Human Antimicrobial Peptides and Proteins
Abstract
:1. Introduction
| Year | Name | Sequence | Source | Activity 2 | Ref. |
|---|---|---|---|---|---|
| 1922 | Lysozyme | KVFERCELARTLKRLGMDGYRGISLANWMCLAKWESGYNTRATNYNAGDRSTDYGIFQINSRYWCNDGKTPGAVNACHLSCSALLQDNIADAVACAKRVVRDPQGIRAWVAWRNRCQNRDVRQYVQGCGV | saliva, tears, intestine | G, F | [20] |
| 1985 | α-Defensin HNP-1 | ACYCRIPACIAGERRYGTCIYQGRLWAFCC | Neutrophils, bone marrow | G, V, F, P, C | [21] |
| 1985 | α-Defensin HNP-2 | CYCRIPACIAGERRYGTCIYQGRLWAFCC | Neutrophils, bone marrow | G, V, F, C | [21] |
| 1985 | α-Defensin HNP-3 | DCYCRIPACIAGERRYGTCIYQGRLWAFCC | Neutrophils, bone marrow | G, V, F, C | [21] |
| 1988 | Histatin 1 | DSHEKRHHGYRRKFHEKHHSHREFPFYGDYGSNYLYDN | saliva | F | [22] |
| 1988 | Histatin 3 | DSHAKRHHGYKRKFHEKHHSHRGYRSNYLYDN | saliva | G, F | [22] |
| 1989 | α-Defensin HNP-4 | VCSCRLVFCRRTELRVGNCLIGGVSFTYCCTRV | neutrophils | G, V, F | [23] |
| 1990 | RNase 2 | KPPQFTWAQWFETQHINMTSQQCTNAMQVINNYQRRCKNQNTFLLTTFANVVNVCGNPNMTCPSNKTRKNCHHSGSQVPLIHCNLTTPSPQNISNCRYAQTPANMFYIVACDNRDQRRDPPQYPVVPVHLDRII | eosinophils | V, P | [24] |
| 1990 | RNase 3 (Eosinophil cationic protein, ECP) | RPPQFTRAQWFAIQHISLNPPRCTIAMRAINNYRWRCKNQNTFLRTTFANVVNVCGNQSIRCPHNRTLNNCHRSRFRVPLLHCDLINPGAQNISNCTYADRPGRRFYVVACDNRDPRDSPRYPVVPVHLDTTI | neutrophils | G, V, P | [24] |
| 1992 | α-Defensin HD-5 | ATCYCRTGRCATRESLSGVCEISGRLYRLCCR | Paneth cells/intestine, female reproductive system | G, V, F | [25] |
| 1993 | α-Defensin HD-6 | AFTCHCRRSCYSTEYSYGTCTVMGINHRFCCL | Paneth cells/intestine | V, F | [26] |
| 1995 | β-Defensin hBD-1 | DHYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK | Kidney, Skin, salivary glands | G, F, C | [27] |
| 1995 | Cathelicidin LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | neutrophils; skin | G, V, F, P, C | [28,29,30] |
| 1997 | β-Defensin hBD-2 | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | skin, lung, epithelia, uterus, salivary glands | G, V, F | [31] |
| 1998 | Granulysin | GRDYRTCLTIVQKLKKMVDKPTQRSVSNAATRVCRTGRSRWRDVCRNFMRRYQSRVTQGLVAGETAQQICEDLR | cytolytic T and NK cells | G, F, P, C | [32] |
| 1999 | Ubiquicidin | KVHGSLARAGKVRGQTPKVAKQEKKKKKTGRAKRRMQYNRRFVNVVPTFGKKKGPNANS | macrophages | G | [33] |
| 2000 | Thrombocidin-1 (TC-1) | AELRCMCIKTTSGIHPKNIQSLEVIGKGTHCNQVEVIATLKDGRKICLDPDAPRIKKIVQKKLAGDES | human blood platelets | G, F | [34] |
| 2000 | Hepcidin 25 (LEAP-1) | DTHFPICIFCCGCCHRSKCGMCCKT | plasma, Urine/Liver | G, F | [35] |
| 2000 | Neuropeptide α-MSH | SYSMEHFRWGKPV | brain | G+, V, F | [36] |
| 2001 | β-Defensin hBD-3 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | Skin, salivary glands | G, V, F | [37] |
| 2001 | β-Defensin hBD-4 | FELDRICGYGTARCRKKCRSQEYRIGRCPNTYACCLRKWDESLLNRTKP | testis, lung, kidney, neutrophils | G | [38] |
| 2001 | Dermcidin | SSLLEKGLDGAKKAVGGLGKLGKDAVEDLESVGKGAVHDVKDVLDSV | eccrine sweat/skin | G, F | [39] |
| 2002 | RNase 7 | KPKGMTSSQWFKIQHMQPSPQACNSAMKNINKHTKRCKDLNTFLHEPFSSVAATCQTPKIACKNGDKNCHQSHGAVSLTMCKLTSGKYPNCRYKEKRQNKSYVVACKPPQKKDSQQFHLVPVHLDRVL | urinary tract; respiratory tract; skin | G, F | [40] |
| 2003 | RNase 5 (angiogenin) | QDNSRYTHFLTQHYDAKPQGRDDRYCESIMRRRGPTSPCKDINTFIHGNKRSIKAICENKNGNPHRENLRISKSSFQVTTCKLHGGSPWPPCQYRATAGFRNVVVACENGLPVHLDQSIFRRPRP | Liver, skin, intestine | G+, F | [41] |
| 2003 | Chemokine CCL20 | SNFDCCLGYTDRILHPKFIVGFTRQLANEGCDINAIIFHTKKKLSVCANPKQTWVKYIVRLLSKKVKNM | skin | G, F, P | [42] |
| 2003 | Chemokine CXCL9 | TPVVRKGRCSCISTNQGTIHLQSLKDLKQFAPSPSCEKIEIIATLKNGVQTCLNPDSADVKELIKKWEKQVSQKKKQKNGKKHQKKKVLKVRKSQRSRQKKTT | blood | G, P | [42] |
| 2005 | Psoriasin (S100A7) | MSNTQAERSIIGMIDMFHKYTRRDDKIDKPSLLTMMKENFPNFLSACDKKGTNYLADVFEKKDKNEDKKIDFSEFLSLLGDIATDYHKQSHGAAPCSGGSQ | Skin, salivary glands, breast | G- | [43] |
| 2006 | RegIIIα | EEPQRELPSARIRCPKGSKAYGSHCYALFLSPKSWTDADLACQKRPSGNLVSVLSGAEGSFVSSLVKSIGNSYSYVWIGLHDPTQGTEPNGEGWEWSSSDVMNYFAWERNPSTISSPGHCASLSRSTAFLRWKDYNCNVRLPYVCKFTD | intestine | G+ | [44] |
| 2008 | Substance P | RPKPQQFFGLM | the nervous system | G, F | [45] |
| 2008 | Drosomycin-like defensin (DLD) | CLAGRLDKQCTCRRSQPSRRSGHEVGRPSPHCGPSRQCGCHMD | oral epithelial cells, skin | F | [46] |
| 2009 | Elafin | AQEPVKGPVSTKPGSCPIILIRCAMLNPPNRCLKDTDCPGIKKCCEGSCGMACFVPQ | γδ T cells | G, F, V | [47] |
| 2010 | β-amyloid peptide 1-42 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVI | brain | G, F | [48] |
| 2011 | Chemerin | ELTEAQRRGLQVALEEFHKHPPVQWAFQETSVESAVDTPFPAGIFVRLEFKLQQTSCRKRDWKKPECKVRPNGRKRKCLACIKLGSEDKVLGRLVHCPIETQVLREAEEHQETQCLRVQRAGEDPHSFYFPGQFAFS | skin | G, F | [49] |
| 2012 | Amylin | KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY | pancreatic β-cells | G | [50] |
| 2012 | KDAMP | RAIGGGLSSVGGGSSTIKY | eyes | G- | [51] |
| 2013 | DEFB114 | DRCTKRYGRCKRDCLESEKQIDICSLPRKICCTEKLYEEDDMF | epididymis | G, F | [19] |
2. Identification of Human Antimicrobial Peptides
2.1. Human Defensins
2.2. Human Histatins: Two Genes Multiple Peptides
2.3. Human Cathelicidins: One Gene Multiple Peptides
2.4. Human Dermcidin
2.5. Human Hepcidins
2.6. Human AMPs Derived from Known Proteins
2.7. Antimicrobial Chemokines and AMPs from Human Immune Cells
2.8. Antimicrobial Neuropeptides
2.9. Beta-Amyloid Peptides
2.10. Human Antimicrobial Proteins
3. Antimicrobial and Anticancer Activities of Human Antimicrobial Peptides
3.1. Antibacterial Activities
3.3. Antifungal Activity
3.4. Antiparasitic Activity
3.5. Anticancer Activity
3.6. Cytotoxic Effects of Human AMPs
3.7. Other Biological Functions of Human AMPs
4. Three-Dimensional Structures of Human Antimicrobial Peptides
| APD ID | Peptide name | Length | Net charge | Pho% | Boman index | Structure class |
|---|---|---|---|---|---|---|
| 2257 | Lysozyme | 130 | +8 | 40 | 2.28 | α |
| 505 | Histatin 5 | 24 | +5 | 8 | 4.81 | α |
| 780 | Lactoferricin | 49 | +10 | 36 | 3.14 | α |
| 310 | LL-37 | 37 | +6 | 35 | 2.99 | α |
| 433 | Dermcidin | 47 | −2 | 38 | 1.11 | α |
| 1161 | Granulysin | 74 | +11 | 33 | 3.5 | α |
| 2072 | Psoriasin/S100A7 | 101 | −1 | 32 | 2.3 | α |
| 1676 | β-Amyloid peptide 1-42 | 42 | −3 | 45 | 0.77 | α |
| 176 | HNP-1 | 30 | +3 | 53 | 1.07 | β |
| 177 | HNP-2 | 29 | +3 | 51 | 1.17 | β |
| 178 | HNP-3 | 30 | +2 | 50 | 1.42 | β |
| 179 | HNP-4 | 33 | +4 | 51 | 1.4 | β |
| 180 | HD-5 | 32 | +4 | 40 | 2.6 | β |
| 181 | HD-6 | 32 | +2 | 40 | 1.71 | β |
| 192 | Hepcidin 20 | 20 | +3 | 60 | 0.46 | β |
