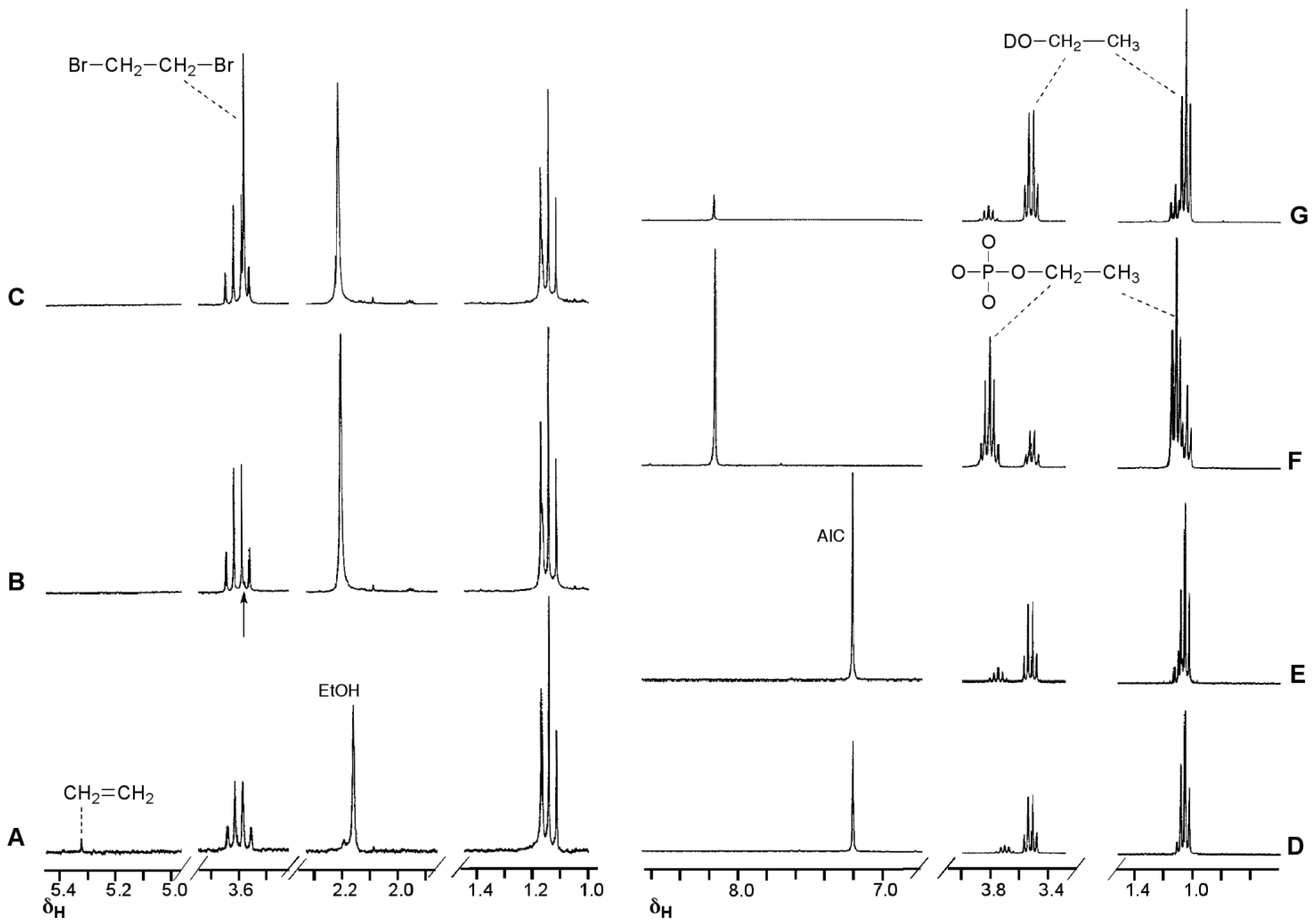
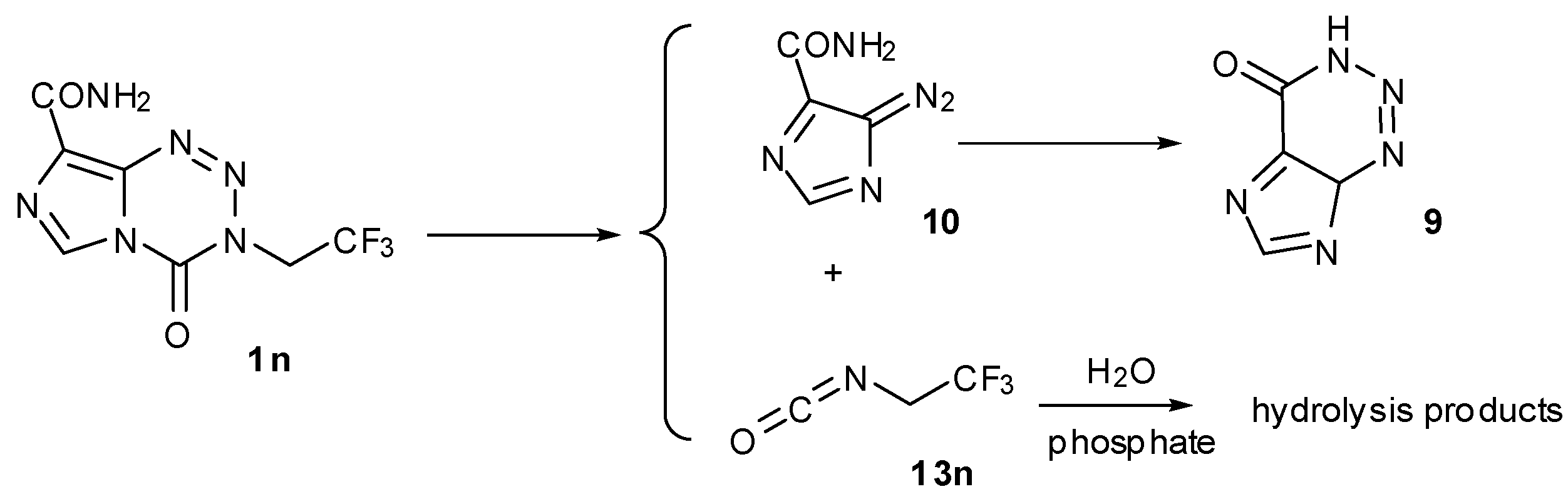
4. Kinetic Considerations
4.1. Prodrug Activation Kinetics

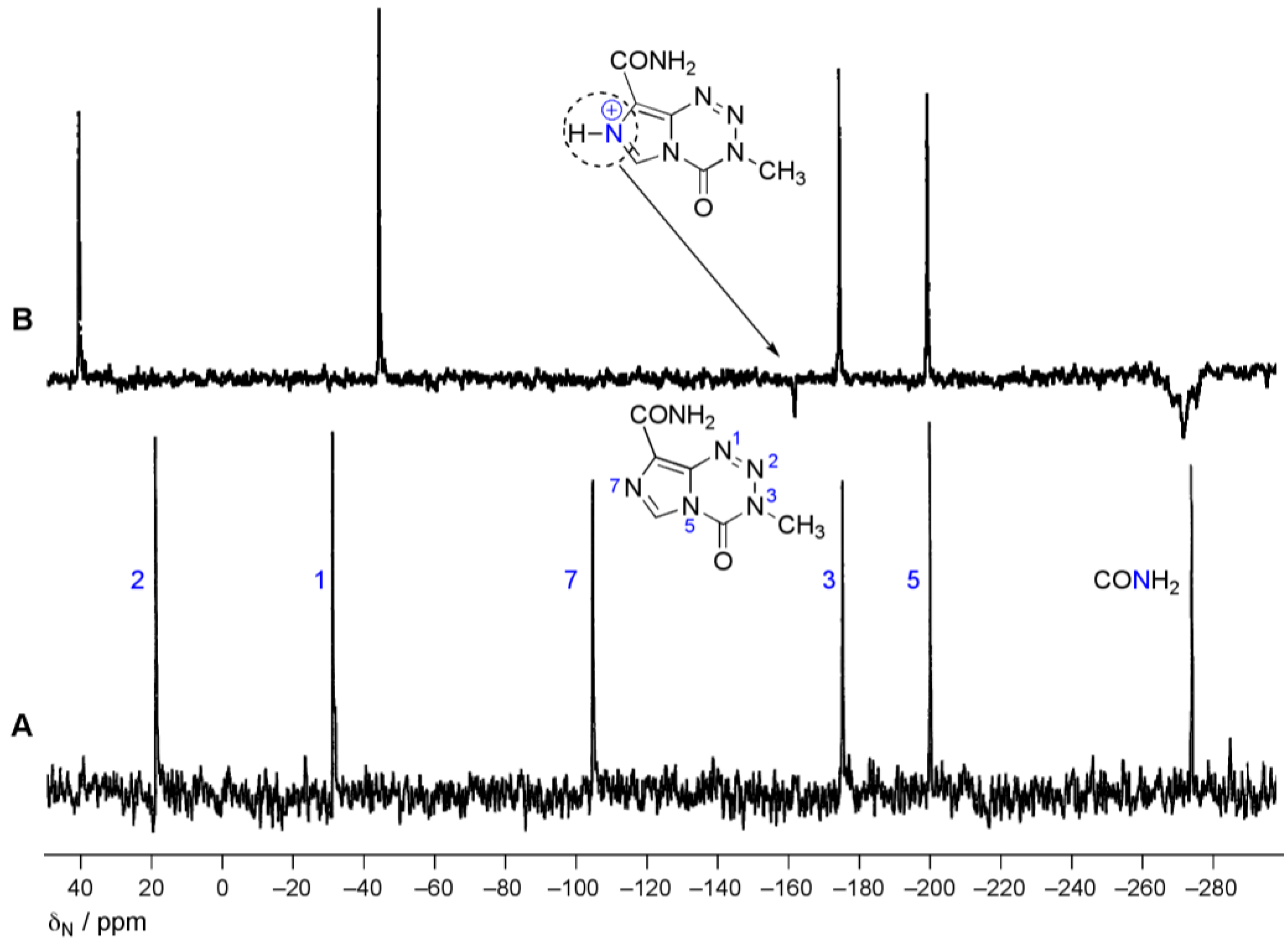
4.2. Ultimate Electrophile Lifetime
5. Temozolomide Co-Crystals
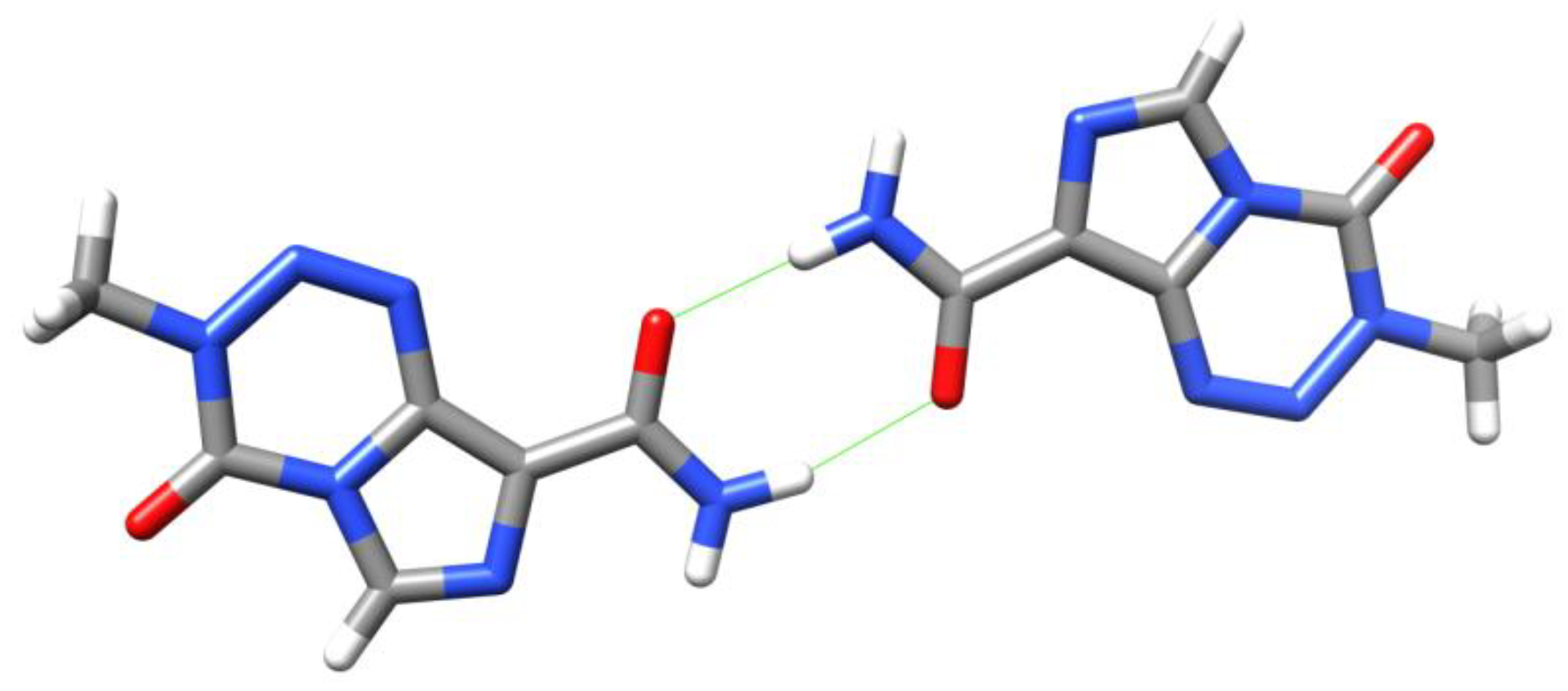
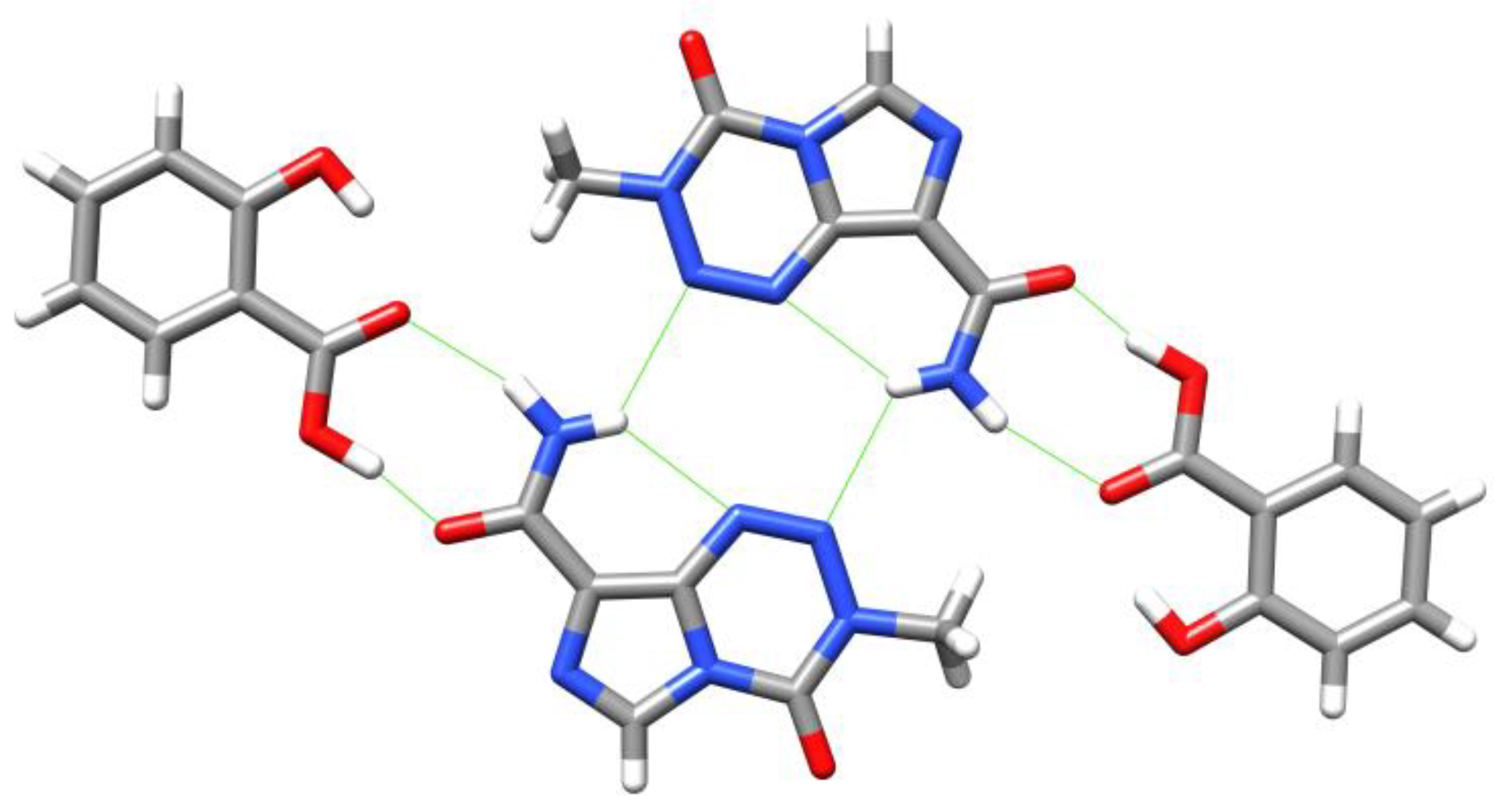
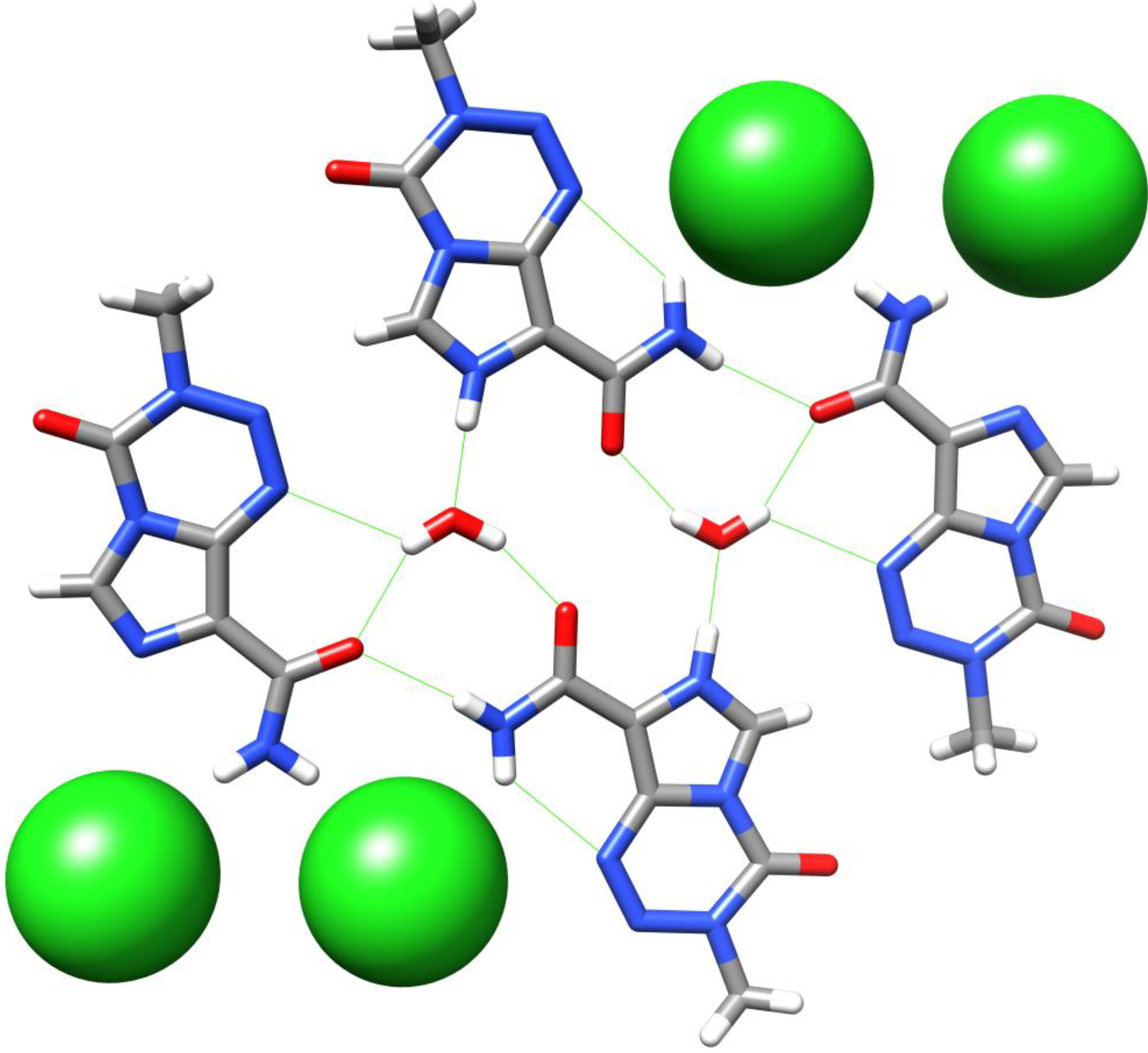
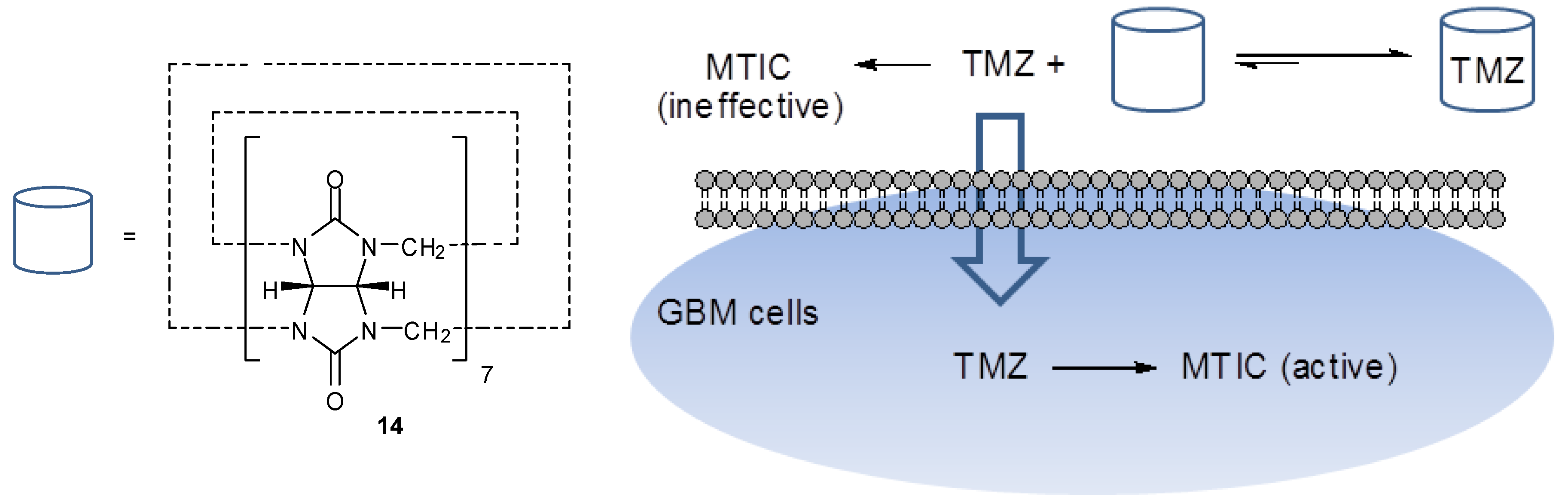
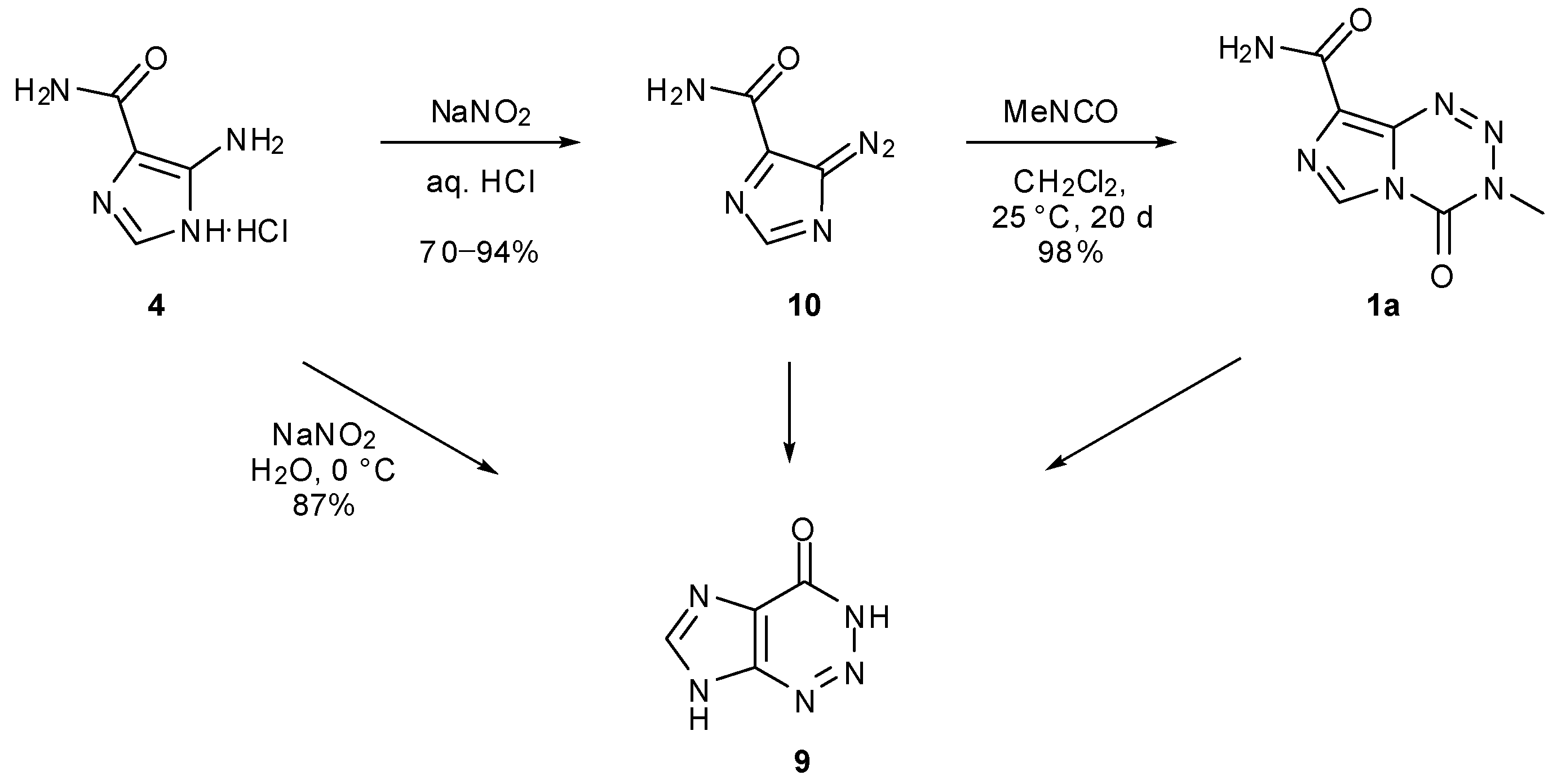
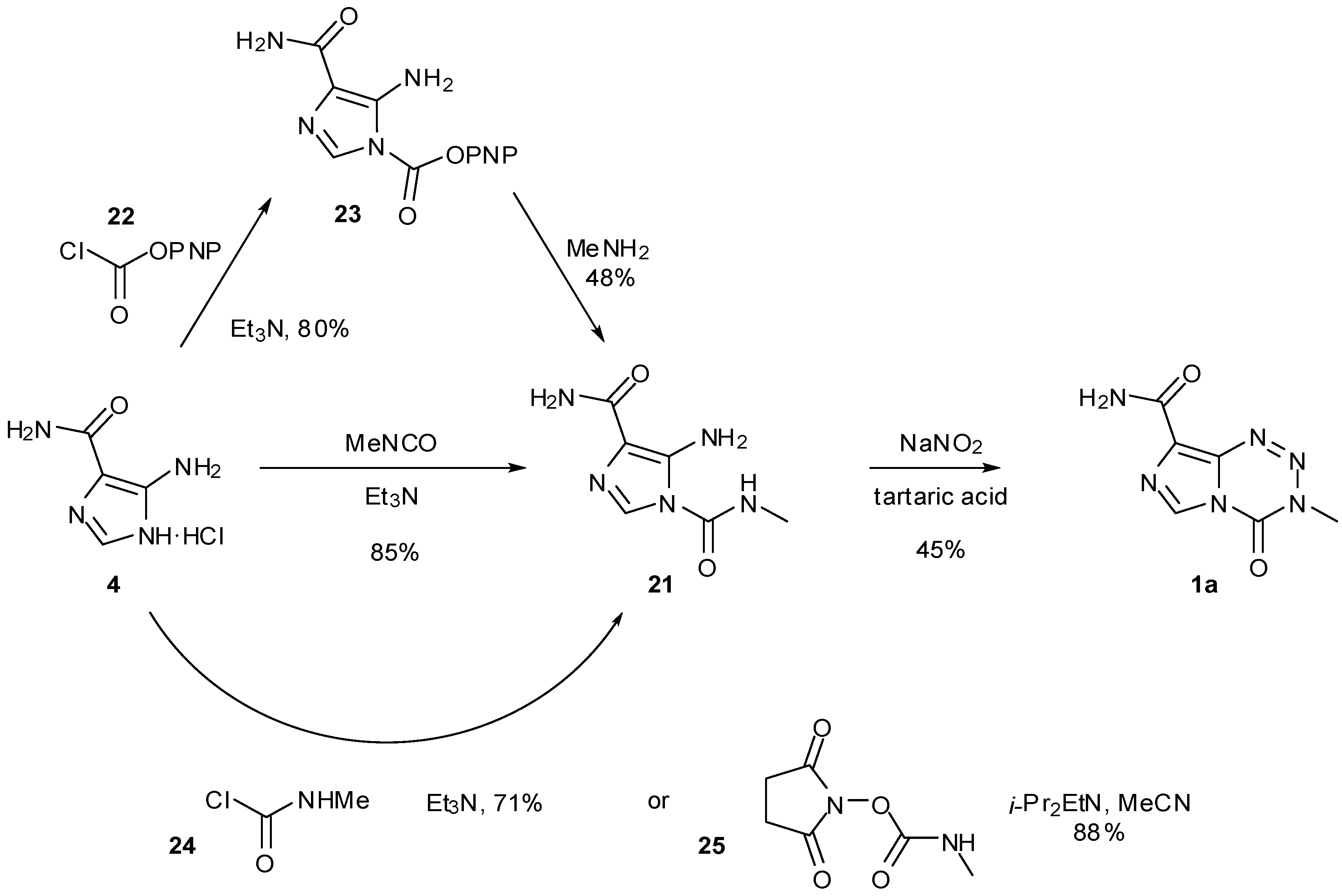
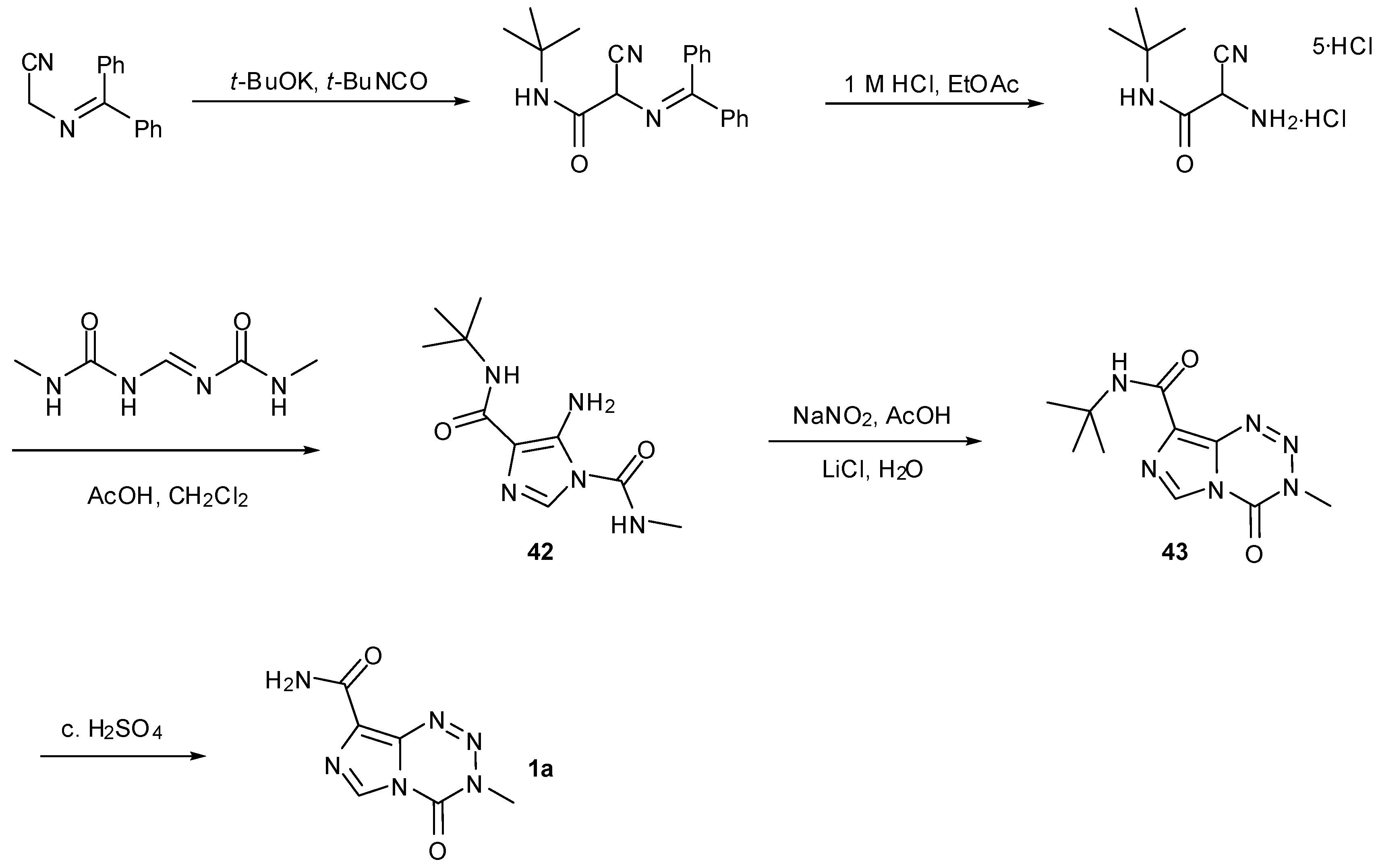
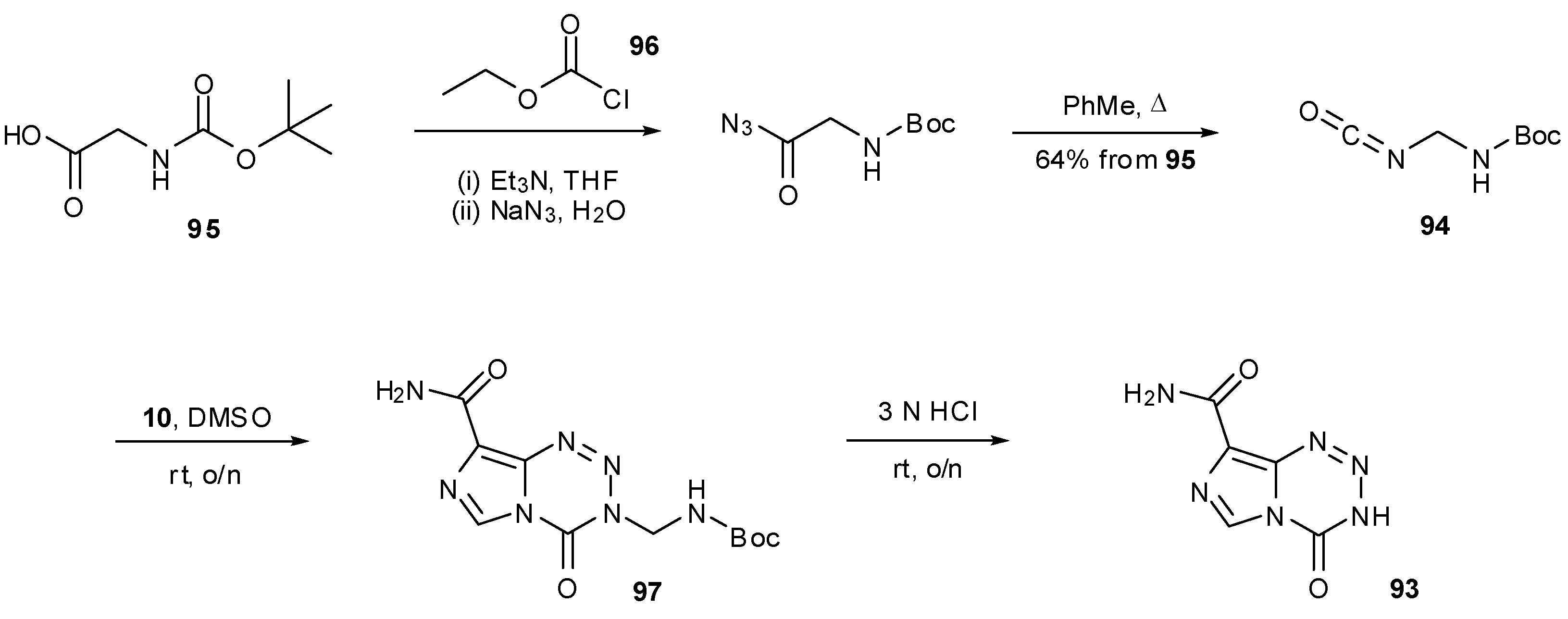
6. Synthesis of Temozolomide and the Imidazotetrazine Core
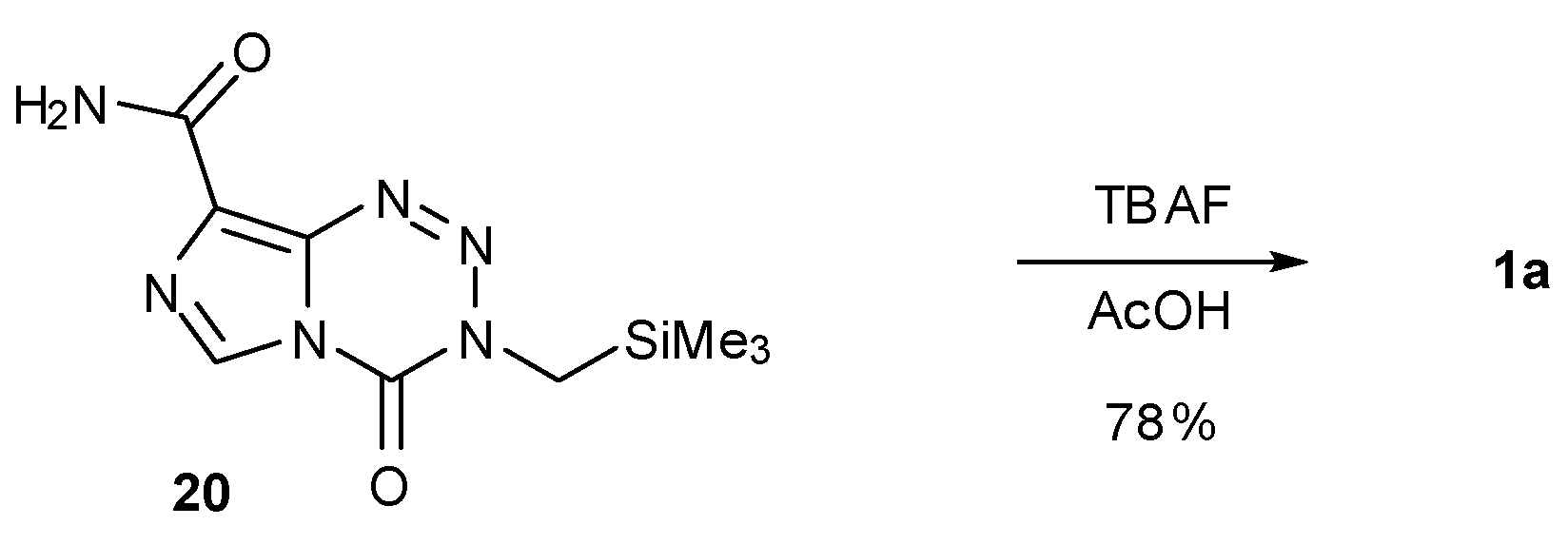
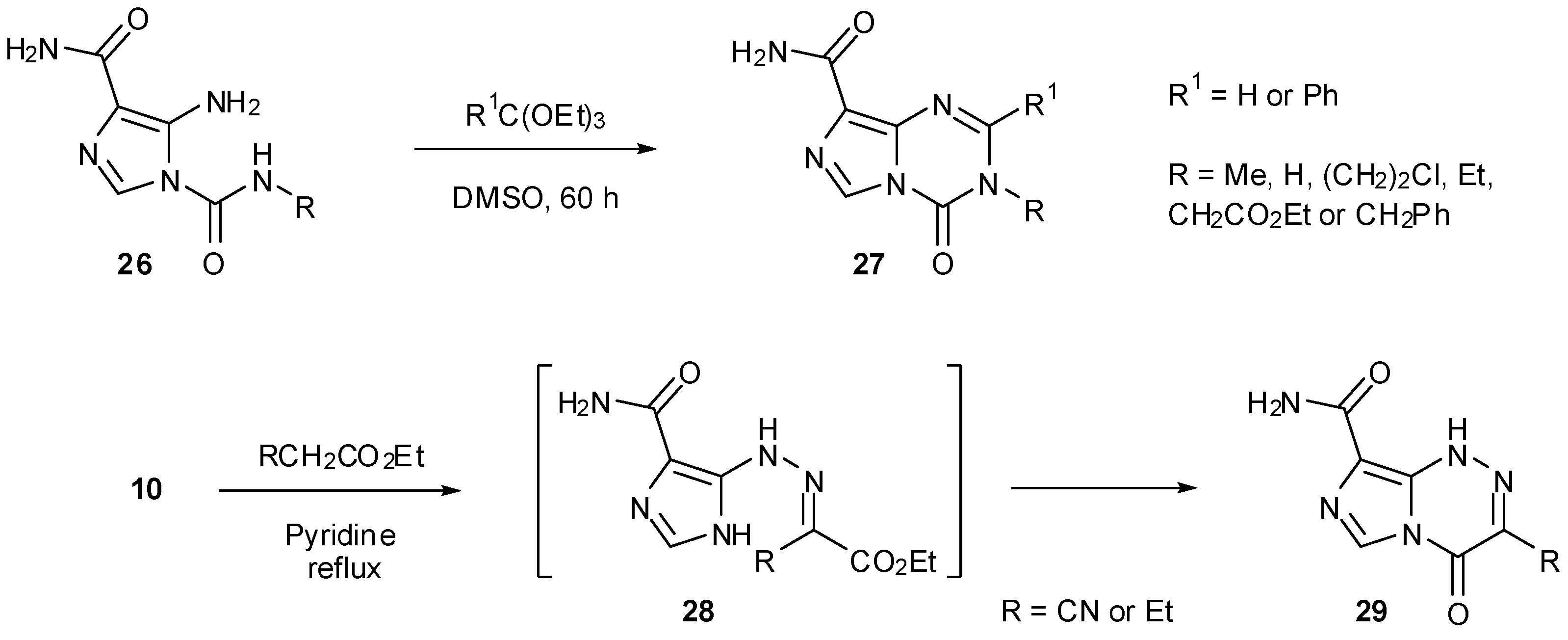
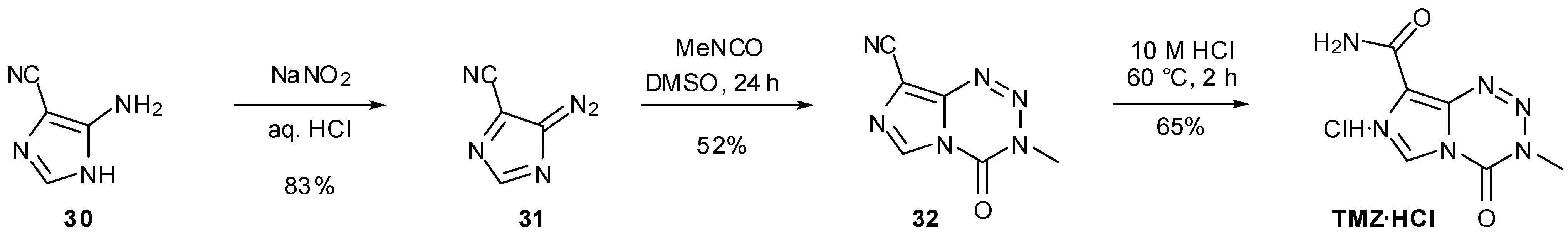
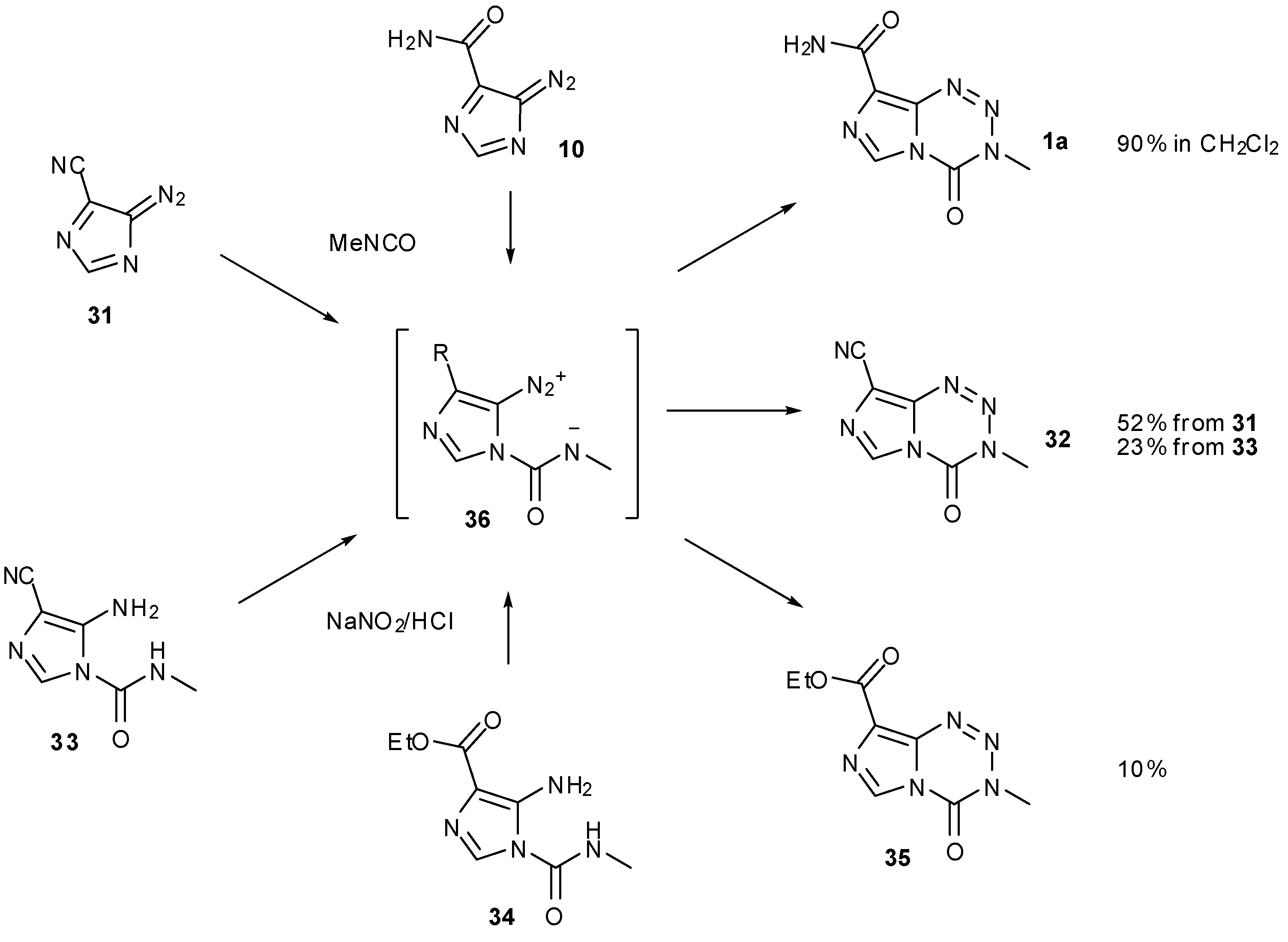
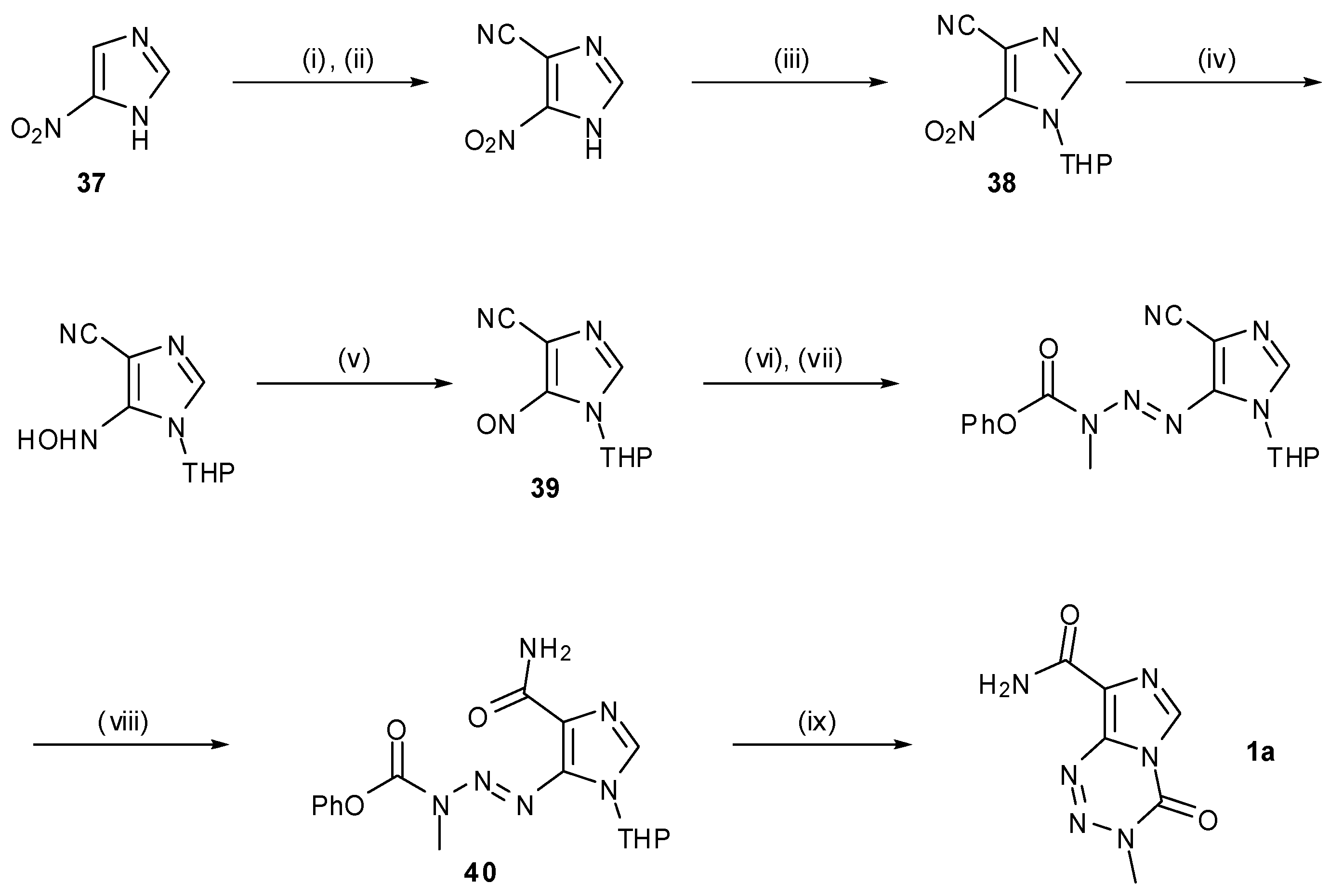
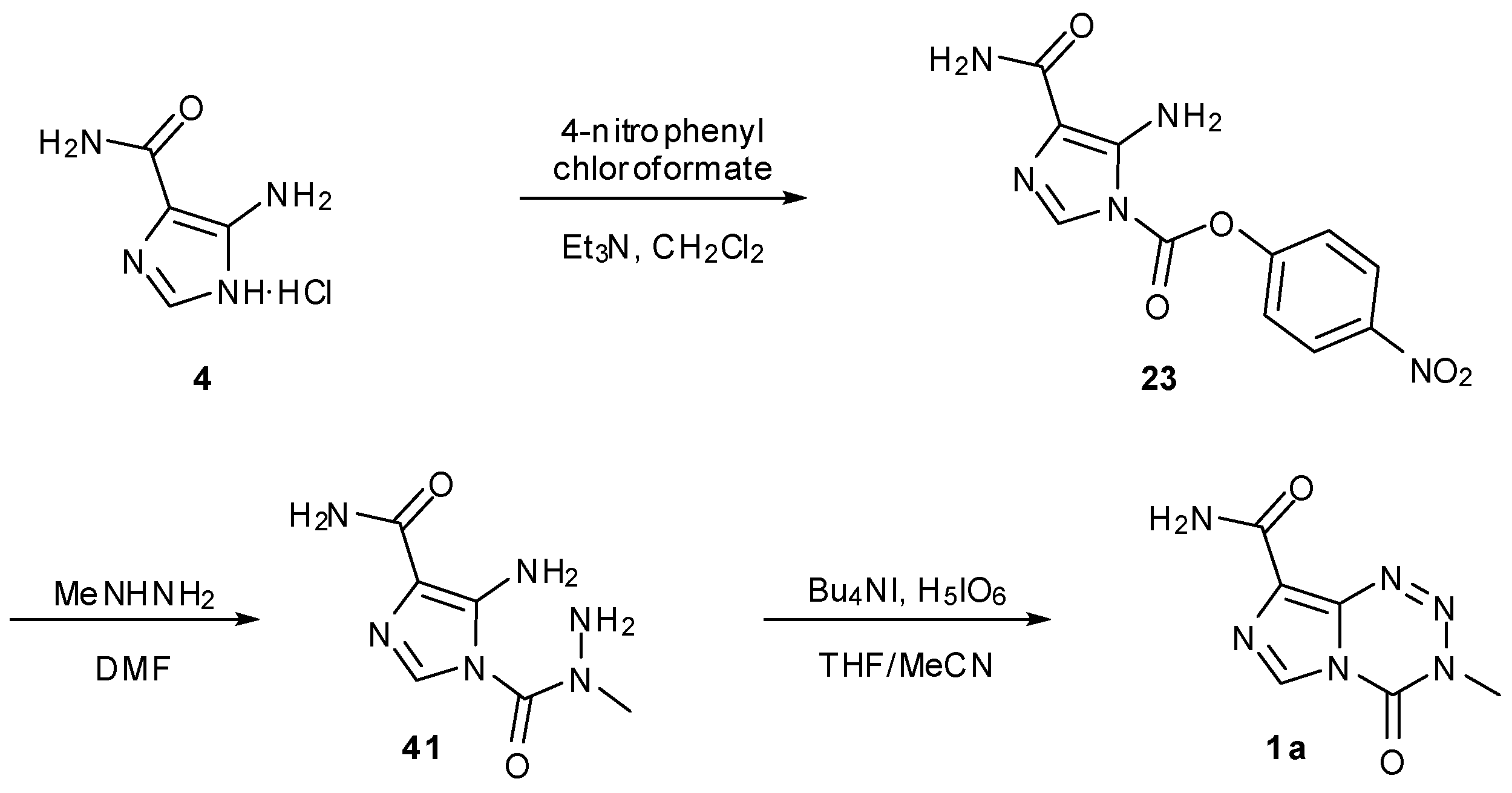
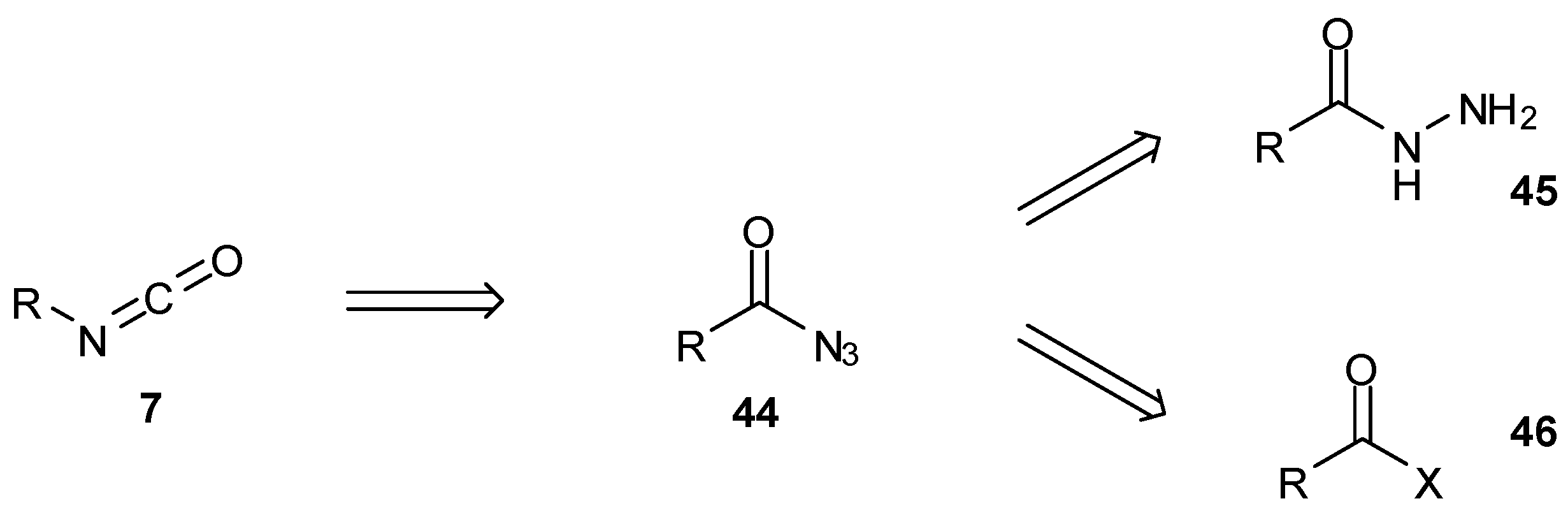
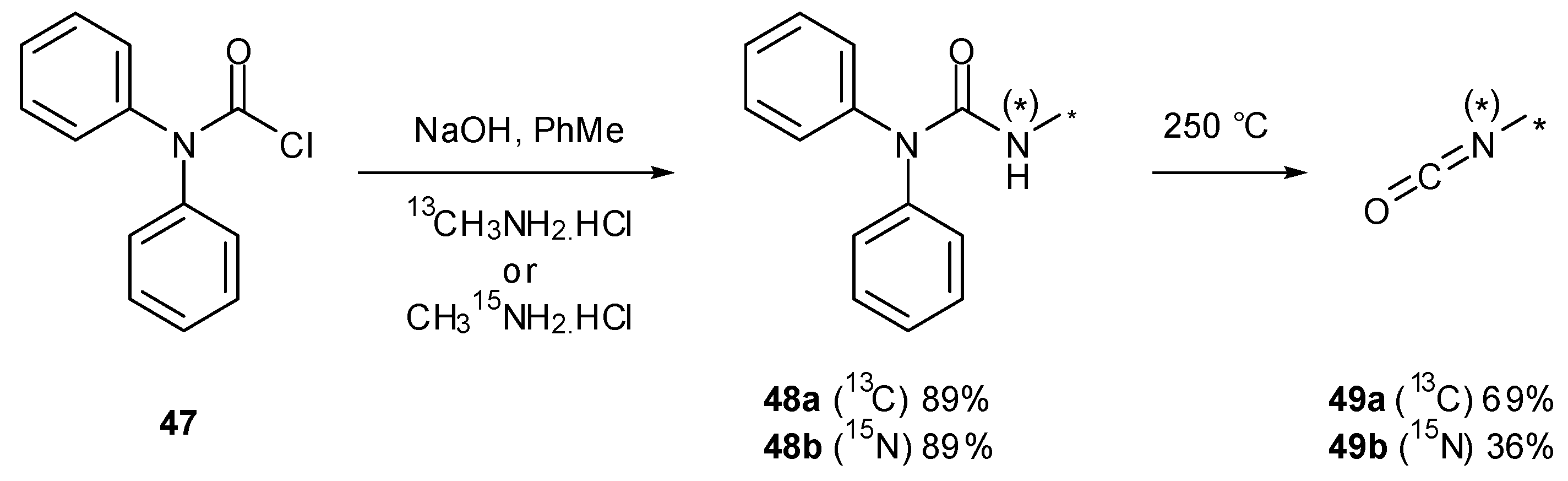
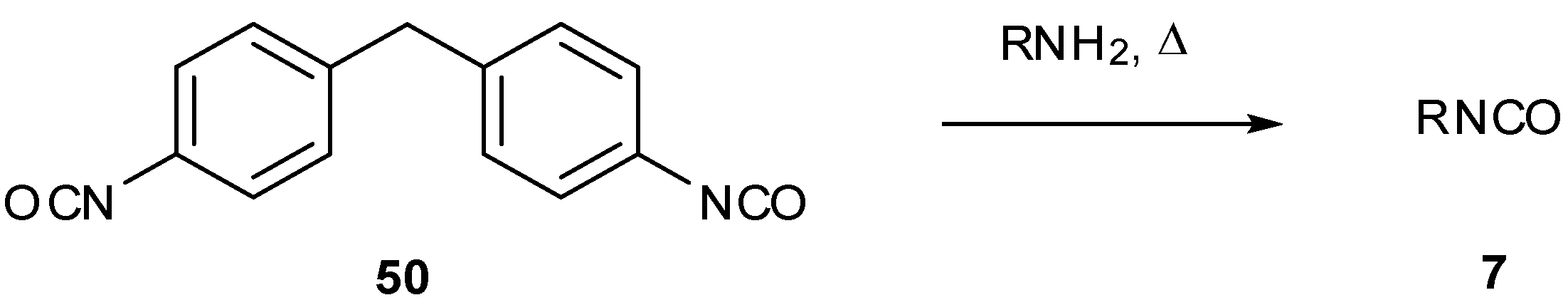
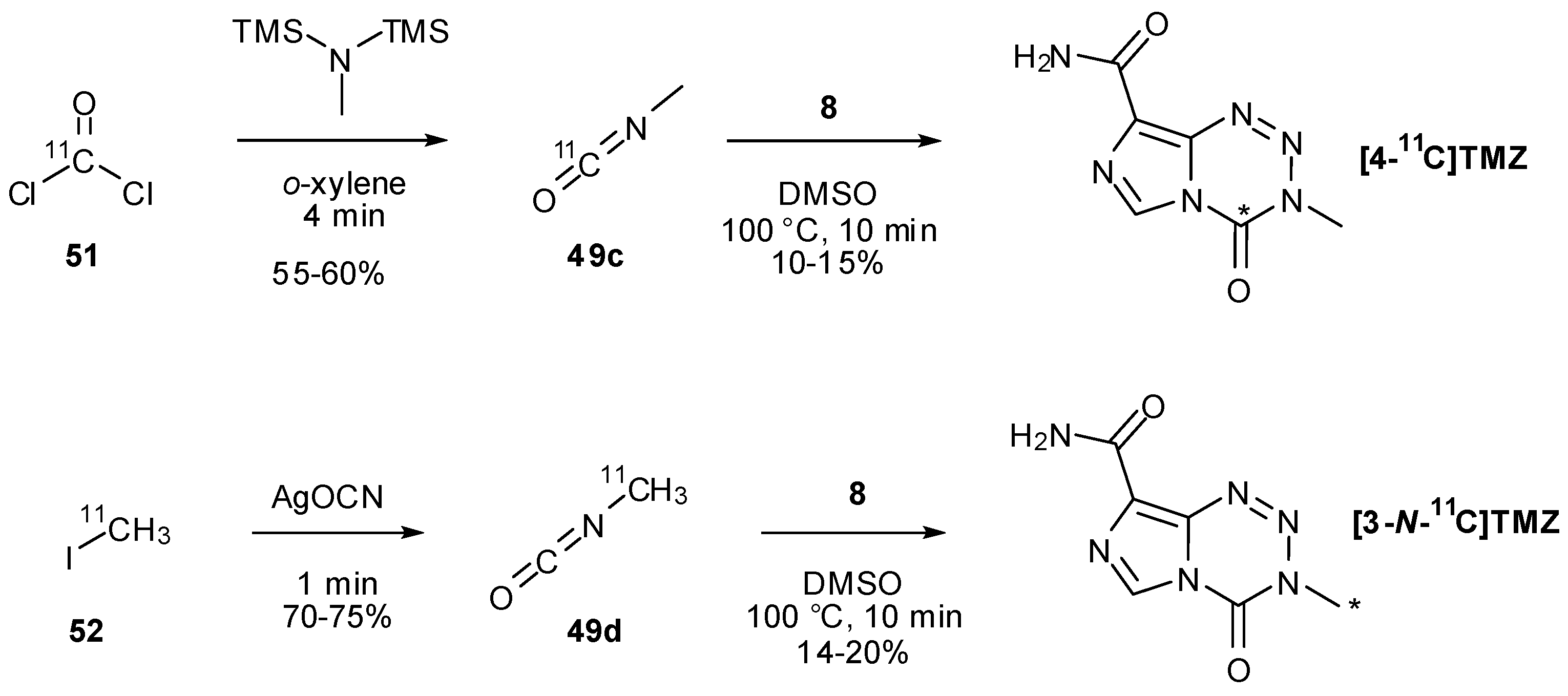
7. Synthesis of Structural Analogues
7.1. Alternative Cores
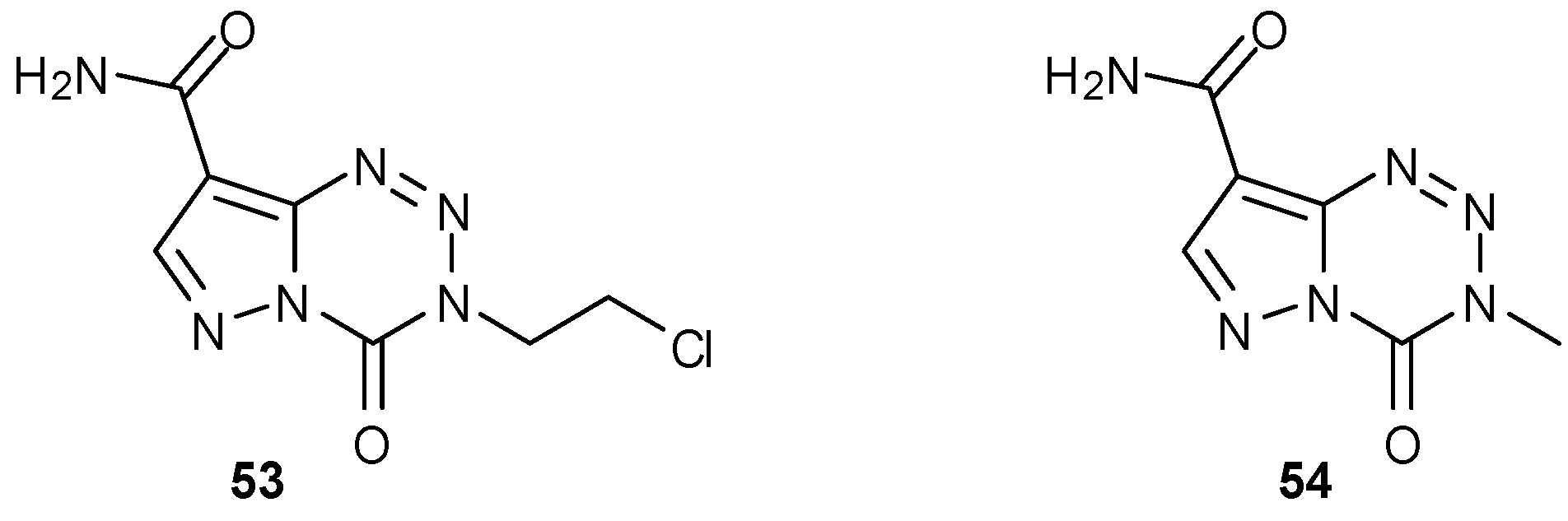
7.2. 6- and 8-Analogues
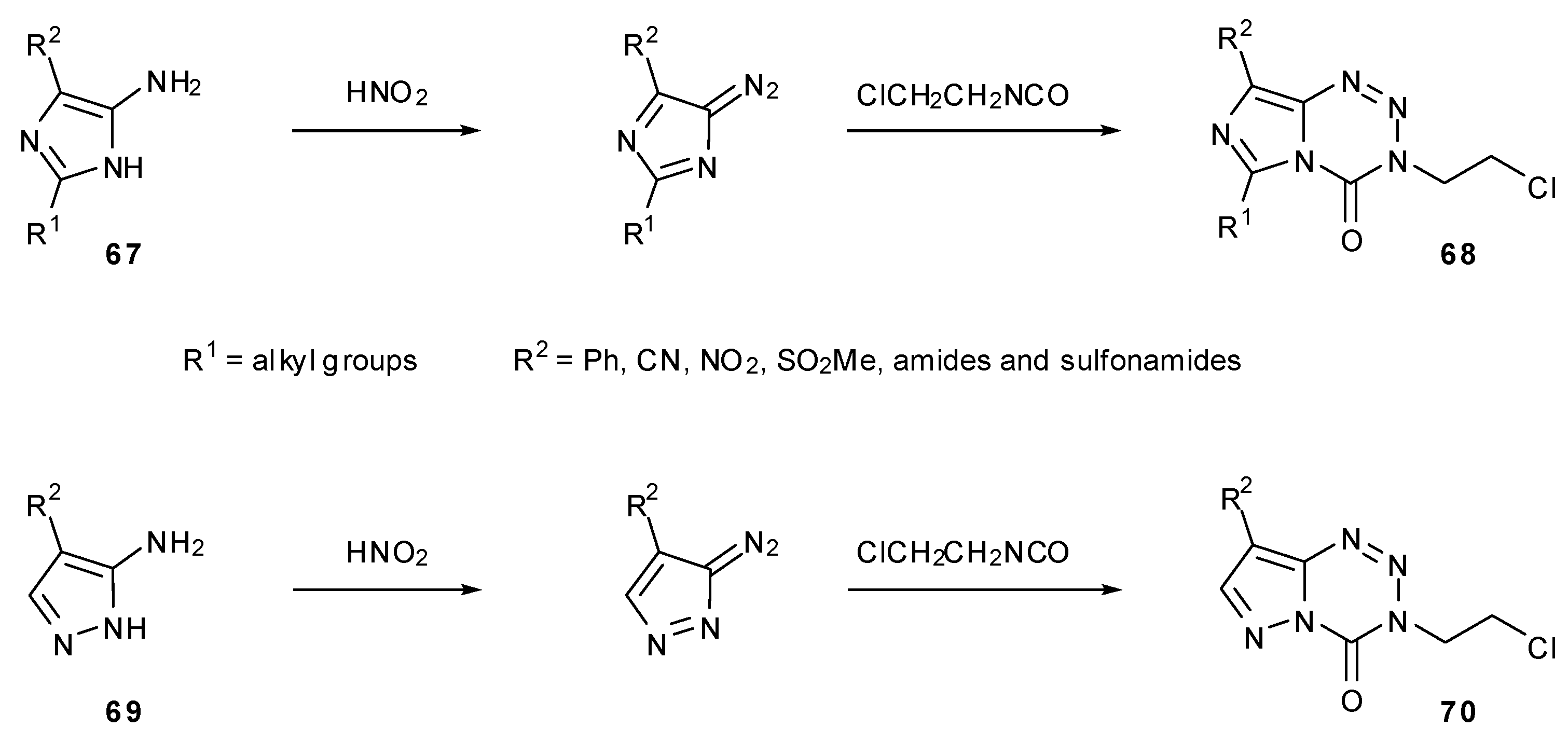
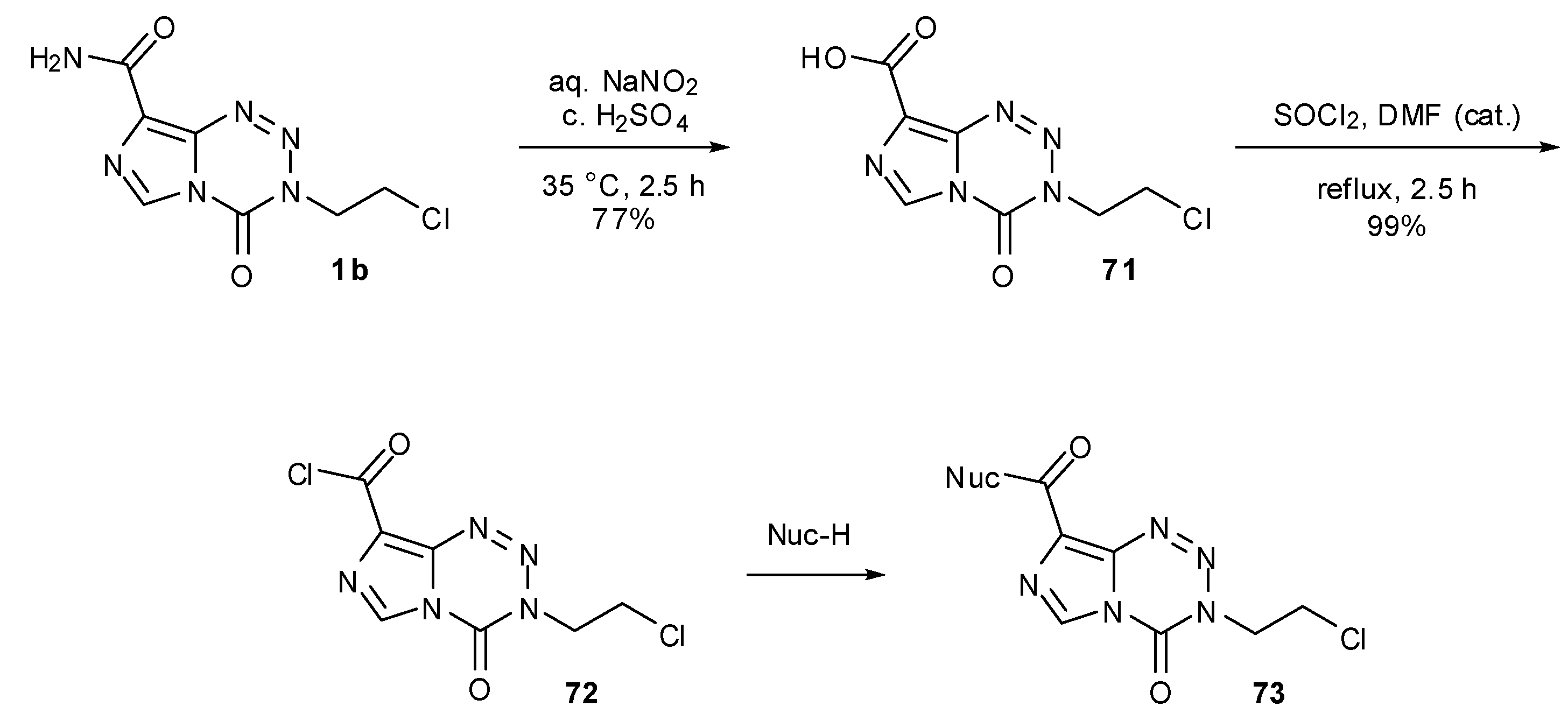
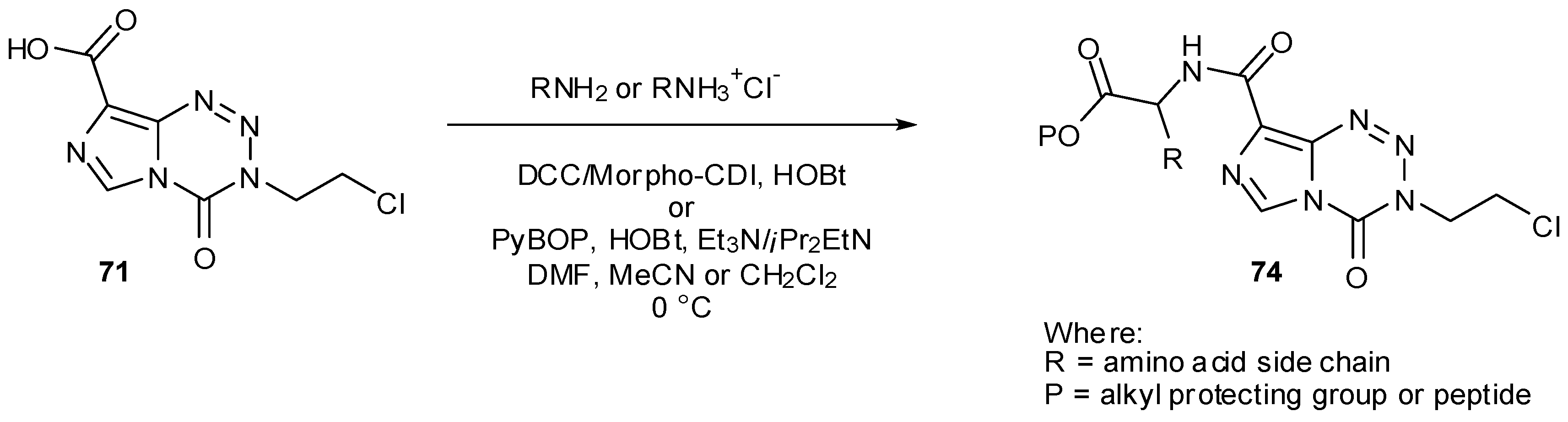
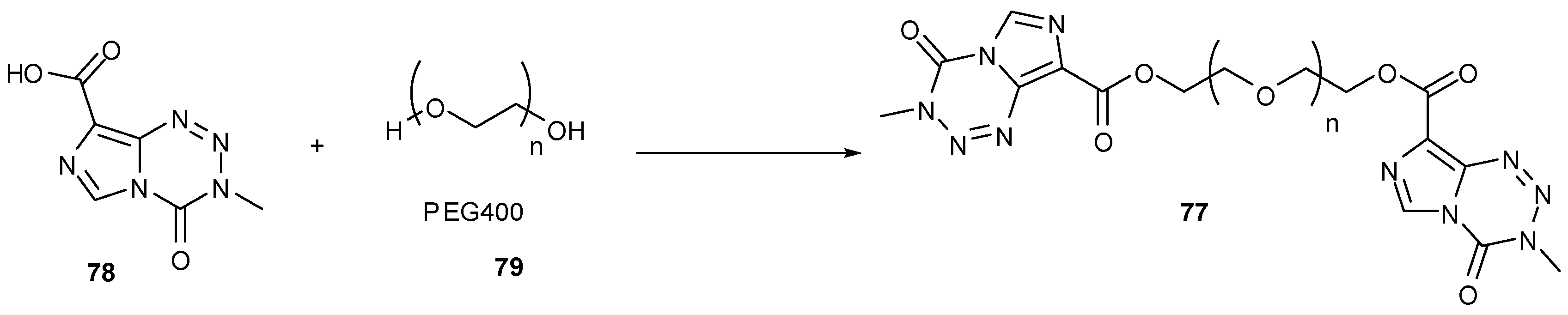
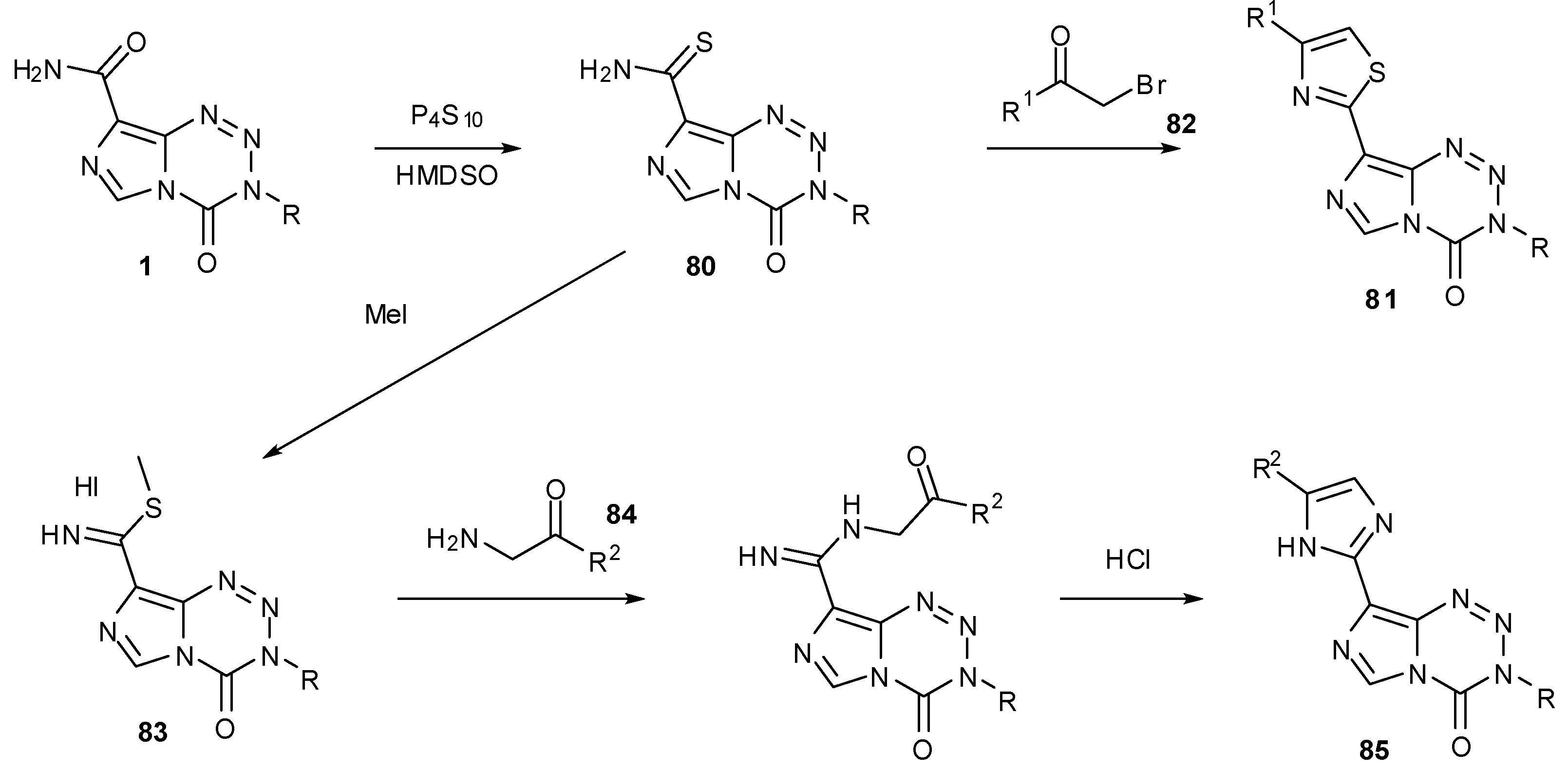
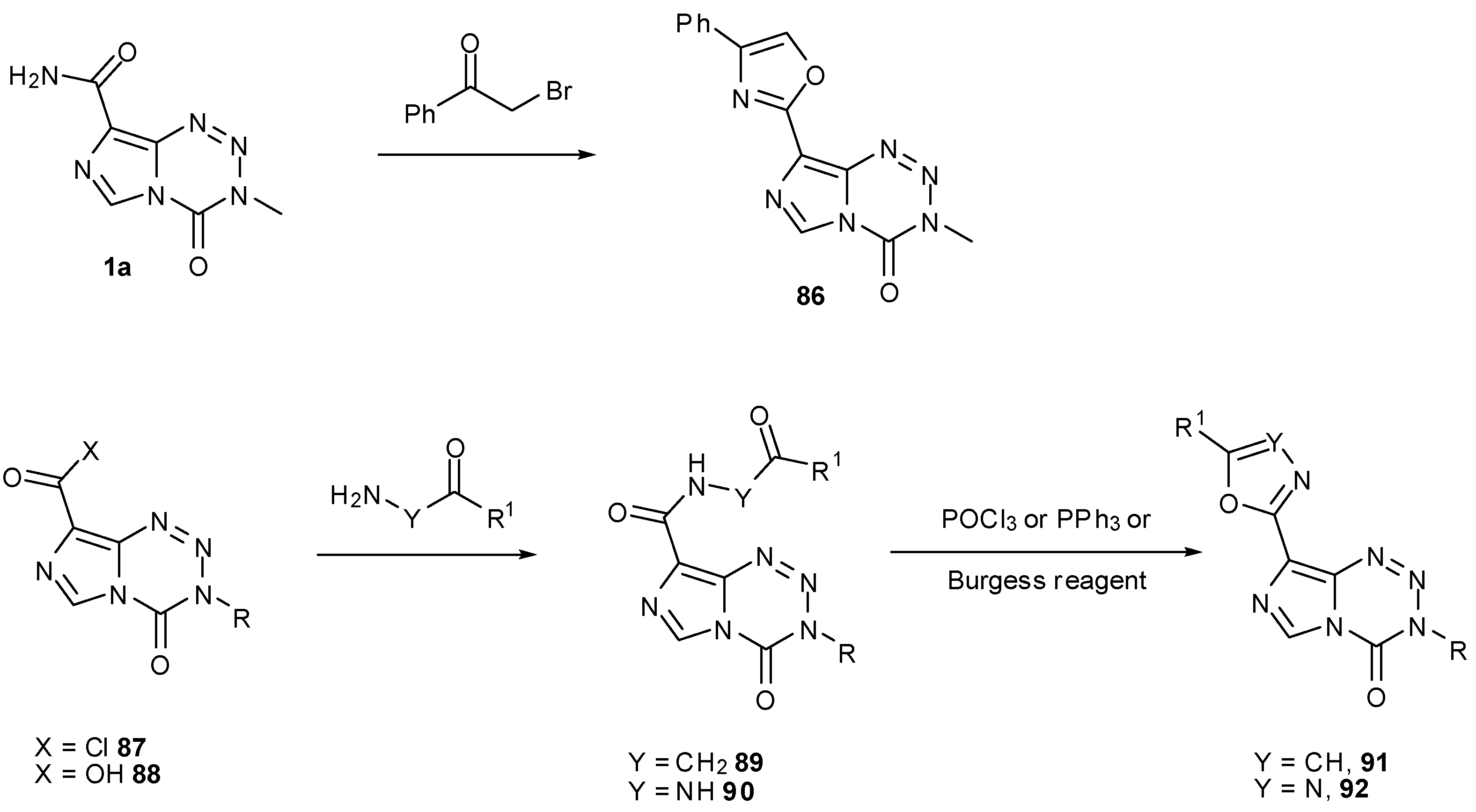
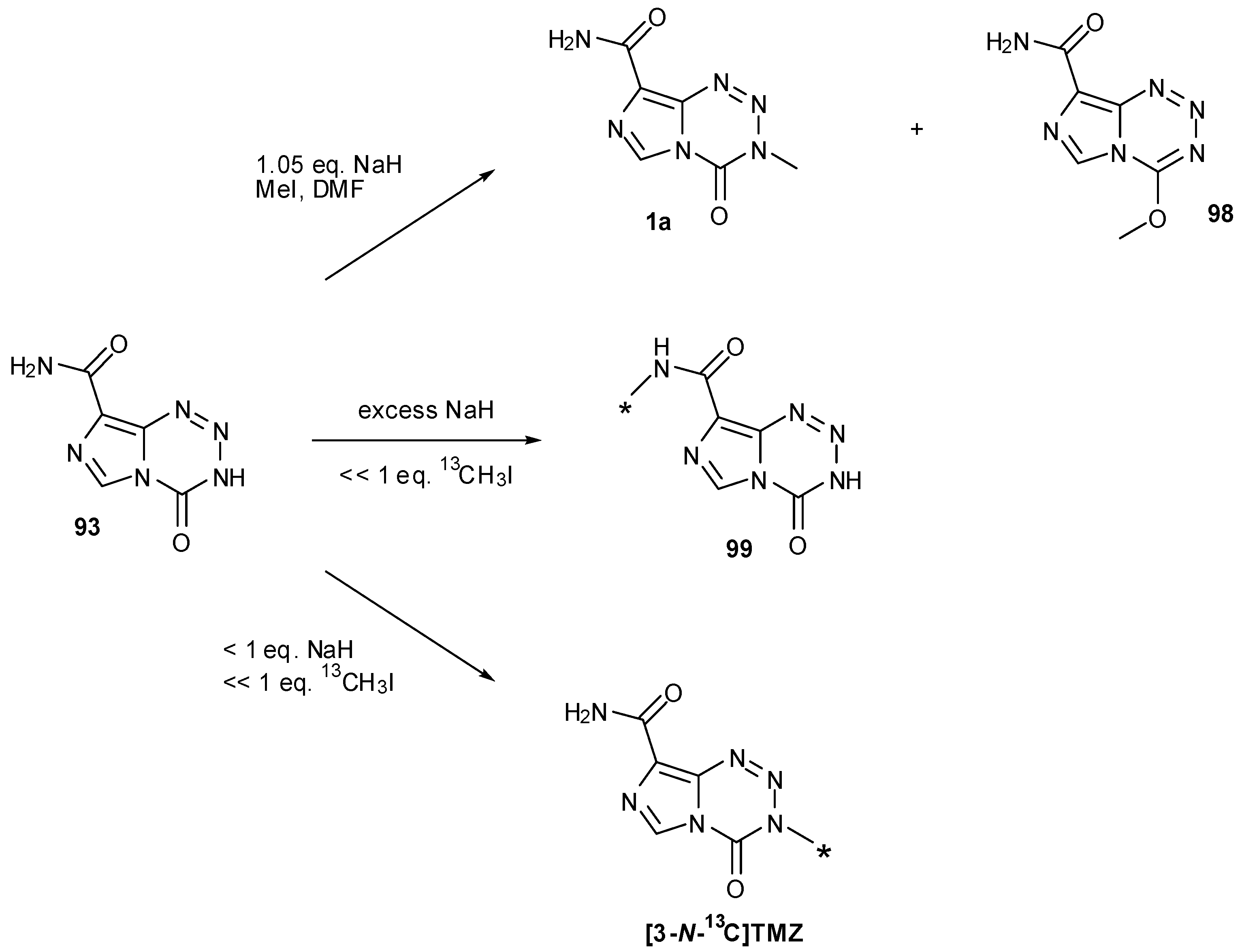
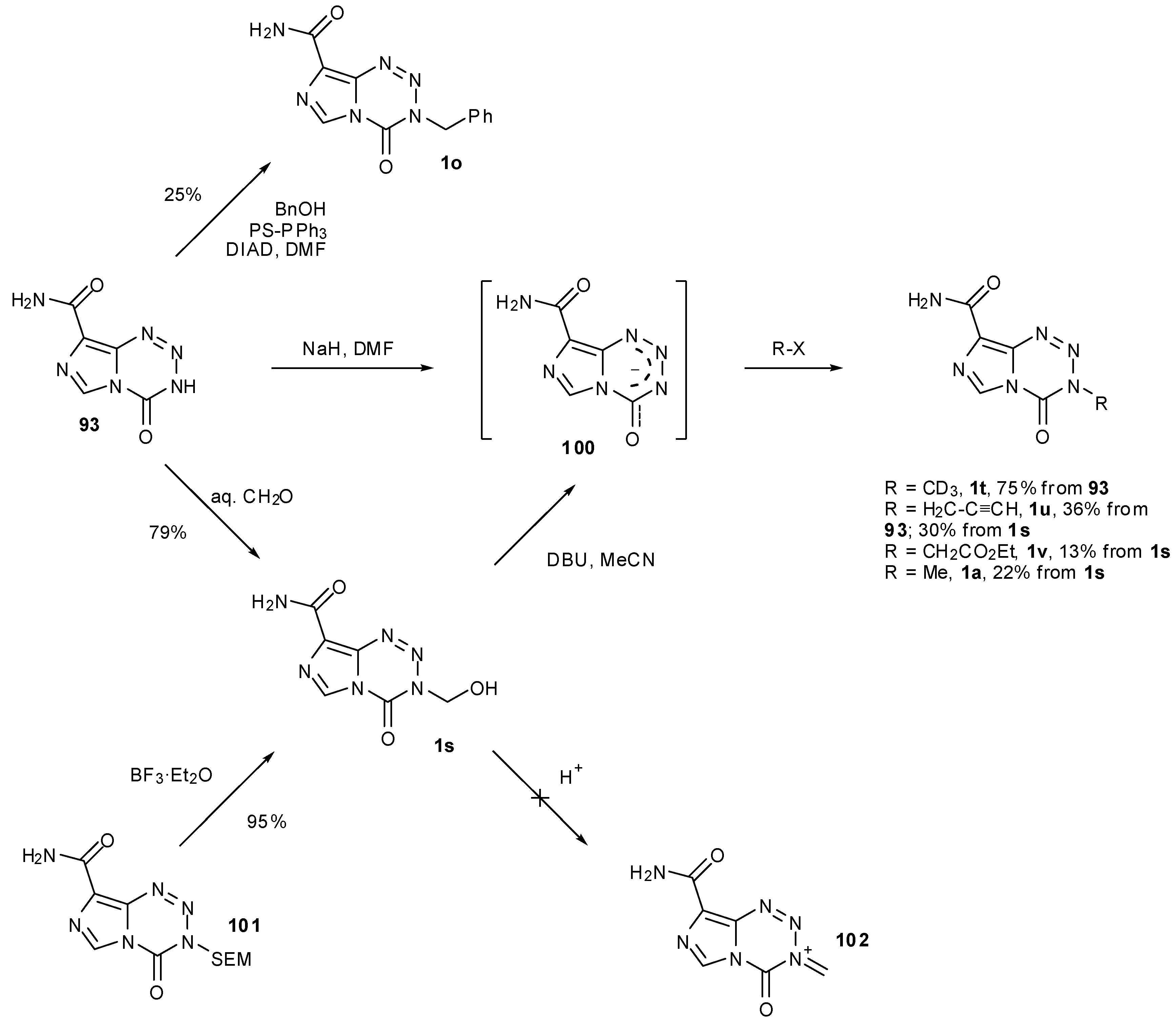
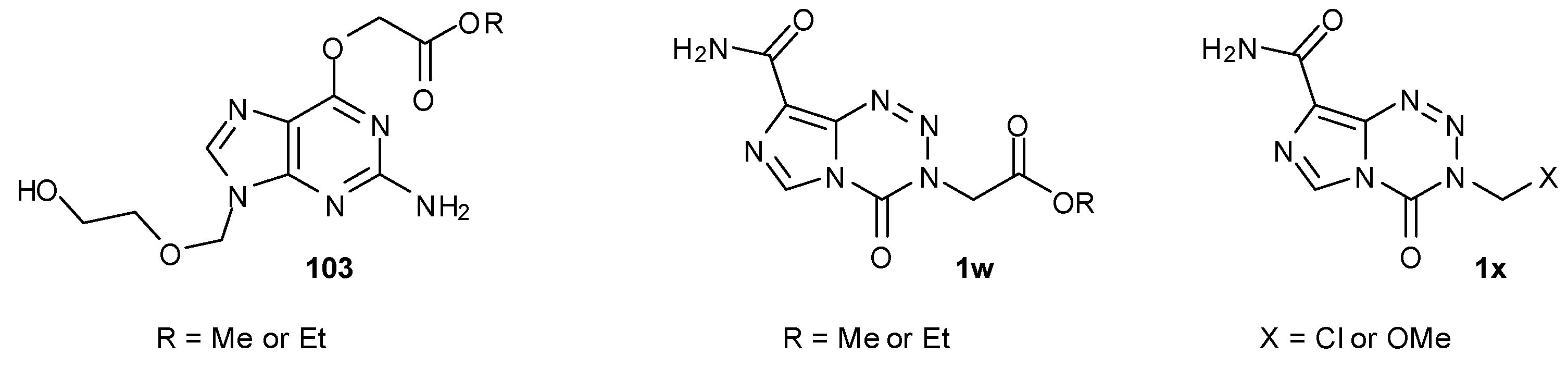
7.3. 3-Analogues
8. Design of MGMT/MMR-Independent Anti-Cancer Agents
8.1. Introduction to NGP Analogues
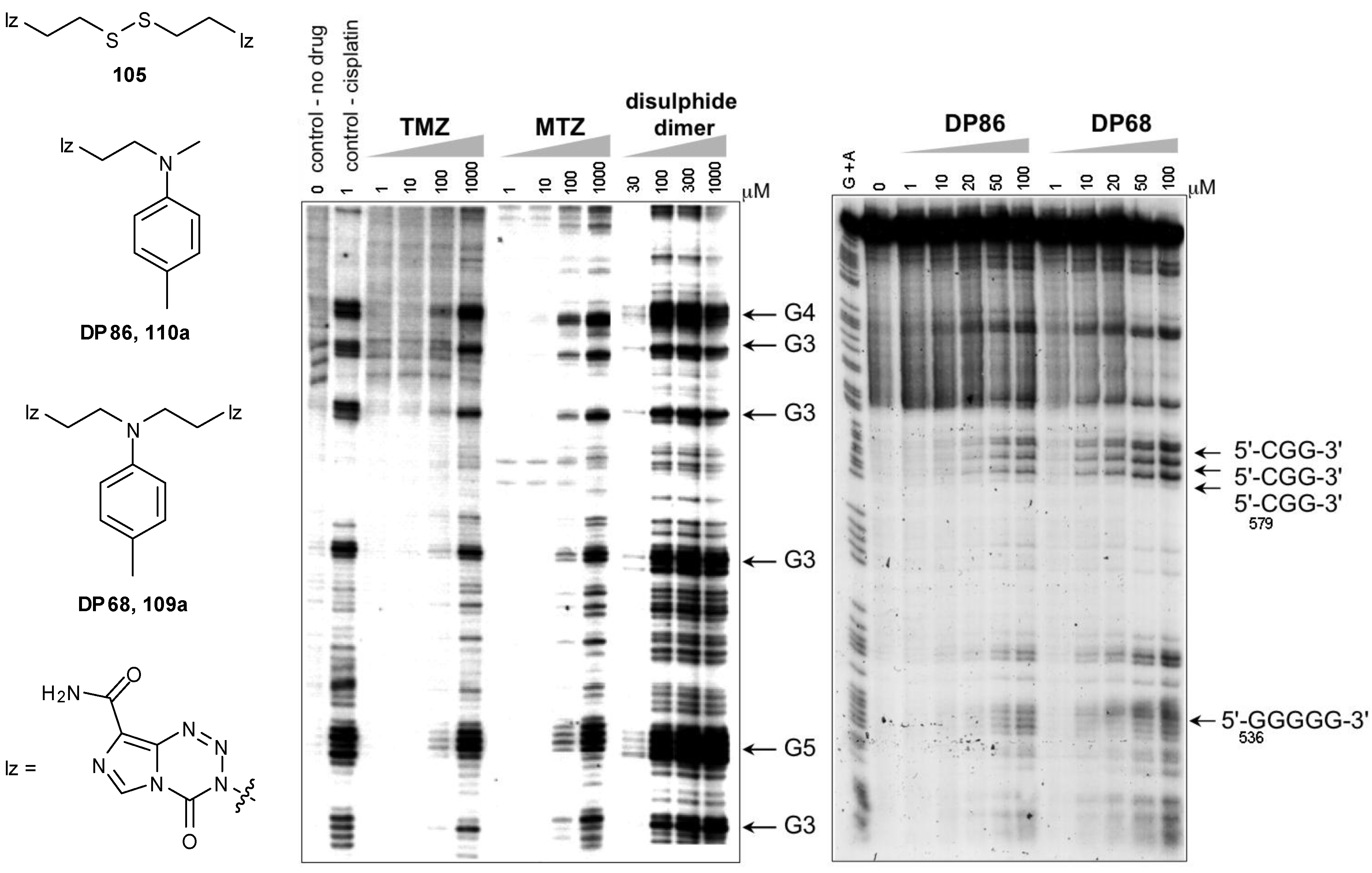
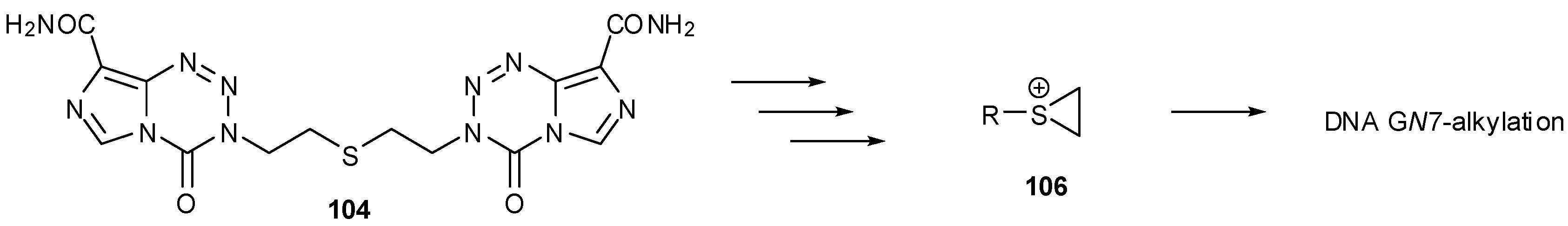
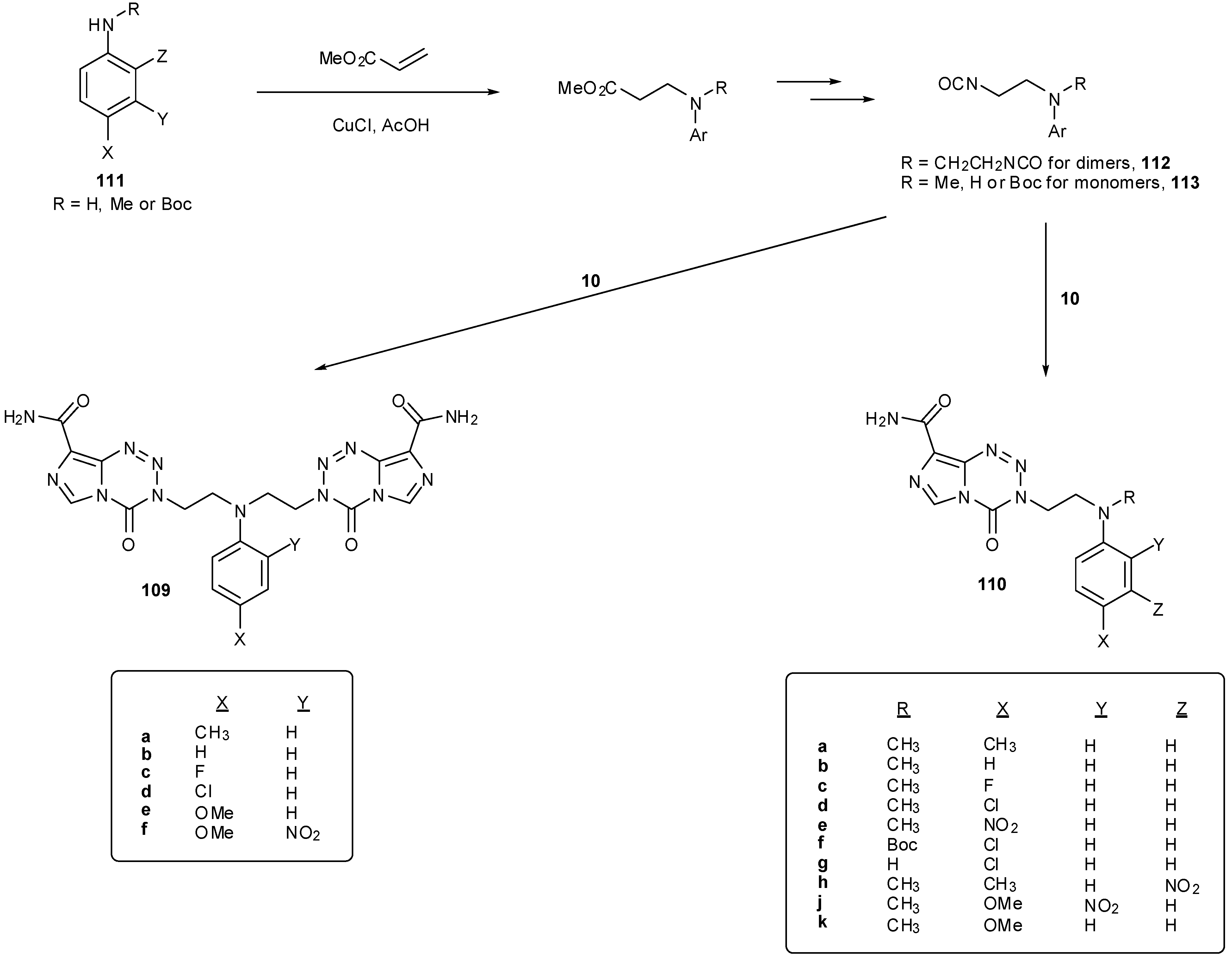
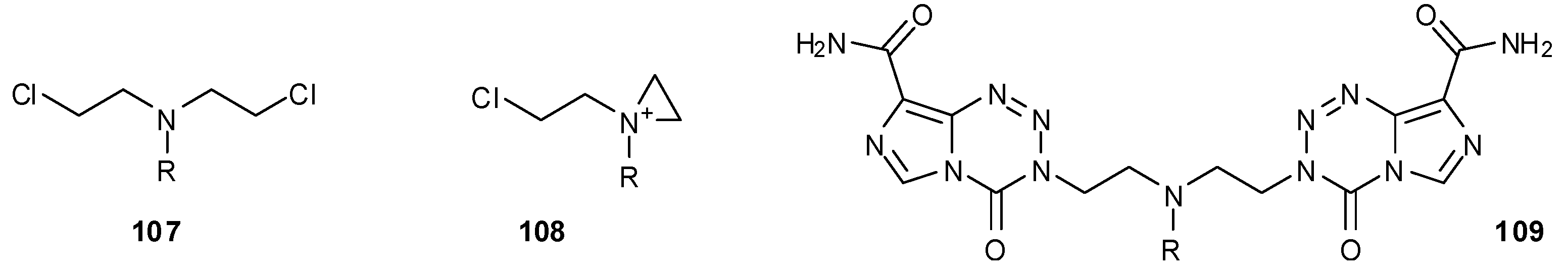
8.2. Synthesis of Novel N-Linked Imidazotetrazine Dimers
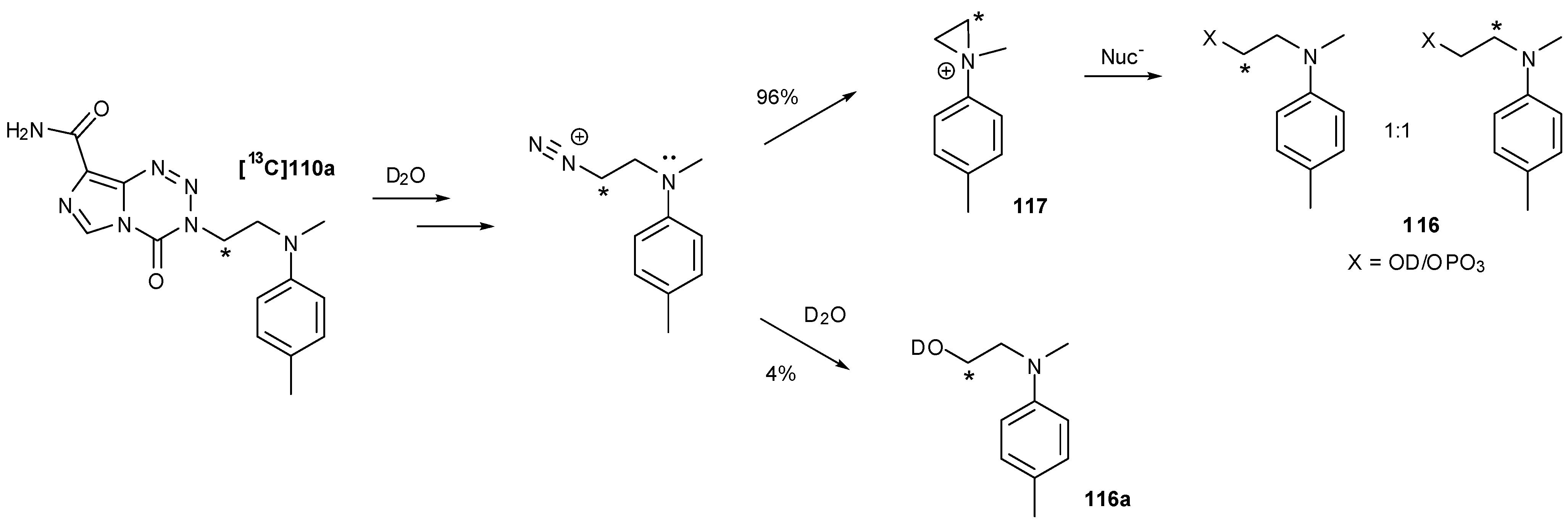
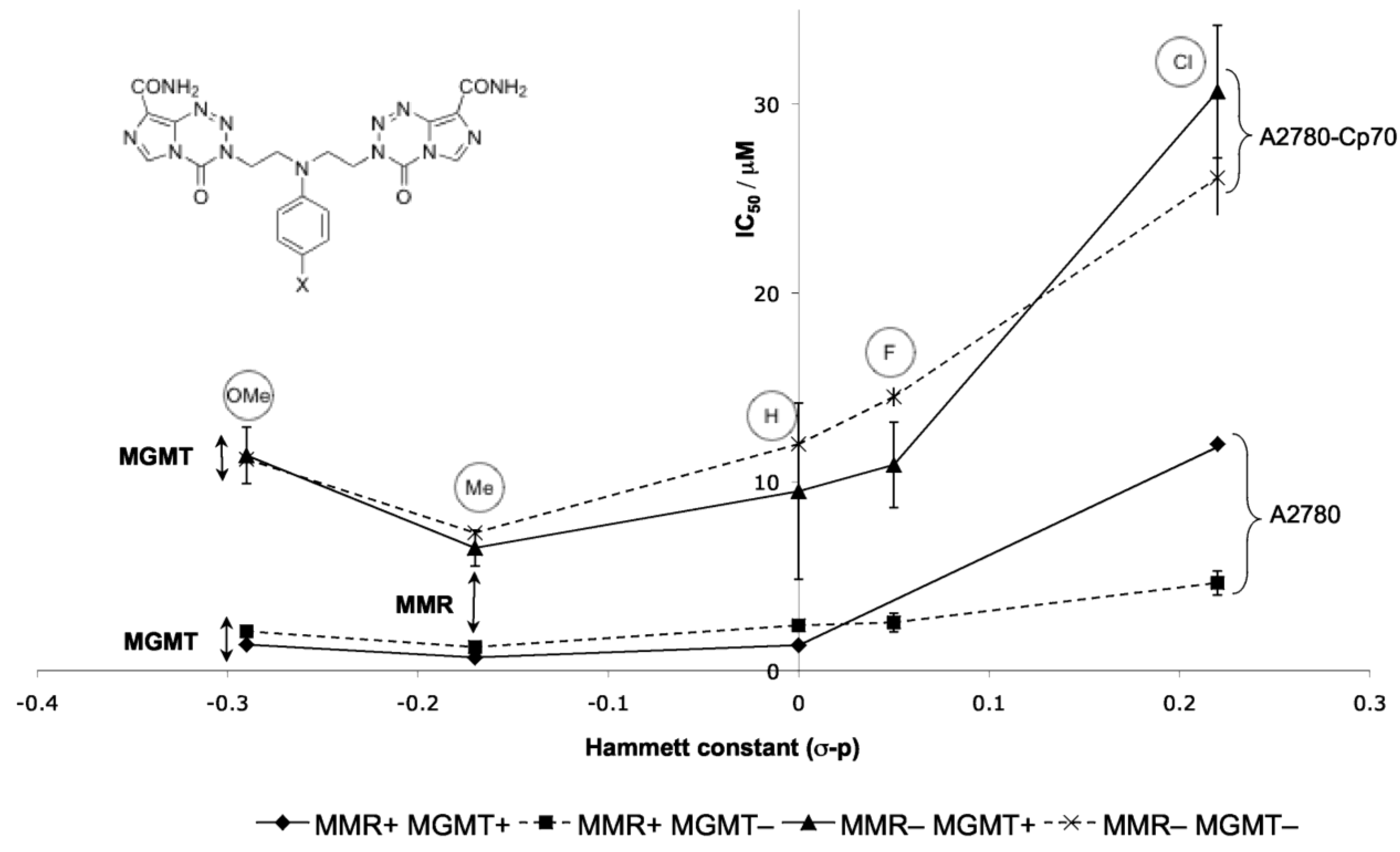
8.3. Properties and Activity of N-Linked Compounds
| 109c | 109d | 109e | 110d | MTZ | DTIC | MEL | CHB | BCNU | CCNU | CP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 109c | 1 | 0.92 | 0.92 | 0.83 | 0.35 | 0.72 | 0.4 | 0.33 | 0.32 | 0.06 | 0.32 |
| 109d | 0.92 | 1 | 0.81 | 0.79 | 0.38 | 0.64 | 0.36 | 0.29 | 0.27 | 0.05 | 0.35 |
| 109e | 0.92 | 0.81 | 1 | 0.77 | 0.46 | 0.71 | 0.59 | 0.53 | 0.45 | 0.10 | 0.42 |
| 110d | 0.83 | 0.79 | 0.77 | 1 | 0.42 | 0.62 | 0.35 | 0.33 | 0.25 | 0.08 | 0.27 |
9. Conclusions
Acknowledgments
Conflict of Interest
References
- Newlands, E.S.; Stevens, M.F.G.; Wedge, S.R.; Wheelhouse, R.T.; Brock, C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef]
- Stevens, M.F.G. Temozolomide: From cytotoxic to molecularly-targeted agent. In Cancer Drug Design and Discovery; Neidle, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 157–172. [Google Scholar]
- Darkes, M.J.M.; Plosker, G.L.; Jarvis, B. Temozolomide a review of its use in the treatment of malignant gliomas, malignant melanoma and other advanced cancers. Am. J. Cancer 2002, 1, 55–80. [Google Scholar]