| 193 | Hepcidin 25 (LEAP-1) | 25 | +2 | 52 | 0.89 | β |
| 2095 | SLPI | 107 | +12 | 34 | 1.87 | β |
| 451 | hBD-1 | 36 | +4 | 36 | 1.3 | αβ |
| 524 | hBD-2 | 41 | +7 | 36 | 0.9 | αβ |
| 283 | hBD-3 | 45 | +11 | 33 | 2.87 | αβ |
| 811 | LEAP-2 | 40 | +4 | 40 | 2.94 | αβ |
| 2067 | RNase 5 | 125 | +11 | 28 | 2.99 | αβ |
| 2073 | RNase 7 | 128 | +16 | 32 | 2.16 | αβ |
| 2071 | RegIIIα | 149 | +1 | 33 | 1.77 | αβ |
| 2085 | CCL1 | 73 | +10 | 41 | 2.25 | αβ |
| 2086 | CCL8 | 75 | +6 | 37 | 2.27 | αβ |
| 2088 | CCL13 | 75 | +11 | 36 | 1.89 | αβ |
| 2075 | CCL20 | 69 | +8 | 43 | 1.34 | αβ |
| 2187 | CCL27 | 56 | +1 | 41 | 1.57 | αβ |
| 2076 | CXCL1 | 73 | +6 | 38 | 1.51 | αβ |
| 2080 | CXCL10 | 77 | +11 | 36 | 2.25 | αβ |
4.1. The α-Helical Family: Histatins, Cathelicidins, Dermcidin, and Granulysin
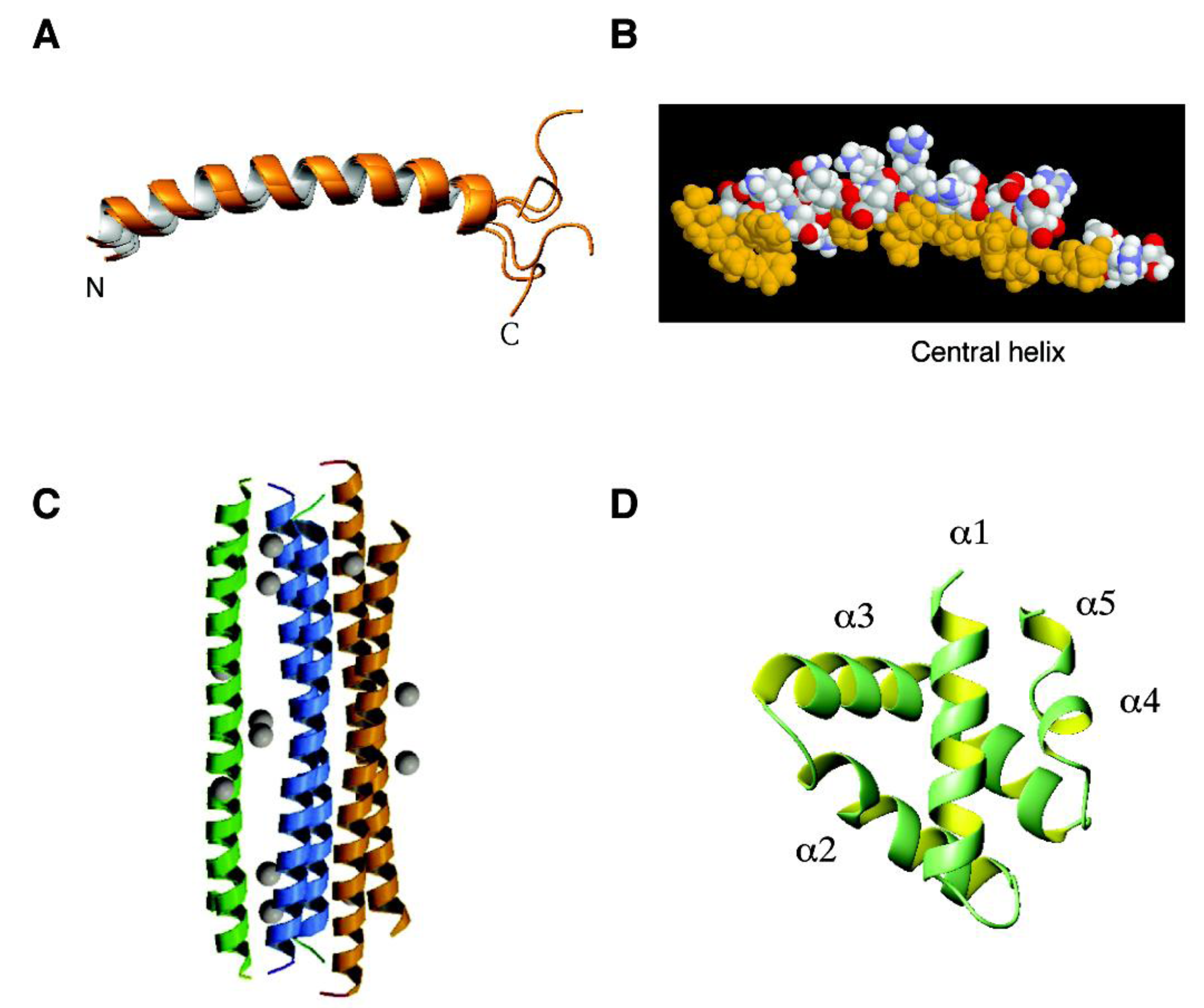
4.2. The β Family: α-Defensins

4.3. The αβ Family: β-Defensins, Antimicrobial Chemokines, RNases, and RegIIIα


5. Mechanism of Action of Human Antimicrobial Peptides
5.1. Targeting Bacterial Cell Wall
5.2. Targeting Bacterial Inner Membranes
5.3. Cell-Penetrating Peptides and Intracellular Targets
6. Concluding Remarks and Potential Therapeutic Strategies
| APD ID | AMP | Structure | Molecular target |
|---|---|---|---|
| 181 | HD-6 | β | Aggregate on bacterial surface |
| 283 | hBD-3 | αβ | Bacterial cell wall (lipid II) |
| 176 | HNP-1 | β | Bacterial cell wall (lipid II) |
| 2257 | Lysozyme | α | Cell wall carbohydrate |
| 2071 | RegIIIα | αβ | Membrane pores |
| 310 | LL-37 | α | Bacterial membranes and/or DNA |
| 433 | Dermcidin | α | Membranes ion channel |
| 2017 | hGAPDH(2-32) | Unknown | Intracellular targets of fungi |
| 505 | Histatin 5 | α | Intracellular mitochondria |
| 2352 | Chromagranin A-derived peptides | Unknown | Cytoplasmic calmodulin of neutrophils |
| 1161 | Granulysin | α | Perforin generates a pore to allow granulysin to enter the cell and kill intracellular bacteria |
| Factor | AMP induced | Cells | Ref |
|---|---|---|---|
| Bacteria/LPS | LL-37, HBD-2 | keratinocytes | [296] |
| TNF-α | LL-37, HBD-2 | keratinocytes | [286] |
| UV Light | LL-37, HBD-2, chemerin | keratinocytes | [286,301] |
| Vitamin D3 | LL-37 | neutrophil progenitors and EBV-transformed B cells | [302,303] |
| Lactose | LL-37 | colonic epithelial cells T84, THP-1 monocytes and macrophages | [304] |
| Short-chain fatty acids | LL-37;pBD-2, pBD-3, pEP2C, and protegrins | human HT-29 colonic epithelial cells and U-937 monocytic cells; | [305,306] |
| Isoleucine | hBD-1;epithelial defensins | human colon cells, HCT-116; bovine kidney epithelial cells | [307,308,309] |
| Arginine | hBD-1 | human colon cells, HCT-116 | [307] |
| Ca2+ | hBD-2, hBD-3 | human keratinocyte monolayers | [310] |
| Zn2+ | LL-37;pBD-1, pBD-2, pBD-3 | Caco-2 cell; Intestinal epithelial cells | [311] |
| Butyrate | LL-37 | colon, gastric and hepatocellular cells | [312] |
| Albumin | hBD-1 | human colon cells, HCT-116 | [307] |
| Cyclic AMP/Butyrate | Chicken β-defensin 9 | macrophages and primary jejunal explants | [297] |
| Phenylbutyrate/1,25-dihydroxyvitamin D3 | cathelicidins | immortalized human bronchial epithelial cell line VA10 | [298] |
Acknowledgements
Conflicts of Interest
References
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Inter. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef]
- Ganz, T.; Lehrer, R.I. Defensins. Curr. Opin. Immunol. 1994, 6, 584–589. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellullar organisms. Nature 2002, 415, 359–365. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Yount, N.Y.; Yeaman, M.R. Emerging themes and therapeutic prospects for anti-infective peptides. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 337–360. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G. APD: The antimicrobial peptide database. Nucleic Acids Res. 2004, 32, D590–D592. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009, 37, D933–D937. [Google Scholar] [CrossRef]
- Marchini, G.; Lindow, S.; Brismar, H.; Ståbi, B.; Berggren, V.; Ulfgren, A.K.; Lonne-Rahm, S.; Agerberth, B.; Gudmundsson, G.H. The newborn infant is protected by an innate antimicrobial barrier: Peptide antibiotics are present in the skin and vernix caseosa. Br. J. Dermatol. 2002, 147, 1127–1134. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Zhong, S.; Tschachler, A.; Mlitz, V.; Karner, S.; Elbe-Bürger, A.; Mildner, M. Fetal Human Keratinocytes Produce Large Amounts of Antimicrobial Peptides: Involvement of Histone-Methylation Processes. J. Invest. Dermatol. 2014. [Google Scholar] [CrossRef]
- Wittersheim, M.; Cordes, J.; Meyer-Hoffert, U.; Harder, J.; Hedderich, J.; Gläser, R. Differential expression and in vivo secretion of the antimicrobial peptides psoriasin (S100A7), RNase 7, human beta-defensin-2 and -3 in healthy human skin. Exp. Dermatol. 2013, 22, 364–366. [Google Scholar] [CrossRef]
- Gläser, R.; Meyer-Hoffert, U.; Harder, J.; Cordes, J.; Wittersheim, M.; Kobliakova, J.; Fölster-Holst, R.; Proksch, E.; Schröder, J.M.; Schwarz, T. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J. Invest. Dermatol. 2009, 129, 641–649. [Google Scholar] [CrossRef]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef]
- Underwood, M.; Bakaletz, L. Innate immunity and the role of defensins in otitis media. Curr. Allergy Asthma Rep. 2011, 11, 499–507. [Google Scholar] [CrossRef]
- Sato, J.; Nishimura, M.; Yamazaki, M.; Yoshida, K.; Kurashige, Y.; Saitoh, M.; Abiko, Y. Expression profile of drosomycin-like defensin in oral epithelium and oral carcinoma cell lines. Arch. Oral Biol. 2013, 58, 279–285. [Google Scholar] [CrossRef]
- Da Silva, B.R.; de Freitas, V.A.; Nascimento-Neto, L.G.; Carneiro, V.A.; Arruda, F.V.; de Aguiar, A.S.; Cavada, B.S.; Teixeira, E.H. Antimicrobial peptide control of pathogenic microorganisms of the oral cavity: A review of the literature. Peptides 2012, 36, 315–321. [Google Scholar] [CrossRef]
- Tollner, T.L.; Bevins, C.L.; Cherr, G.N. Multifunctional glycoprotein DEFB126—A curious story of defensin-clad spermatozoa. Nat. Rev. Urol. 2012, 9, 365–375. [Google Scholar] [CrossRef]
- Yu, H.; Dong, J.; Gu, Y.; Liu, H.; Xin, A.; Shi, H.; Sun, F.; Zhang, Y.; Lin, D.; Diao, H. The novel human β-defensin 114 regulates lipopolysaccharide (LPS)-mediated inflammation and protects sperm from motility loss. J. Biol. Chem. 2013, 288, 12270–12282. [Google Scholar]
- Tollner, T.L.; Yudin, A.I.; Tarantal, A.F.; Treece, C.A.; Overstreet, J.W.; Cherr, G.N. Beta-defensin 126 on the surface of macaque sperm mediates attachment of sperm to oviductal epithelia. Biol. Reprod. 2008, 78, 400–412. [Google Scholar] [CrossRef]
- Fleming, A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. B 1922, 93, 306–317. [Google Scholar] [CrossRef]
- Selsted, M.E.; Harwig, S.S.; Ganz, T.; Schilling, J.W.; Lehrer, R.I. Primary structures of three human neutrophil defensins. J. Clin. Invest. 1985, 76, 1436–1439. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar]
- Wilde, C.G.; Griffith, J.E.; Marra, M.N.; Snable, J.L.; Scott, R.W. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J. Biol. Chem. 1989, 264, 11200–11203. [Google Scholar]
- Hamann, K.J.; Gleich, G.J.; Checkel, J.L.; Loegering, D.A.; McCall, J.W.; Barker, R.L. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J. Immunol. 1990, 144, 3166–3173. [Google Scholar]
- Jones, D.E.; Bevins, C.L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 1992, 267, 23216–23225. [Google Scholar]
- Jones, D.E.; Bevins, C.L. Defensin-6 mRNA in human Paneth cells: Implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993, 315, 187–192. [Google Scholar] [CrossRef]
- Bensch, K.W.; Raida, M.; Mägert, H.J.; Schulz-Knappe, P.; Forssmann, W.G. hBD-1: A novel beta-defensin from human plasma. FEBS Lett. 1995, 368, 331–335. [Google Scholar] [CrossRef]
- Agerberth, B.; Gunne, H.; Odeberg, J.; Kogner, P.; Boman, H.G.; Gudmundsson, G.H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 1995, 92, 195–199. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar]
- Cowland, J.B.; Johnsen, A.H.; Borregaard, N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995, 368, 173–176. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.M. A peptide antibiotic from human skin. Nature 1997, 387, 861. [Google Scholar] [CrossRef]
- Stenger, S.; Hanson, D.A.; Teitelbaum, R.; Dewan, P.; Niazi, K.R.; Froelich, C.J.; Ganz, T.; Thoma-Uszynski, S.; Melián, A.; Bogdan, C.; et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998, 282, 121–125. [Google Scholar] [CrossRef]
- Hieshima, K.; Ohtani, H.; Shibano, M.; Izawa, D.; Nakayama, T.; Kawasaki, Y.; Shiba, F.; Shiota, M.; Katou, F.; Saito, T.; et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 2003, 170, 1452–1461. [Google Scholar] [CrossRef]
- Krijgsveld, J.; Zaat, S.A.; Meeldijk, J.; van Veelen, P.A.; Fang, G.; Poolman, B.; Brandt, E.; Ehlert, J.E.; Kuijpers, A.J.; Engbers, G.H.; et al. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 2000, 275, 20374–20381. [Google Scholar] [CrossRef]
- Krause, A.; Neitz, S.; Mägert, H.J.; Schulz, A.; Forssmann, W.G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef]
- Cutuli, M.; Cristiani, S.; Lipton, J.M.; Catania, A. Antimicrobial effects of alpha-MSH peptides. J. Leukoc. Biol. 2000, 67, 233–239. [Google Scholar]
- Harder, J.; Bartels, J.; Christophers, E.; Schroeder, J.M. Isolation and characterization of human deta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef]
- García, J.R.; Krause, A.; Schulz, S.; Rodríguez-Jiménez, F.J.; Klüver, E.; Adermann, K.; Forssmann, U.; Frimpong-Boateng, A.; Bals, R.; Forssmann, W.G. Human beta-defensin 4: A novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001, 15, 1819–1821. [Google Scholar]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef]
- Harder, J.; Schroder, J.M. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 2002, 277, 46779–46784. [Google Scholar] [CrossRef]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Hoover, D.M.; Staley, P.; Tucker, K.D.; Lubkowski, J.; Oppenheim, J.J. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J. Leukoc. Biol. 2003, 74, 448–455. [Google Scholar] [CrossRef]
- Gläser, R.; Harder, J.; Lange, H.; Bartels, J.; Christophers, E.; Schröder, J.M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005, 6, 57–64. [Google Scholar] [CrossRef]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- El Karim, I.A.; Linden, G.J.; Orr, D.F.; Lundy, F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J. Neuroimmunol. 2008, 200, 11–16. [Google Scholar] [CrossRef]
- Simon, A.; Kullberg, B.J.; Tripet, B.; Boerman, O.C.; Zeeuwen, P.; van der Ven-Jongekrijg, J.; Verweij, P.; Schalkwijk, J.; Hodges, R.; van der Meer, J.W.; et al. Drosomycin-like defensin, a human homologue of Drosophila melanogaster drosomycin with antifungal activity. Antimicrob. Agents Chemother. 2008, 52, 1407–1412. [Google Scholar] [CrossRef]
- Marischen, L.; Wesch, D.; Schröder, J.M.; Wiedow, O.; Kabelitz, D. Human γδ T cells produce the protease inhibitor and antimicrobial peptide elafin. Scand. J. Immunol. 2009, 70, 547–552. [Google Scholar] [CrossRef]
- Soscia, S.J.; Kirby, J.E.; Washicosky, K.J.; Tucker, S.M.; Ingelsson, M.; Hyman, B.; Burton, M.A.; Goldstein, L.E.; Duong, S.; Tanzi, R.E.; et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 2010, 5, e9505. [Google Scholar] [CrossRef]
- Kulig, P.; Kantyka, T.; Zabel, B.A.; Banas, M.; Chyra, A.; Stefanska, A.; Tu, H.; Allen, S.J.; Handel, T.M.; Kozik, A.; et al. Regulation of chemerin chemoattractant and antibacterial activity by human cysteine cathepsins. J. Immunol. 2011, 187, 1403–1410. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Chen, J.C.; Cui, Y.X.; Zhou, B.; Chen, Y.X.; Zhao, Y.F.; Li, Y.M. Antimicrobial activity of human islet amyloid polypeptides: An insight into amyloid peptides’ connection with antimicrobial peptides. Biol. Chem. 2012, 393, 641–646. [Google Scholar]
- Tam, C.; Mun, J.J.; Evans, D.J.; Fleiszig, S.M. Cytokeratins mediate epithelial innate defense through their antimicrobial properties. J. Clin. Invest. 2012, 122, 3665–3677. [Google Scholar] [CrossRef]
- Wang, G. Database-guided discovery of potent peptides to combat HIV-1 or superbugs. Pharmaceuticals 2013, 6, 728–758. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, I.; Lehrer, R.I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996, 396, 319–322. [Google Scholar] [CrossRef]
- Ouellette, A.J.; Greco, R.M.; James, M.; Frederick, D.; Naftilan, J.; Fallon, J.T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J. Cell Biol. 1989, 108, 1687–1695. [Google Scholar] [CrossRef]
- Ganz, T. Defensins in the urinary tract and other tissues. J. Infect. Dis. 2001, 183 Suppl 1, S41–S42. [Google Scholar] [CrossRef]