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000, 6, 2585–2597. [Google Scholar]
- Threadgill, M.D. The Chemistry of azolotetrazinones. In The Chemistry of Antitumour Agents; Wilman, D.E.V., Ed.; Blackie & Son Ltd.: Bel Air, CA, USA, 1990; pp. 187–201. [Google Scholar]
- Gaudilliere, B.; Berna, P. Chapter 30. To market, to market. Annu. Rep. Med. Chem. 2000, 35, 331–356. [Google Scholar] [CrossRef]
- Baker, S.D.; Wirth, M.; Statkevich, P.; Reidenberg, P.; Alton, K.; Sartorius, S.E.; Dugan, M.; Cutler, D.; Batra, V.; Grochow, L.B.; et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin. Cancer Res. 1999, 5, 309–317. [Google Scholar]
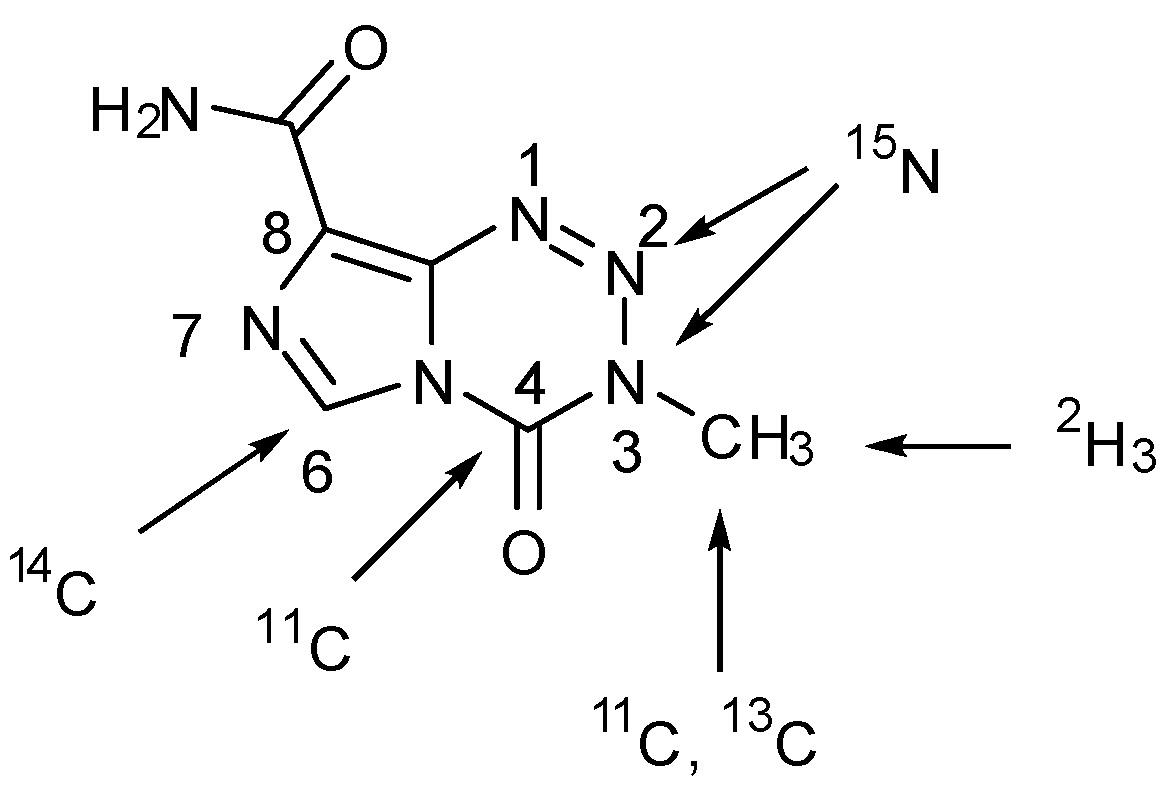
- Brown, G.D.; Luthra, S.K.; Brock, C.S.; Stevens, M.F.G.; Price, P.M.; Brady, F. Antitumor imidazotetrazines. 40. Radiosyntheses of [4-11C-carbonyl]- and [3-N-11C-methyl]-8-carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (temozolomide) for positron emission tomography (PET) Studies. J. Med. Chem. 2002, 45, 5448–5457. [Google Scholar]
- Stevens, M.F.G.; Hickman, J.A.; Langdon, S.P.; Chubb, D.; Vickers, L.; Stone, R.; Baig, G.; Goddard, C.; Gibson, N.W.; Slack, J.A.; et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987, 47, 5846–5852. [Google Scholar]
- Tsang, L.L.H.; Quarterman, C.P.; Gescher, A.; Slack, J.A. Comparison of the cytotoxicity in vitro of temozolomide and dacarbazine, prodrugs of 3-methyl-(triazen-1-yl)imidazole-4-carboxamide. Cancer Chemother. Pharmacol. 1991, 27, 342–346. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar]
- Ramirez, Y.P.; Weatherbee, J.L.; Wheelhouse, R.T.; Ross, A.H. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals 2013, 6, 1475–1506. [Google Scholar]
- Happold, C.; Roth, P.; Wick, W.; Schmidt, N.; Florea, A.-M.; Silginer, M.; Reifenberger, G.; Weller, M. Distinct molecular mechanisms of acquired resistance to temozolomide in glioblastoma cells. J. Neurochem. 2012, 122, 444–455. [Google Scholar] [Green Version]
- Barvaux, V.A.; Ranson, M.; Brown, R.; McElhinney, R.S.; McMurry, T.B.H.; Margison, G.P. Dual repair modulation reverses temozolomide resistance in vitro. Mol. Cancer Ther. 2004, 3, 123–127. [Google Scholar]
- Wheelhouse, R.T.; Stevens, M.F.G. Decomposition of the antitumour drug temozolomide in deuteriated phosphate buffer: methyl group transfer is accompanied by deuterium exchange. Chem. Commun. 1993, 1177–1178. [Google Scholar] [CrossRef]
- Denny, B.J.; Wheelhouse, R.T.; Stevens, M.F.G.; Tsang, L.L.; Slack, J.A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry 1994, 33, 9045–9051. [Google Scholar] [CrossRef]
- Wheelhouse, R.T.; Denny, B.J.; Stevens, M.F.G. Novel approaches in anticancer drug design. In Proceedings of the International Symposium on Novel Approaches in Cancer Therapy, Heidelberg, Germany, December 1993; Zeller, W.J., D’Incalci, M., Newell, D.R., Eds.; Contrib. to Oncol. 1995; 49, pp. 40–49. [Google Scholar]
- Campbell, J.R. Diazomethane-d2 (CD2N2). Chem. Ind. 1972, 540. [Google Scholar]