- Valore, E.V.; Park, C.H.; Quayle, A.J.; Wiles, K.R.; McCray, P.B., Jr.; Ganz, T. Human beta-defensin-1: An antimicrobial peptide of urogenital tissues. J. Clin. Invest. 1998, 101, 1633–1642. [Google Scholar] [CrossRef]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Bals, R.; Wang, X.; Wu, Z.; Freeman, T.; Bafna, V.; Zasloff, M.; Wilson, J.M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Invest. 1998, 102, 874–880. [Google Scholar] [CrossRef]
- Jia, H.P.; Schutte, B.C.; Schudy, A.; Linzmeier, R.; Guthmiller, J.M.; Johnson, G.K.; Tack, B.F.; Mitros, J.P.; Rosenthal, A.; Ganz, T.; et al. Discovery of new human beta-defensins using a genomics-based approach. Gene 2001, 263, 211–218. [Google Scholar] [CrossRef]
- García, J.R.; Jaumann, F.; Schulz, S.; Krause, A.; Rodríguez-Jiménez, J.; Forssmann, U.; Adermann, K.; Klüver, E.; Vogelmeier, C.; Becker, D.; et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001, 306, 257–264. [Google Scholar] [CrossRef]
- Scheetz, T.; Bartlett, J.A.; Walters, J.D.; Schutte, B.C.; Casavant, T.L.; McCray, P.B., Jr. Genomics-based approaches to gene discovery in innate immunity. Immunol. Rev. 2002, 190, 137–145. [Google Scholar] [CrossRef]
- Huang, L.; Leong, S.S.; Jiang, R. Soluble fusion expression and characterization of bioactive human beta-defensin 26 and 27. Appl. Microbiol. Biotechnol. 2009, 84, 301–308. [Google Scholar] [CrossRef]
- Schulz, A.; Klüver, E.; Schulz-Maronde, S.; Adermann, K. Engineering disulfide bonds of the novel human beta-defensins hBD-27 and hBD-28: Differences in disulfide formation and biological activity among human beta-defensins. Biopolymers 2005, 80, 34–49. [Google Scholar] [CrossRef]
- Xin, A.; Zhao, Y.; Yu, H.; Shi, H.; Liu, H.; Diao, H.; Zhang, Y. Soluble fusion expression, characterization and localization of human β-defensin 6. Mol. Med. Rep. 2014, 9, 149–155. [Google Scholar]
- Lemaitre, B.; Reichhart, J.M.; Hoffmann, J.A. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 1997, 94, 14614–14619. [Google Scholar] [CrossRef]
- Selsted, M.E. Theta-defensins: cyclic antimicrobial peptides produced by binary ligation of truncated alpha-defensins. Curr Protein Pept Sci. 2004, 5, 365–371. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Yuan, J.; Osapay, G.; Osapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 1999, 286, 498–502. [Google Scholar] [CrossRef]
- Cole, A.M.; Wang, W.; Waring, A.J.; Lehrer, R.I. Retrocyclins: Using past as prologue. Curr. Protein Pept. Sci. 2004, 5, 373–381. [Google Scholar] [CrossRef]
- Yang, C.; Boone, L.; Nguyen, T.X.; Rudolph, D.; Limpakarnjanarat, K.; Mastro, T.D.; Tappero, J.; Cole, A.M.; Lal, R.B. Theta-Defensin pseudogenes in HIV-1-exposed, persistently seronegative female sex-workers from Thailand. Infect Genet Evol. 2005, 5, 11–15. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Cole, A.M.; Selsted, M.E. θ-Defensins: Cyclic peptides with endless potential. J. Biol. Chem. 2012, 287, 27014–27019. [Google Scholar] [CrossRef]
- Troxler, R.F.; Offner, G.D.; Xu, T.; Vanderspek, J.C.; Oppenheim, F.G. Structural relationship between human salivary histatins. J. Dent. Res. 1990, 69, 2–6. [Google Scholar] [CrossRef]
- Sabatini, L.M.; Azen, E.A. Histatins, a family of salivary histidine-rich proteins, are encoded by at least two loci (HIS1 and HIS2). Biochem. Biophys. Res. Commun. 1989, 160, 495–502. [Google Scholar] [CrossRef]
- vanderSpek, J.C.; Wyandt, H.E.; Skare, J.C.; Milunsky, A.; Oppenheim, F.G.; Troxler, R.F. Localization of the genes for histatins to human chromosome 4q13 and tissue distribution of the mRNAs. Am. J. Hum. Genet. 1989, 45, 381–387. [Google Scholar]
- Nizet, V.; Ohtake, T.; Lauth, X.; Trowbridge, J.; Rudisill, J.; Dorschner, R.A.; Pestonjamasp, V.; Piraino, J.; Huttner, K.; Gallo, R.L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001, 414, 454–457. [Google Scholar] [CrossRef]
- Braff, M.H.; Zaiou, M.; Fierer, J.; Nizet, V.; Gallo, R.L. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect. Immun. 2005, 73, 6771–6781. [Google Scholar] [CrossRef]
- Lee, P.H.; Ohtake, T.; Zaiou, M.; Murakami, M.; Rudisill, J.A.; Lin, K.H.; Gallo, R.L. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc. Natl. Acad. Sci. USA 2005, 102, 3750–3755. [Google Scholar]
- Romeo, D.; Skerlavaj, B.; Bolognesi, M.; Gennaro, R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J. Biol. Chem. 1988, 263, 9573–9575. [Google Scholar]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Gudmundsson, G.H.; Agerberth, B.; Odeberg, J.; Bergman, T.; Olsson, B.; Salcedo, R. The human gene FALL-39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 1996, 238, 325–332. [Google Scholar]
- Sørensen, O.E.; Gram, L.; Johnsen, A.H.; Andersson, E.; Bangsbøll, S.; Tjabringa, G.S.; Hiemstra, P.S.; Malm, J.; Egesten, A.; Borregaard, N. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: A novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 2003, 278, 28540–28546. [Google Scholar] [CrossRef]
- Zhao, H.; Lee, W.H.; Shen, J.H.; Li, H.; Zhang, Y. Identification of novel semenogelin I-derived antimicrobial peptide from liquefied human seminal plasma. Peptides 2008, 29, 505–511. [Google Scholar] [CrossRef]
- Yamasaki, K.; Schauber, J.; Coda, A.; Lin, H.; Dorschner, R.A.; Schechter, N.M.; Bonnart, C.; Descargues, P.; Hovnanian, A.; Gallo, R.L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006, 20, 2068–2080. [Google Scholar] [CrossRef]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta 2014. [Google Scholar] [CrossRef]
- Tailor, R.H.; Acland, D.P.; Attenborough, S.; Cammue, B.P.; Evans, I.J.; Osborn, R.W.; Ray, J.A.; Rees, S.B.; Broekaert, W.F. A novel family of small cysteine-rich antimicrobial peptides from seed of Impatiens balsamina is derived from a single precursor protein. J. Biol. Chem. 1997, 272, 24480–24487. [Google Scholar] [CrossRef]
- Scocchi, M.; Bontempo, D.; Boscolo, S.; Tomasinsig, L.; Giulotto, E.; Zanetti, M. Novel cathelicidins in horse leukocytes. FEBS Lett. 1999, 457, 459–464. [Google Scholar] [CrossRef]
- Anderson, R.C.; Yu, P.L. Isolation and characterisation of proline/arginine-rich cathelicidin peptides from ovine neutrophils. Biochem. Biophys. Res. Commun. 2003, 312, 1139–1146. [Google Scholar] [CrossRef]
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef]