- Lunt, E.; Newton, C.G.; Smith, C.; Stevens, G.P.; Stevens, M.F.G.; Straw, C.G.; Walsh, R.J.A.; Warren, P.J.; Fizames, C.; Lavelle, F.; et al. Antitumor imidazotetrazines. 14. Synthesis and antitumor activity of 6- and 8-substituted imidazo[5,1-d]-1,2,3,5-tetrazinones and 8-substituted pyrazolo[5,1-d]-1,2,3,5-tetrazinones. J. Med. Chem. 1987, 30, 357–366. [Google Scholar] [CrossRef]
- Stevens, M.F.G.; Hickman, J.A.; Stone, R.; Gibson, N.W.; Baig, G.U.; Lunt, E.; Newton, C.G. Antitumor imidazotetrazines. 1. Synthesis and chemistry of 8-carbamoyl-3-(2-chloroethyl)imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one, a novel broad-spectrum antitumor agent. J. Med. Chem. 1984, 27, 196–201. [Google Scholar]
- Lown, J.W.; Chauhan, S.M.S. Synthesis of specifically 15N- and 13C-labeled antitumor (2-haloethyl)nitrosoureas. The study of their conformations in solution by nitrogen-15 and carbon-13 nuclear magnetic resonance and evidence for stereoelectronic control in their aqueous decomposition. J. Org. Chem. 1981, 46, 5309–5321. [Google Scholar] [CrossRef]
- Hartley, J.A.; Gibson, N.W.; Kohn, K.W.; Mattes, W.B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986, 46, 1943–1947. [Google Scholar]
- Horgan, C.M.T.; Tisdale, M.J. Antitumour imidazotetrazines—IV An investigation into the mechanism of antitumour activity of a novel and potent antitumour agent, mitozolomide (CCRG 81010, M & B 39565; NSC 353451). Biochem. Pharmacol. 1984, 33, 2185–2192. [Google Scholar] [CrossRef]
- Clark, A.S.; Deans, B.; Stevens, M.F.G.; Tisdale, M.J.; Wheelhouse, R.T.; Denny, B.J.; Hartley, J.A. Antitumor imidazotetrazines. 32. Synthesis of novel imidazotetrazinones and related bicyclic heterocycles to probe the mode of action of the antitumor drug temozolomide. J. Med. Chem. 1995, 38, 1493–1504. [Google Scholar]
- Wang, Y.; Wheelhouse, R.T.; Zhao, L.; Langnel, D.A.F.; Stevens, M.F.G. Antitumour imidazotetrazines. Part 36. Conversion of 5-amino-imidazole-4-carboxamide to imidazo[5,1-d][1,2,3,5]tetrazin-4(3H)-ones and imidazo[1,5-a][1,3,5]triazin-4(3H)-ones related in structure to the antitumour agents temozolomide and mitozolomide. J. Chem. Soc. Perkin Trans. 1998, 1, 1669–1675. [Google Scholar]
- Wheelhouse, R.T.; Wilman, D.E.V; Thomson, W.; Stevens, M.F.G. Antitumour imidazotetrazines. Part 31. The synthesis of isotopically labelled temozolomide and a multinuclear (1H, 13C, 15N) magnetic resonance investigation of temozolomide and mitozolomide. J. Chem. Soc. Perkin Trans. 1995, 1, 249–252. [Google Scholar]
- Babu, N.J.; Sanphui, P.; Nath, N.K.; Khandavilli, U.B.R.; Nangia, A. Temozolomide hydrochloride dihydrate. CrystEngComm 2013, 15, 666–671. [Google Scholar] [CrossRef]
- McGarrity, J.F.; Smyth, T. Hydrolysis of diazomethane—Kinetics and mechanism. J. Am. Chem. Soc. 1980, 102, 7303–7308. [Google Scholar] [CrossRef]
- Newlands, E.S.; Blackledge, G.; Slack, J.A.; Goddard, C.; Brindley, C.J.; Holden, L.; Stevens, M.F.G. Phase-I clinical trial of mitozolomide. Cancer Treat. Rep. 1985, 69, 801–805. [Google Scholar]
- Zhang, D.; Tian, A.; Xue, X.; Wang, M.; Qiu, B.; Wu, A. The effect of temozolomide/poly(lactide-co-glycolide) (PLGA)/nano-hydroxyapatite microspheres on glioma U87 cells behavior. Int. J. Mol. Sci. 2012, 13, 1109–1125. [Google Scholar]
- Panda, B.; Maikap, G.C.; Agarwal, S.K.; Singh, M.K.; Jaggi, M. Crystalline temozolomide monohydrate and process for preparation thereof. Int. Pat. WO2008111092A1, 18 September 2008. [Google Scholar]
- Adin, I.; Iustain, C. Novel crystalline forms of temozolomide. US Pat. US20100022609A1, 28 January 2010. [Google Scholar]
- Lowe, P.R.; Sansom, C.E.; Schwalbe, C.H.; Stevens, M.F.G.; Clark, A.S. Antitumor imidazotetrazines. 25. Crystal structure of 8-carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (temozolomide) and structural comparisons with the related drugs mitozolomide and DTIC. J. Med. Chem. 1992, 35, 3377–3382. [Google Scholar] [CrossRef]
- Babu, N.J.; Reddy, L.S.; Aitipamula, S.; Nangia, A. Polymorphs and polymorphic cocrystals of temozolomide. Chem. Asian J. 2008, 3, 1122–1133. [Google Scholar] [CrossRef]
- Babu, N.J.; Sanphui, P.; Nangia, A. Crystal engineering of stable temozolomide cocrystals. Chem. Asian J. 2012, 7, 2274–2285. [Google Scholar]
- Sanphui, P.; Babu, N.J.; Nangia, A. Temozolomide cocrystals with carboxamide coformers. Cryst. Growth Des. 2013, 13, 2208–2219. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Wei, J.; Zhou, R.; Chen, Z.; Liang, H. Synthesis and crystal structure of a cadmium complex of temozolomide. J. Chem. Res. 2012, 36, 520–522. [Google Scholar] [CrossRef]
- Appel, E.A.; Rowland, M.J.; Loh, X.J.; Heywood, R.M.; Watts, C.; Scherman, O.A. Enhanced stability and activity of temozolomide in primary glioblastoma multiforme cells with cucurbit[n]uril. Chem. Commun. 2012, 48, 9843–9845. [Google Scholar]
- Ege, G.; Gilbert, K. [7+2]- and [11+2]-Cycloaddition reactions of diazo-azoles with isocyanates to azolo[5,1-d][1,2,3,5]tetrazine-4-ones. Tetrahedron Lett. 1979, 20, 4253–4256. [Google Scholar]
- Shealy, Y.F.; Struck, R.F.; Holum, L.B.; Montgomery, J.A. Synthesis of potential anticancer agents. XXIX. 5-Diazoimidazole-4-carboxamide and 5-diazo-ν-triazole-4-carboxamide. J. Org. Chem. 1961, 26, 2396–2401. [Google Scholar] [CrossRef]
- Woolley, D.W.; Shaw, E. Some imidazo-1,2,3-triazenes and their biological relationship to the purines. J. Biol. Chem. 1951, 189, 401–410. [Google Scholar]
- Horton, K.; Stevens, M.F.G. Triazenes and related products. Part 23. New photo-products from 5-diazoimidazole-4-carboxamide (diazo-IC). J. Chem. Soc. Perkin Trans. 1981, 1, 1433–1436. [Google Scholar] [CrossRef]
- Wang, Y.; Stevens, M.F.G. Synthetic studies of 8-carbamoylimidazo-[5,1-d]-1,2,3,5-tetrazin-4(3H)-one: A key derivative of antitumour drug temozolomide. Bioorganic Med. Chem. Lett. 1996, 3, 185–188. [Google Scholar] [CrossRef]
- Pletsas, D.; Karodia, N.; Wheelhouse, R.T.; University of Bradford, Bradford, UK. Unpublished data.
- Arrowsmith, J.; Jennings, S.A.; Clark, A.S.; Stevens, M.F.G. Antitumor imidazotetrazines. 41. Conjugation of the antitumor agents mitozolomide and temozolomide to peptides and lexitropsins bearing DNA major and minor groove-binding structural motifs. J. Med. Chem. 2002, 45, 5458–5470. [Google Scholar]