- Castiglioni, B.; Scocchi, M.; Zanetti, M.; Ferretti, L. Six antimicrobial peptide genes of the cathelicidin family map to bovine chromosome 22q24 by fluorescence in situ hybridization. Cytogenet. Cell Genet. 1996, 75, 240–242. [Google Scholar] [CrossRef]
- Murakami, M.; Ohtake, T.; Dorschner, R.A.; Schittek, B.; Garbe, C.; Gallo, R.L. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Invest. Dermatol. 2002, 119, 1090–1095. [Google Scholar] [CrossRef]
- Rieg, S.; Garbe, C.; Sauer, B.; Kalbacher, H.; Schittek, B. Dermcidinis constitutively produced by eccrine sweat glands and is not induced in epidermal cells under inflammatory skin conditions. Br. J. Dermatol. 2004, 151, 534–539. [Google Scholar] [CrossRef]
- Rieg, S.; Saborowski, V.; Kern, W.V.; Jonas, D.; Bruckner-Tuderman, L.; Hofmann, S.C. Expression of the sweat-derived innate defence antimicrobial peptide dermcidin is not impaired in Staphylococcus aureus colonization or recurrent skin infections. Clin. Exp. Dermatol. 2013. [Google Scholar] [CrossRef]
- Schittek, B. The multiple facets of dermcidin in cell survival and host defense. J. Innate Immun. 2012, 4, 349–360. [Google Scholar]
- Ghosh, R.; Maji, U.K.; Bhattacharya, R.; Sinha, A.K. The role of dermcidin isoform 2: A two-faceted atherosclerotic risk factor for coronary artery disease and the effect of acetyl salicylic acid on it. Thrombosis 2012, 2012, 987932. [Google Scholar]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001, 276, 7806–7801. [Google Scholar] [CrossRef]
- Jordan, J.B.; Poppe, L.; Haniu, M.; Arvedson, T.; Syed, R.; Li, V.; Kohno, H.; Kim, H.; Schnier, P.D.; Harvey, T.S.; et al. Hepcidin revisited, disulfide connectivity, dynamics, and structure. J. Biol. Chem. 2009, 284, 24155–24167. [Google Scholar] [CrossRef]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar]
- Roetto, A.; Papanikolaou, G.; Politou, M.; Alberti, F.; Girelli, D.; Christakis, J.; Loukopoulos, D.; Camaschella, C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat. Genet. 2003, 33, 21–22. [Google Scholar]
- Krause, A.; Sillard, R.; Kleemeier, B.; Klüver, E.; Maronde, E.; Conejo-García, J.R.; Forssmann, W.G.; Schulz-Knappe, P.; Nehls, M.C.; Wattler, F.; et al. Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Sci. 2003, 12, 143–152. [Google Scholar]
- Park, C.B.; Kim, M.S.; Kim, S.C. A novel antimicrobial peptide from Bufo bufo gargarizans. Biochem. Biophys. Res. Commun. 1996, 218, 408–413. [Google Scholar] [CrossRef]
- Minn, I.; Kim, H.S.; Kim, S.C. Antimicrobial peptides derived from pepsinogens in the stomach of the bullfrog, Rana catesbeiana. Biochim. Biophys. Acta 1998, 1407, 31–39. [Google Scholar] [CrossRef]
- Cole, A.M.; Kim, Y.H.; Tahk, S.; Hong, T.; Weis, P.; Waring, A.J.; Ganz, T. Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett. 2001, 504, 5–10. [Google Scholar] [CrossRef]
- Park, C.J.; Park, C.B.; Hong, S.S.; Lee, H.S.; Lee, S.Y.; Kim, S.C. Characterization and cDNA cloning of two glycine- and histidine-rich antimicrobial peptides from the roots of shepherd’s purse, Capsella. bursa-pastoris. Plant. Mol. Biol. 2000, 44, 187–197. [Google Scholar] [CrossRef]
- Chang, C.I.; Pleguezuelos, O.; Zhang, Y.A.; Zou, J.; Secombes, C.J. Identification of a novel cathelicidin gene in the rainbow trout, Oncorhynchus. mykiss. Infect. Immun. 2005, 73, 5053–5064. [Google Scholar] [CrossRef]
- Bayer, A.; Freund, S.; Jung, G. Post-translational heterocyclic backbone modifications in the 43-peptide antibiotic microcin B17. Structure elucidation and NMR study of a 13C,15N-labelled gyrase inhibitor. Eur. J. Biochem. 1995, 234, 414–426. [Google Scholar]
- Sousa1, J.C.; Berto1, R.F.; Gois, E.A.; Fontenele-Cardi, N.C.; Honório-Júnior, J.E.; Konno, K.; Richardson, M.; Rocha, M.F.; Camargo, A.A.; Pimenta, D.C.; et al. Leptoglycin: A new Glycine/Leucine-rich antimicrobial peptide isolated from the skin secretion of the South American frog Leptodactylus. pentadactylus (Leptodactylidae). Toxicon 2009, 54, 23–32. [Google Scholar] [CrossRef]
- Couillault, C.; Pujol, N.; Reboul, J.; Sabatier, L.; Guichou, J.F.; Kohara, Y.; Ewbank, J.J. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat. Immunol. 2004, 5, 488–494. [Google Scholar] [CrossRef]
- Hao, X.; Yang, H.; Wei, L.; Yang, S.; Zhu, W.; Ma, D.; Yu, H.; Lai, R. Amphibian cathelicidin fills the evolutionary gap of cathelicidin in vertebrate. Amino Acids 2012, 43, 677–685. [Google Scholar] [CrossRef]
- Lorenzini, D.M.; da Silva, P.I., Jr.; Fogaça, A.C.; Bulet, P.; Daffre, S. Acanthoscurrin: A novel glycine-rich antimicrobial peptide constitutively expressed in the hemocytes of the spider Acanthoscurria. gomesiana. Dev. Comp. Immunol. 2003, 27, 781–791. [Google Scholar] [CrossRef]
- Zeng, Y. Procambarin: A glycine-rich peptide found in the haemocytes of red swamp crayfish Procambarus clarkii and its response to white spot syndrome virus challenge. Fish. Shellfish Immunol. 2013, 35, 407–412. [Google Scholar] [CrossRef]
- Lee, S.Y.; Moon, H.J.; Kurata, S.; Natori, S.; Lee, B.L. Purification and cDNA cloning of an antifungal protein from the hemolymph of Holotrichia. diomphalia larvae. Biol. Pharm. Bull. 1995, 18, 1049–1052. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; van den Barselaar, M.T.; Roest, M.; Nibbering, P.H.; van Furth, R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 1999, 66, 423–428. [Google Scholar]
- Linde, C.M.; Grundström, S.; Nordling, E.; Refai, E.; Brennan, P.J.; Andersson, M. Conserved structure and function in the granulysin and NK-lysin peptide family. Infect. Immun. 2005, 73, 6332–6339. [Google Scholar] [CrossRef]
- Drannik, A.G.; Nag, K.; Sallenave, J.M.; Rosenthal, K.L. Antiviral activity of trappin-2 and elafin in vitro and in vivo against genital herpes. J. Virol. 2013, 87, 7526–7538. [Google Scholar] [CrossRef]
- Strub, J.M.; Garcia-Sablone, P.; Lonning, K.; Taupenot, L.; Hubert, P.; van Dorsselaer, A.; Aunis, D.; Metz-Boutigue, M.H. Processing of chromogranin B in bovine adrenal medulla. Identification of secretolytin, the endogenous C-terminal fragment of residues 614–626 with antibacterial activity. Eur. J. Biochem. 1995, 229, 356–368. [Google Scholar] [CrossRef]
- Lugardon, K.; Raffner, R.; Goumon, Y.; Corti, A.; Delmas, A.; Bulet, P.; Aunis, D.; Metz-Boutigue, M.H. Antibacterial and antifungal activities of vasostatin-1, the N-terminal fragment of chromogranin A. J. Biol. Chem. 2000, 275, 10745–10753. [Google Scholar]