- Wang, Y.; Stevens, M.F.G.; Thomson, W. Alternative syntheses of the antitumour drug temozolomide avoiding the use of methyl isocyanate. J. Chem. Soc. Chem. Commun. 1994, 1687–1688. [Google Scholar]
- Wang, Y.; Stevens, M.F.G.; Chan, T.; DiBenedetto, D.; Ding, Z.; Gala, D.; Hou, D.; Kugelman, M.; Leong, W.; Kuo, S.; et al. Antitumor imidazotetrazines. 35. New synthetic routes to the antitumor drug temozolomide. J. Org. Chem. 1997, 62, 7288–7294. [Google Scholar] [CrossRef]
- Wang, Y.; Stevens, M.F.G.; Thomson, W.T.; Shutts, B.P. Antitumour imidazotetrazines. Part 33. New syntheses of the antitumour drug temozolomide using “masked” methyl isocyanates. J. Chem. Soc. Perkin Trans. 1995, 1, 2783–2787. [Google Scholar]
- Wang, Y.; Lowe, P.R.; Thomson, W.T.; Clark, J.; Stevens, M.F.G. A new route to the antitumour drug temozolomide, but not thiotemozolomide. Chem. Commun. 1997, 363–364. [Google Scholar]
- Turchetta, S.; De Ferra, L.; Zenoni, M.; Anibaldi, M. Process for preparing temozolomide. Eur. Pat. EP2151442A2, 10 February 2010. [Google Scholar]
- Pathi, S.L.; Rao, D.R.; Kankan, R.N. Process for the preparation of temozolomide and analogs. US Pat. 2009. [Google Scholar]
- Etlin, O.; Alnabari, M.; Sery, Y.; Danon, E.; Arad, O.; Kaspi, J. Process for preparing temozolomide. US Pat. US20060183898A1, 17 August 2006. [Google Scholar]
- Wanner, M.J.; Koomen, G. A new synthesis of temozolomide. J. Chem. Soc. Perkin Trans. 2002, 1, 1877–1880. [Google Scholar] [CrossRef]
- Kuo, S.-C. Synthesis of temozolomide and analogs. US Pat. US6844434B2, 18 Janaury 2005. [Google Scholar]
- Kuo, S.-C.; Mas, J.L.; Hou, D. Synthesis of temozolomide and analogs. US Pat. US7087751B2, 8 August 2006. [Google Scholar]
- Łaszcz, M.; Kubiszewski, M.; Jedynak, Ł.; Kaczmarska, M.; Kaczmarek, L.; Łuniewski, W.; Gabarski, K.; Witkowska, A.; Kuziak, K.; Malińska, M. Identification and physicochemical characteristics of temozolomide process-related impurities. Molecules 2013, 18, 15344–15356. [Google Scholar] [CrossRef]
- Schwartzman, S.; Lima, D.A. Method of generating lower alkyl and cycloalkyl isocyanates. US Pat. US4141913A, 27 February 1979. [Google Scholar]
- Palle, R.V.; Marathe, A.M.; Manda, A. Process for preparing temozolomide. US Pat. US2007225496A1, 27 September 2007. [Google Scholar]
- Mormann, W.; Leukel, G. A simple and versatile synthesis of trimethylsiloxy-substituted isocyanates. Synthesis-Stuttgart 1988, 12, 990–992. [Google Scholar] [CrossRef]
- Tsang, L.L.H.; Farmer, P.B.; Gescher, A.; Slack, J.A. Characterisation of urinary metabolites of temozolomide in humans and mice and evaluation of their cytotoxicity. Cancer Chemother. Pharmacol. 1990, 26, 429–436. [Google Scholar] [CrossRef]
- Reyderman, L.; Statkevich, P.; Thonoor, C.M.; Patrick, J.; Batra, V.K.; Wirth, M. Disposition and pharmacokinetics of temozolomide in rat. Xenobiotica 2004, 34, 487–500. [Google Scholar]
- Ningaraj, N.S.; Sankpal, U.T.; Khaitan, D.; Meister, E.A.; Vats, T. Activation of KATP channels increases anticancer drug delivery to brain tumors and survival. Eur. J. Pharmacol. 2009, 602, 188–193. [Google Scholar]