- Briolat, J.; Wu, S.D.; Mahata, S.K.; Gonthier, B.; Bagnard, D.; Chasserot-Golaz, S.; Helle, K.B.; Aunis, D.; Metz-Boutigue, M.H. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell. Mol. Life Sci. 2005, 62, 377–385. [Google Scholar] [CrossRef]
- Goumon, Y.; Strub, J.M.; Moniatte, M.; Nullans, G.; Poteur, L.; Hubert, P.; van Dorsselaer, A.; Aunis, D.; Metz-Boutigue, M.H. The C-terminal bisphosphorylated proenkephalin-A-(209-237)-peptide from adrenal medullary chromaffin granules possesses antibacterial activity. Eur. J. Biochem. 1996, 235, 516–525. [Google Scholar]
- Vindrola, O.; Padrós, M.R.; Sterin-Prync, A.; Ase, A.; Finkielman, S.; Nahmod, V. Proenkephalin system in human polymorphonuclear cells. Production and release of a novel 1.0-kD peptide derived from synenkephalin. J. Clin. Invest. 1990, 86, 531–537. [Google Scholar] [CrossRef]
- Metz-Boutigue, M.H.; Goumon, Y.; Strub, J.M.; Lugardon, K.; Aunis, D. Antimicrobial chromogranins and proenkephalin-A-derived peptides. Ann. N. Y. Acad. Sci. 2003, 992, 168–178. [Google Scholar]
- Shimizu, M.; Shigeri, Y.; Tatsu, Y.; Yoshikawa, S.; Yumoto, N. Enhancement of antimicrobial activity of neuropeptide Y by N-terminal truncation. Antimicrob. Agents Chemother. 1998, 42, 2745–2746. [Google Scholar]
- Hansen, C.J.; Burnell, K.K.; Brogden, K.A. Antimicrobial activity of Substance P and Neuropeptide Y against laboratory strains of bacteria and oral microorganisms. J. Neuroimmunol. 2006, 177, 215–218. [Google Scholar] [CrossRef]
- Allaker, R.P.; Zihni, C.; Kapas, S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol. Med. Microbiol. 1999, 23, 289–293. [Google Scholar] [CrossRef]
- Chu, H.; Pazgier, M.; Jung, G.; Nuccio, S.P.; Castillo, P.A.; de Jong, M.F.; Winter, M.G.; Winter, S.E.; Wehkamp, J.; Shen, B.; et al. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 2012, 337, 477–481. [Google Scholar] [CrossRef]
- Wada, A.; Wong, P.F.; Hojo, H.; Hasegawa, M.; Ichinose, A.; Llanes, R.; Kubo, Y.; Senba, M.; Ichinose, Y. Alarin but not its alternative-splicing form, GALP (Galanin-like peptide) has antimicrobial activity. Biochem. Biophys. Res. Commun. 2013, 434, 223–227. [Google Scholar] [CrossRef]
- Brogden, K.A.; Guthmiller, J.M.; Salzet, M.; Zasloff, M. The nervous system and innate immunity: The neuropeptide connection. Nat. Immunol. 2005, 6, 558–564. [Google Scholar]
- Khemtémourian, L.; Killian, J.A.; Höppener, J.W.; Engel, M.F. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in beta-cell death in type 2 diabetes mellitus. Exp. Diabetes Res. 2008, 2008, 421287. [Google Scholar]
- Shahnawaz, M.; Soto, C. Microcin amyloid fibrils A are reservoir of toxic oligomeric species. J. Biol. Chem. 2012, 287, 11665–11676. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Bonville, C.A.; Dyer, K.D.; Rosenberg, H.F. Evolution of antiviral activity in the ribonuclease A gene superfamily: Evidence for a specific interaction between eosinophil-derived neurotoxin (EDN/RNase 2) and respiratory syncytial virus. Nucleic Acids Res. 1998, 26, 5327–5332. [Google Scholar] [CrossRef]
- Pulido, D.; Torrent, M.; Andreu, D.; Nogués, M.V.; Boix, E. Two human host defense ribonucleases against mycobacteria, the eosinophil cationic protein (RNase 3) and RNase 7. Antimicrob. Agents Chemother. 2013, 57, 3797–3805. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lin, Y.M.; Chang, T.W.; Wu, S.J.; Lee, Y.S.; Chang, M.D.; Chen, C.; Wu, S.H.; Liao, Y.D. The flexible and clustered lysine residues of human ribonuclease 7 are critical for membrane permeability and antimicrobial activity. J. Biol. Chem. 2007, 282, 4626–4633. [Google Scholar] [CrossRef]
- Spencer, J.D.; Schwaderer, A.L.; Dirosario, J.D.; McHugh, K.M.; McGillivary, G.; Justice, S.S.; Carpenter, A.R.; Baker, P.B.; Harder, J.; Hains, D.S. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011, 80, 174–180. [Google Scholar] [CrossRef]
- Spencer, J.D.; Schwaderer, A.L.; Wang, H.; Bartz, J.; Kline, J.; Eichler, T.; DeSouza, K.R.; Sims-Lucas, S.; Baker, P.; Hains, D.S. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013, 83, 615–625. [Google Scholar]
- Nielsen, K.L.; Dynesen, P.; Larsen, P.; Jakobsen, L.; Andersen, P.S.; Frimodt-Møller, N. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated Escherichia coli urinary tractinfections. Infect Immun. 2014, 82, 1572–1578. [Google Scholar] [CrossRef]
- Chromek, M.; Slamová, Z.; Bergman, P.; Kovács, L.; Podracká, L.; Ehrén, I.; Hökfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef]
- Becknell, B.; Spencer, J.D.; Carpenter, A.R.; Chen, X.; Singh, A.; Ploeger, S.; Kline, J.; Ellsworth, P.; Li, B.; Proksch, E.; et al. Expression and antimicrobial function of beta-defensin 1 in the lower urinary tract. PLoS One. 2013, 8, e77714. [Google Scholar]
- Wiedłocha, A. Following angiogenin during angiogenesis: A journey from the cell surface to the nucleolus. Arch. Immunol. Ther. Exp. (Warsz) 1999, 47, 299–305. [Google Scholar]
- Avdeeva, S.V.; Chernukha, M.U.; Shaginyan, I.A.; Tarantul, V.Z.; Naroditsky, B.S. Human angiogenin lacks specific antimicrobial activity. Curr. Microbiol. 2006, 53, 477–478. [Google Scholar] [CrossRef]
- Rudolph, B.; Podschun, R.; Sahly, H.; Schubert, S.; Schröder, J.M.; Harder, J. Identification of RNase 8 as a novel human antimicrobial protein. Antimicrob. Agents Chemother. 2006, 50, 3194–3196. [Google Scholar] [CrossRef]
- Boix, E.; Torrent, M.; Sánchez, D.; Nogués, M.V. The antipathogen activities of eosinophil cationic protein. Curr. Pharm. Biotechnol. 2008, 9, 141–152. [Google Scholar] [CrossRef]
- Hoffmann, H.J.; Olsen, E.; Etzerodt, M.; Madsen, P.; Thøgersen, H.C.; Kruse, T.; Celis, J.E. Psoriasin binds calcium and is upregulated by calcium to levels that resemble those observed in normal skin. J. Invest. Dermatol. 1994, 103, 370–375. [Google Scholar]
- Porre, S.; Heinonen, S.; Mäntyjärvi, R.; Rytkönen-Nissinen, M.; Perola, O.; Rautiainen, J.; Virtanen, T. Psoriasin, a calcium-binding protein with chemotactic properties is present in the third trimester amniotic fluid. Mol. Hum. Reprod. 2005, 11, 87–92. [Google Scholar] [CrossRef]
- Ostergaard, M.; Rasmussen, H.H.; Nielsen, H.V.; Vorum, H.; Orntoft, T.F.; Wolf, H.; Celis, J.E. Proteome profiling of bladder squamous cell carcinomas: Identification of markers that define their degree of differentiation. Cancer Res. 1997, 57, 4111–4117. [Google Scholar]
- Narushima, Y.; Unno, M.; Nakagawara, K.; Mori, M.; Miyashita, H.; Suzuki, Y.; Noguchi, N.; Takasawa, S.; Kumagai, T.; Yonekura, H.; et al. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII α, RegIII β, RegIII γ. Gene 1997, 185, 159–168. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, N.; Wu, Q.; Wang, B. HMGN2: A novel antimicrobial effector molecule of human mononuclear leukocytes? J. Leukoc. Biol. 2005, 78, 1136–1141. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Dyer, K.D.; Bonville, C.A.; Rosenberg, H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 1998, 177, 1458–64. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Dyer, K.D.; Adams, A.G.; Leto, T.L.; Rosenberg, H.F. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998, 26, 3358–63. [Google Scholar] [CrossRef]
- Shugars, D.C. Endogenous mucosal antiviral factors of the oral cavity. J. Infect. Dis. 1999, 179, S431–S435. [Google Scholar] [CrossRef]
- Wang, W.; Owen, S.M.; Rudolph, D.L.; Cole, A.M.; Hong, T.; Waring, A.J.; Lal, R.B.; Lehrer, R.I. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J. Immunol. 2004, 173, 515–520. [Google Scholar] [CrossRef]
- Dugan, A.S.; Maginnis, M.S.; Jordan, J.A.; Gasparovic, M.L.; Manley, K.; Page, R.; Williams, G.; Porter, E.; O'Hara, B.A.; Atwood, W.J. Human alpha-defensins inhibit BK virus infection by aggregating virions and blocking binding to host cells. J. Biol. Chem. 2008, 283, 31125–31132. [Google Scholar] [CrossRef]
- Quiñones-Mateu, M.E.; Lederman, M.M.; Feng, Z.; Chakraborty, B.; Weber, J.; Rangel, H.R.; Marotta, M.L.; Mirza, M.; Jiang, B.; Kiser, P.; et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 2003, 17, F39–F48. [Google Scholar]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Söderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef]
- Wang, G.; Watson, K.M.; Buckheit, R.W., Jr. Anti-human immunodeficiency virus type 1 activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob. Agents Chemother. 2008, 52, 3438–3440. [Google Scholar] [CrossRef]
- Groot, F.; Sanders, R.W.; ter Brake, O.; Nazmi, K.; Veerman, E.C.; Bolscher, J.G.; Berkhout, B. Histatin 5-derived peptide with improved fungicidal properties enhances human immunodeficiency virus type 1 replication by promoting viral entry. J. Virol. 2006, 80, 9236–9243. [Google Scholar] [CrossRef]
- Wang, G. Natural antimicrobial peptides as promising anti-HIV candidates. Curr. Topics Peptide Protein Res. 2012, 13, 93–110. [Google Scholar]
- López-García, B.; Lee, P.H.; Yamasaki, K.; Gallo, R.L. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J. Invest. Dermatol. 2005, 125, 108–115. [Google Scholar] [CrossRef]
- Den Hertog, A.L.; van Marle, J.; Veerman, E.C.; Valentijn-Benz, M.; Nazmi, K.; Kalay, H.; Grün, C.H.; Van’t Hof, W.; Bolscher, J.G.; Nieuw Amerongen, A.V. The human cathelicidin peptide LL-37 and truncated variants induce segregation of lipids and proteins in the plasma membrane of Candida albicans. Biol. Chem. 2006, 387, 1495–1502. [Google Scholar]
- Dabirian, S.; Taslimi, Y.; Zahedifard, F.; Gholami, E.; Doustdari, F.; Motamedirad, M.; Khatami, S.; Azadmanesh, K.; Nylen, S.; Rafati, S. Human neutrophil peptide-1 (HNP-1): A new anti-leishmanial drug candidate. PLoS Negl. Trop. Dis. 2013, 7, e2491. [Google Scholar] [CrossRef]
- Söbirk, S.K.; Mörgelin, M.; Egesten, A.; Bates, P.; Shannon, O.; Collin, M. Human chemokines as antimicrobial peptides with direct parasiticidal effect on Leishmania mexicana in vitro. PLoS One 2013, 8, e58129. [Google Scholar]
- Rico-Mata, R.; De Leon-Rodriguez, L.M.; Avila, E.E. Effect of antimicrobial peptides derived from human cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp. Parasitol. 2013, 133, 300–306. [Google Scholar] [CrossRef]
- Love, M.S.; Millholland, M.G.; Mishra, S.; Kulkarni, S.; Freeman, K.B.; Pan, W.; Kavash, R.W.; Costanzo, M.J.; Jo, H.; Daly, T.M.; et al. Platelet factor 4 activity against P. falciparum and its translation to nonpeptidic mimics as antimalarials. Cell Host Microbe. 2012, 12, 815–823. [Google Scholar] [CrossRef]
- Baker, M.A.; Maloy, W.L.; Zasloff, M.; Jacob, L.S. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993, 53, 3052–3057. [Google Scholar]
- Winder, D.; Günzburg, W.H.; Erfle, V.; Salmons, B. Expression of antimicrobial peptides has an antitumour effect in human cells. Biochem. Biophys. Res. Commun. 1998, 242, 608–612. [Google Scholar] [CrossRef]
- Lichtenstein, A.; Ganz, T.; Selsted, M.E.; Lehrer, R.I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood 1986, 68, 1407–1410. [Google Scholar]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides—Challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar]
- Kishi, A.; Takamori, Y.; Ogawa, K.; Takano, S.; Tomita, S.; Tanigawa, M.; Niman, M.; Kishida, T.; Fujita, S. Differential expression of granulysin and perforin by NK cells in cancer patients and correlation of impaired granulysin expression with progression of cancer. Cancer Immunol. Immunother. 2002, 50, 604–614. [Google Scholar] [CrossRef]
- Sekiguchi, N.; Asano, N.; Ito, T.; Momose, K.; Momose, M.; Ishida, F. Elevated serum granulysin and its clinical relevance in mature NK-cell neoplasms. Int. J. Hematol. 2012, 96, 461–468. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Y.S.; Pan, W.B.; Xiao, B.; Wen, Y.J.; Chen, X.C.; Chen, L.J.; Deng, H.X.; You, J.; Kan, B.; et al. Human alpha-defensin-1 inhibits growth of human lung adenocarcinoma xenograft in nude mice. Mol. Cancer Ther. 2008, 7, 1588–1597. [Google Scholar] [CrossRef]
- Mizukawa, N.; Sugiyama, K.; Fukunaga, J.; Ueno, T.; Mishima, K.; Takagi, S.; Sugahara, T. Defensin-1, a peptide detected in the saliva of oral squamous cell carcinoma patients. Anticancer Res. 1998, 18, 4645–4649. [Google Scholar]
- Müller, C.A.; Markovic-Lipkovski, J.; Klatt, T.; Gamper, J.; Schwarz, G.; Beck, H.; Deeg, M.; Kalbacher, H.; Widmann, S.; Wessels, J.T.; et al. Human alpha-defensins HNPs-1, -2, and -3 in renal cell carcinoma: Influences on tumor cell proliferation. Am. J. Pathol. 2002, 160, 1311–1324. [Google Scholar] [CrossRef]
- Sun, C.Q.; Arnold, R.; Fernandez-Golarz, C.; Parrish, A.B.; Almekinder, T.; He, J.; Ho, S.M.; Svoboda, P.; Pohl, J.; Marshall, F.F.; et al. Human beta-defensin-1, a potential chromosome 8p tumor suppressor: Control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res. 2006, 66, 8542–8549. [Google Scholar]