- Moseley, C.K.; Carlin, S.M.; Neelamegam, R.; Hooker, J.M. An efficient and practical radiosynthesis of [11C]temozolomide. Org. Lett. 2012, 14, 5872–8575. [Google Scholar] [CrossRef]
- Cheng, C.C.; Elslager, E.F.; Werbel, L.M.; Priebe, S.R.; Leopold, W.R., III. Pyrazole derivatives. 5. Synthesis and antineoplastic activity of 3-(2-chloroethyl)-3,4-dihydro-4-oxopyrazolo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide and related compounds. J. Med. Chem. 1986, 29, 1544–1547. [Google Scholar] [CrossRef]
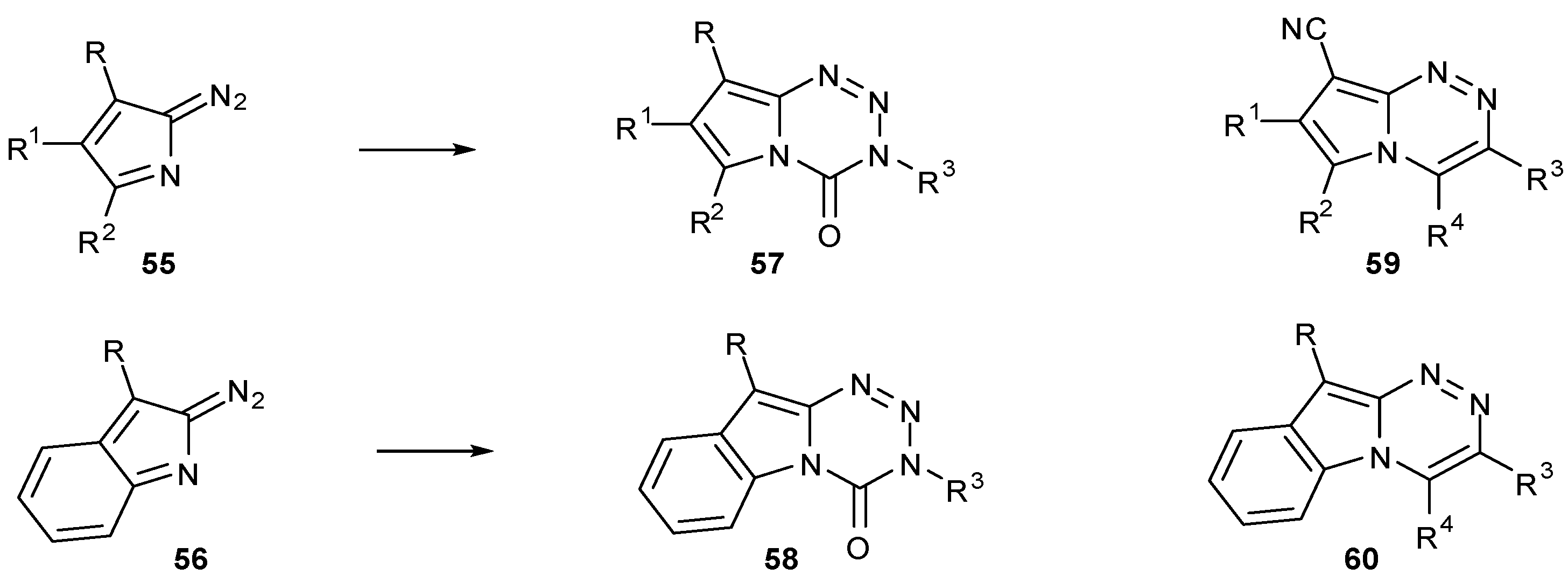
- Diana, P.; Barraja, P.; Lauria, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G. Pyrrolo[2,1-d][1,2,3,5]tetrazines, a new class of azolotetrazines related to the antitumor drug temozolomide. Synthesis-Stuttgart 1999, 12, 2082–2086. [Google Scholar]
- Barraja, P.; Diana, P.; Lauria, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G. 2-Diazoindoles: building blocks for the synthesis of antineoplastic agents. Farmaco 2002, 57, 97–100. [Google Scholar] [CrossRef]
- Barraja, P.; Diana, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G. Synthesis and antiproliferative activity of [1,2,3,5]tetrazino[5,4-a]indoles, a new class of azolo-tetrazinones. Bioorg. Med. Chem. 2005, 13, 295–300. [Google Scholar] [CrossRef]
- Diana, P.; Barraja, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G. Pyrrolo[2,1-d][1,2,3,5]tetrazine-4(3H)-ones, a new class of azolotetrazines with potent antitumor activity. Bioorg. Med. Chem. 2003, 11, 2371–2380. [Google Scholar] [CrossRef]
- Tripathi, G.; Mistra, J.P. QSAR studies on pyrrolo[2,1-d][1,2,3,5]tetrazinones, a new class of azolotetrazines. Indian J. Chem. 2005, 44, 1398–1400. [Google Scholar]
- Diana, P.; Barraja, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G. Pyrrolo[2,1-c][1,2,4]triazines from 2-diazopyrroles: Synthesis and antiproliferative activity. Eur. J. Med. Chem. 2002, 37, 267–272. [Google Scholar] [CrossRef]
- Barraja, P.; Diana, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G. Synthesis and antiproliferative activity of [1,2,4]triazino[4,3-a]indoles. Anticancer Res. 2004, 24, 3775–3779. [Google Scholar]
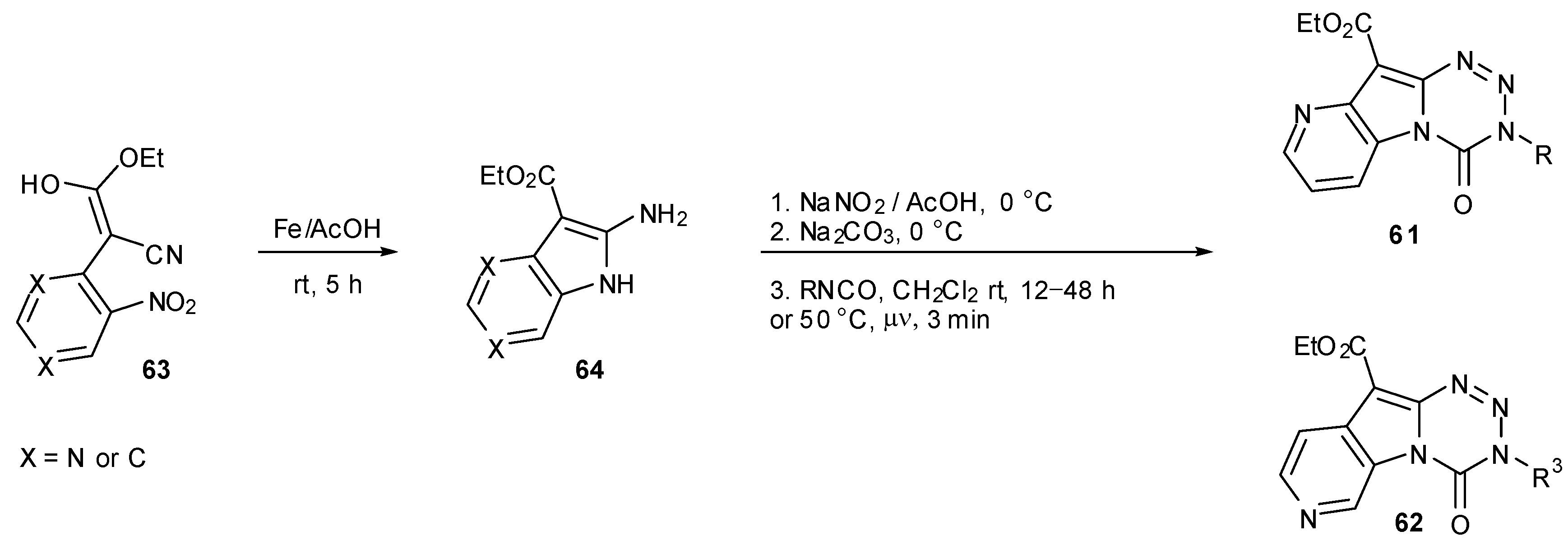
- Diana, P.; Stagno, A.; Barraja, P.; Carbone, A.; Montalbano, A.; Martorana, A.; Dattolo, G.; Cirrincione, G. Pyrido[4',3':4,5]pyrrolo[2,1-d][1,2,3,5]tetrazine a new class of temozolomide heteroanalogues. Arkivoc 2009, 1–11. [Google Scholar]
- Diana, P.; Stagno, A.; Barraja, P.; Montalbano, A.; Carbone, A.; Dattolo, G.; Cirrincione, G. Pyrido[2',3':4,5]pyrrolo[2,1-d][1,2,3,5]tetrazine-4(3H)-ones, a new class of temozolomide heteroanalogues. Arkivoc 2009, 177–186. [Google Scholar]
- Maggio, B.; Raffa, D.; Raimondi, M.V.; Plescia, F.; Cascioferro, S.; Daidone, G. Synthesis of alkyl-5,8-dimethyl-6-phenyl-5,6-dihydropyrazolo[3,4-f][1,2,3,5]tetrazepin-4(3H)-ones of pharmaceutical interest. Arkivoc 2006, 120–126. [Google Scholar]
- Zhu, Y.-Q.; Wu, C.; Li, H.-B.; Zou, X.-M.; Si, X.-K.; Hu, F.-Z.; Yang, H.-Z. Design, synthesis, and quantitative structure–activity relationship study of herbicidal analogues of pyrazolo[5,1-d][1,2,3,5]tetrazin-4(3H)ones. J. Agric. Food Chem. 2007, 55, 1364–1369. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Cheng, J.; Zou, X.-M.; Hu, F.-Z.; Xiao, T.-H.; Yang, H.-Z. Design, synthesis and quantitative structure–activity relationship study of herbicidal analogues of pyrazolotetrazinones. Chin. J. Chem. 2008, 28, 1044–1049. [Google Scholar]
- Horspool, K.R.; Stevens, M.F.; Newton, C.G.; Lunt, E.; Walsh, R.J.; Pedgrift, B.L.; Baig, G.U.; Lavelle, F.; Fizames, C. Antitumor imidazotetrazines. 20. Preparation of the 8-acid derivative of mitozolomide and its utility in the preparation of active antitumor agents. J. Med. Chem. 1990, 33, 1393–1399. [Google Scholar]
- Liu, D.; Yang, J.-G.; Cheng, J.; Zhao, L.-X. Synthesis and antitumor activity of 3-methyl-4-oxo-3,4-dihydroimidazo [5,1-d][1,2,3,5]tetrazine-8-carboxylates and -carboxamides. Molecules 2010, 15, 9427–9437. [Google Scholar] [CrossRef]
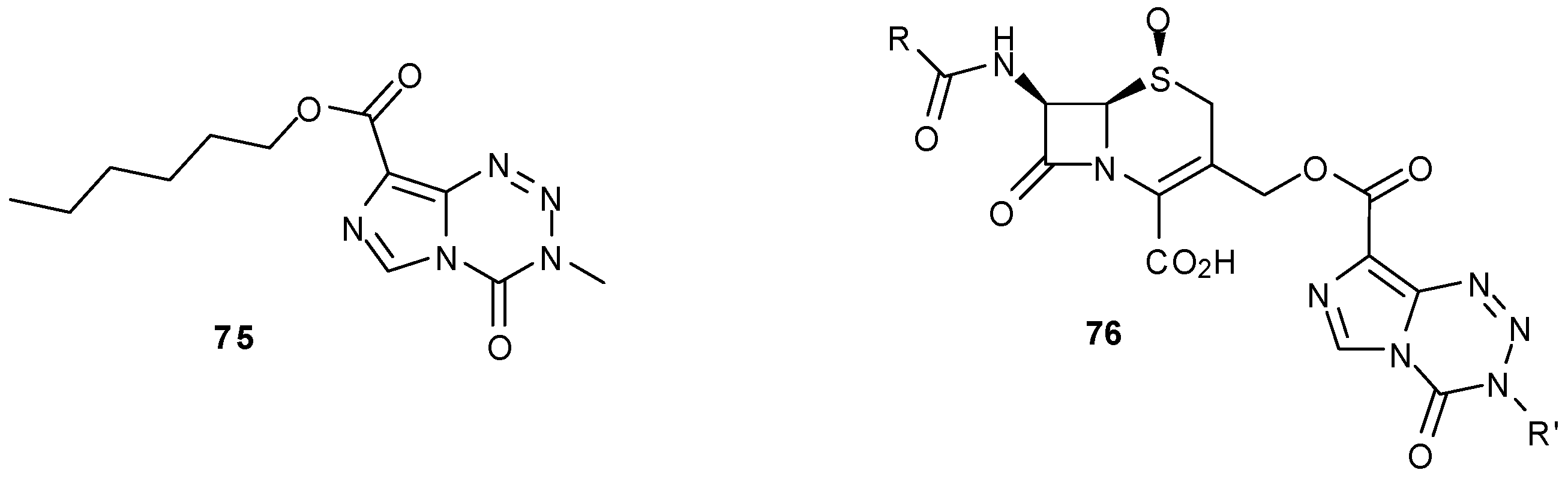
- Suppasansatorn, P.; Wang, G.; Conway, B.R.; Wang, W.; Wang, Y. Skin delivery potency and antitumor activities of temozolomide ester prodrugs. Cancer Lett. 2006, 244, 42–52. [Google Scholar] [CrossRef]
- Wang, Y. Synthesis of temozolomide esters as potent anticancer pro-drugs for topical and transdermal applications in treatments of cancers. US Pat. US2006047117A1, 2 March 2006. [Google Scholar]
- Wang, Y.; Lambert, P.; Zhao, L.; Wang, D. Synthesis and antibacterial activity of dual-action agents of a β-lactam antibiotic with cytotoxic agent mitozolomide or temozolomide. Eur. J. Med. Chem. 2002, 37, 323–332. [Google Scholar] [CrossRef]
- Li, R.; Tang, D.; Zhang, J.; Wu, J.; Wang, L.; Dong, J. The temozolomide derivative 2T-P400 inhibits glioma growth via administration route of intravenous injection. J. Neurooncol. 2014, 116, 25–30. [Google Scholar]
- Hummersone, M.G.; Cousin, D. 3-Substituted-8-substituted-3H-imidazo[5,1-d][1,2,3,5]tetrazin-4-one compounds and their use. US Pat. US20120083513A1, 5 April 2012. [Google Scholar]
- Stevens, M.F.G.; Cousin, D.; Jennings, S.; McCarroll, A.J.; Williams, J.G.; Hummersone, M.G.; Zhang, J. 3-Substituted-4-oxo-3,4-dihydro-imidazo-[5,1-d][1,2,3,5]tetrazine-8-carboxylic acid amides and their use. Int. Pat. WO2009077741A2, 25 June 2009. [Google Scholar]
- Stevens, M.G.F.; Cousin, D.; Jennings, S.; McCarroll, A.J.; Williams, J.G.; Hummersone, M.G.; Zhang, J. 3-Substituted-4-oxo-3,4-dihydro-imidazo[5,1-d]1,2,3,5-tetrazine-8-carboxylic acid amides and their Use. US Pat. US20130338104A1, 19 December 2013. [Google Scholar]
- Hummersone, M.G.; Cousin, D. Methods and intermediates for the synthesis of 4-oxo-3,4-dihydro-imidazo[5,1-d][1,2,3,5]tetrazines. Int. Pat. WO2011107726A1, 9 September 2011. [Google Scholar]
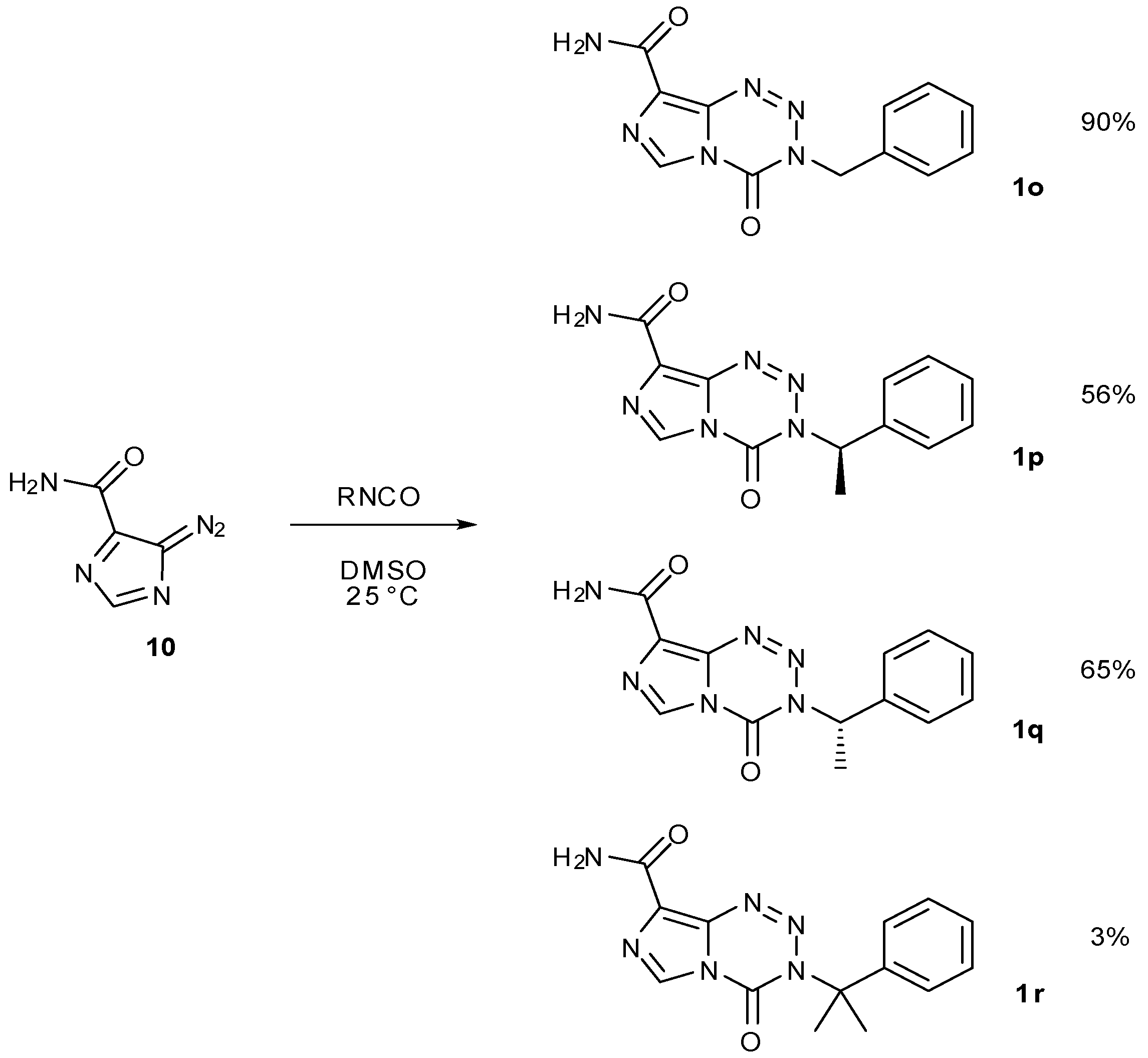
- Cousin, D.; Stevens, M.F.G.; Hummersone, M.G. Antitumour imidazotetrazines. Synthesis and chemistry of 4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide (nor-temozolomide): An intermediate for the preparation of the antitumour drug temozolomide and analogues, avoiding the use of isocyan. Med. Chem. Comm. 2012, 3, 1419–1422. [Google Scholar]
- Pletsas, D.; Wheelhouse, R.T.; Pletsa, V.; Nicolaou, A.; Jenkins, T.C.; Bibby, M.C.; Kyrtopoulos, S.A. Polar, functionalized guanine-O6 derivatives resistant to repair by O6-alkylguanine-DNA alkyltransferase: implications for the design of DNA-modifying drugs. Eur. J. Med. Chem. 2006, 41, 330–339. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.G.; Hummersone, M.; Madhusudan, S.; Laughton, C.A.; Bradshaw, T.D. Certain imidazotetrazines escape O6-methylguanine-DNA methyltransferase and mismatch repair. Oncology 2011, 80, 195–207. [Google Scholar] [CrossRef]
- Arrowsmith, J.; Jennings, S.A.; Langnel, D.A.F.; Wheelhouse, R.T.; Stevens, M.F.G. Antitumour imidazotetrazines. Part 39. Synthesis of bis(imidazotetrazine)s with saturated spacer groups. J. Chem. Soc. Perkin Trans. 2000, 1, 4432–4438. [Google Scholar]
- Henkel, J.G.; Amato, G.S. Methyl mercapturate episulfonium ion: a model reactive metabolite of dihaloethanes. J. Med. Chem. 1988, 31, 1279–1282. [Google Scholar] [CrossRef]
- Arrowsmith, J. Antitumour imidazotetrazines major and minor groove interactions. Ph.D. Thesis, The University of Nottingham, Nottingham, UK, 1997. [Google Scholar]
- Garelnabi, E.A.E.; Pletsas, D.; Li, L.; Kiakos, K.; Karodia, N.; Hartley, J.A.; Phillips, R.M.; Wheelhouse, R.T. A strategy for imidazotetrazine prodrugs with anti-cancer activity independent of MGMT and MMR. ACS Med. Chem. Lett. 2012, 3, 965–968. [Google Scholar]
- Wheelhouse, R.T.; Pletsas, D. Aminoalkyl-imidazotetrazines for treatment of cancer. Int. Pat. WO2009127815A1, 22 October 2009. [Google Scholar]
- Pletsas, D.; Garelnabi, E.A.E.; Li, L.; Phillips, R.M.; Wheelhouse, R.T. Synthesis and quantitative structure–activity relationship of imidazotetrazine prodrugs with activity independent of O6-methylguanine-DNA-methyltransferase, DNA mismatch repair, and p53. J. Med. Chem. 2013, 56, 7120–7132.96. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, Y.P.; Mladek, A.C.; Phillips, R.M.; Gyntherd, M.; Rautio, J.; Ross, A.H.; Wheelhouse, R.T.; Sakaria, J.N. Evaluation of novel imidazotetrazine analogues designed to overcome temozolomide resistance and glioblastoma regrowth. Submitted for publication.
- Johnson, B.E.; Mazor, T.; Hong, C.; Barnes, M.; Aihara, K.; McLean, C.Y.; Fouse, S.D.; Yamamoto, S.; Ueda, H.; Tatsuno, K.; et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014, 343, 189–193. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moody, C.L.; Wheelhouse, R.T. The Medicinal Chemistry of Imidazotetrazine Prodrugs. Pharmaceuticals 2014, 7, 797-838. https://doi.org/10.3390/ph7070797
Moody CL, Wheelhouse RT. The Medicinal Chemistry of Imidazotetrazine Prodrugs. Pharmaceuticals. 2014; 7(7):797-838. https://doi.org/10.3390/ph7070797
Chicago/Turabian StyleMoody, Catherine L., and Richard T. Wheelhouse. 2014. "The Medicinal Chemistry of Imidazotetrazine Prodrugs" Pharmaceuticals 7, no. 7: 797-838. https://doi.org/10.3390/ph7070797