- Bullard, R.S.; Gibson, W.; Bose, S.K.; Belgrave, J.K.; Eaddy, A.C.; Wright, C.J.; Hazen-Martin, D.J.; Lage, J.M.; Keane, T.E.; Ganz, T.A.; et al. Functional analysis of the host defense peptide Human Beta Defensin-1: New insight into its potential role in cancer. Mol. Immunol. 2008, 45, 839–848. [Google Scholar] [CrossRef]
- Winter, J.; Pantelis, A.; Reich, R.; Martini, M.; Kraus, D.; Jepsen, S.; Allam, J.P.; Novak, N.; Wenghoefer, M. Human beta-defensin-1, -2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Invest. 2011, 29, 196–201. [Google Scholar]
- Wu, W.K.; Wang, G.; Coffelt, S.B.; Betancourt, A.M.; Lee, C.W.; Fan, D.; Wu, K.; Yu, J.; Sung, J.J.; Cho, C.H. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int. J. Cancer. 2010, 127, 1741–1747. [Google Scholar] [CrossRef]
- van den Broek, I.; Sparidans, R.W.; Engwegen, J.Y.; Cats, A.; Depla, A.C.; Schellens, J.H.; Beijnen, J.H. Evaluation of human neutrophil peptide-1, -2 and -3 as serum markers for colorectal cancer. Cancer Biomark. 2010, 7, 109–115. [Google Scholar]
- Albrethsen, J.; Bøgebo, R.; Gammeltoft, S.; Olsen, J.; Winther, B.; Raskov, H. Upregulated expression of human neutrophil peptides 1, 2 and 3 (HNP 1–3) in colon cancer serum and tumours: A biomarker study. BMC Cancer 2005, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Albrethsen, J.; Møller, C.H.; Olsen, J.; Raskov, H.; Gammeltoft, S. Human neutrophil peptides 1, 2 and 3 are biochemical markers for metastatic colorectal cancer. Eur. J. Cancer 2006, 42, 3057–3064. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef]
- Ren, S.X.; Shen, J.; Cheng, A.S.; Lu, L.; Chan, R.L.; Li, Z.J.; Wang, X.J.; Wong, C.C.; Zhang, L.; Ng, S.S.; et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One 2013, 8, e63641. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, J.; Hou, J.; Cao, F.; Zhang, Y.; Rasmussen, J.T.; Heegaard, C.W.; Gilbert, G.E. Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia. J. Thromb. Haemost. 2010, 8, 773–782. [Google Scholar] [CrossRef]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar]
- Svensson, D.; Westman, J.; Wickström, C.; Jönsson, D.; Herwald, H.; Nilsson, B.O. Human endogenous peptide p33 inhibits detrimental effects of LL-37 on osteoblast viability. J. Periodontal Res. 2014. [Google Scholar] [CrossRef]
- Hiemstra, T.F.; Charles, P.D.; Gracia, T.; Hester, S.S.; Gatto, L.; Al-Lamki, R.; Floto, R.A.; Su, Y.; Skepper, J.N.; Lilley, K.S.; et al. Human Urinary Exosomes as Innate Immune Effectors. J. Am. Soc. Nephrol. 2014, in press. [Google Scholar]
- Yung, S.C.; Murphy, P.M. Antimicrobial chemokines. Front Immunol. 2012, 3, 276. [Google Scholar]
- Yang, D.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef]
- Ciornei, C.D.; Tapper, H.; Bjartell, A.; Sternby, N.H.; Bodelsson, M. Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: A laboratory study. BMC Cardiovasc. Disord. 2006, 6, 49. [Google Scholar] [CrossRef]
- Barlow, P.G.; Li, Y.; Wilkinson, T.S.; Bowdish, D.M.; Lau, Y.E.; Cosseau, C.; Haslett, C.; Simpson, A.J.; Hancock, R.E.; Davidson, D.J. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006, 80, 509–520. [Google Scholar] [CrossRef]
- Aarbiou, J.; Tjabringa, G.S.; Verhoosel, R.M.; Ninaber, D.K.; White, S.R.; Peltenburg, L.T.; Rabe, K.F.; Hiemstra, P.S. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm. Res. 2006, 55, 119–127. [Google Scholar] [CrossRef]
- Okumura, K.; Itoh, A.; Isogai, E.; Hirose, K.; Hosokawa, Y.; Abiko, Y.; Shibata, T.; Hirata, M.; Isogai, H. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett. 2004, 212, 185–194. [Google Scholar] [CrossRef]
- Ciornei, C.D.; Egesten, A.; Bodelsson, M. Effects of human cathelicidin antimicrobial peptide LL-37 on lipopolysaccharide-induced nitric oxide release from rat aorta in vitro. Acta Anaesthesiol. Scand. 2003, 47, 213–220. [Google Scholar] [CrossRef]
- Chamorro, C.I.; Weber, G.; Grönberg, A.; Pivarcsi, A.; Ståhle, M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J. Invest. Dermatol. 2009, 129, 937–944. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Hirata, M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J. Immunol. 2006, 176, 3044–3052. [Google Scholar] [CrossRef]
- Lee, W.Y.; Savage, J.R.; Zhang, J.; Jia, W.; Oottamasathien, S.; Prestwich, G.D. Prevention of anti-microbial peptide LL-37-induced apoptosis and ATP release in the urinary bladder by a modified glycosaminoglycan. PLoS One 2013, 8, e77854. [Google Scholar]
- Suzuki, K.; Murakami, T.; Kuwahara-Arai, K.; Tamura, H.; Hiramatsu, K.; Nagaoka, I. Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells. Int. Immunol. 2011, 23, 185–193. [Google Scholar] [CrossRef]
- Ren, S.X.; Cheng, A.S.; To, K.F.; Tong, J.H.; Li, M.S.; Shen, J.; Wong, C.C.; Zhang, L.; Chan, R.L.; Wang, X.J.; et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012, 72, 6512–6523. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Blaauboer, M.E.; Nazmi, K.; Scheres, N.; Bolscher, J.G.; Veerman, E.C. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol. Chem. 2010, 391, 541–548. [Google Scholar]
- Wang, G. NMR of membrane-associated peptides and proteins. Curr. Protein Pept. Sci. 2008, 9, 50–69. [Google Scholar] [CrossRef]
- Wang, G. NMR of membrane proteins. In “Advances in Protein and Peptide Sciences” (edited by Dunn BM). Bentham Sci. 2013, 1, 128–188. [Google Scholar]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zasloff, M. A Database View of Natural Antimicrobial Peptides: Nomenclature, Classification and Amino acid Sequence Analysis. In Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Wang, G., Ed.; CABI: Wallingford, UK, 2010; pp. 1–21. [Google Scholar]
- Rose, P.W.; Bi, C.; Bluhm, W.F.; Christie, C.H.; Dimitropoulos, D.; Dutta, S.; Green, R.K.; Goodsell, D.S.; Prlic, A.; Quesada, M.; et al. The RCSB Protein Data Bank: New resources for research and education. Nucleic Acids Res. 2013, 41, D475–D482. [Google Scholar] [CrossRef]
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554. [Google Scholar]
- Raj, P.A.; Edgerton, M.; Levine, M.J. Salivary histatin 5: Dependence of sequence, chain length, and helical conformation for candidacidal activity. J. Biol. Chem. 1990, 265, 3898–3905. [Google Scholar]
- Rothstein, D.M.; Spacciapoli, P.; Tran, L.T.; Xu, T.; Roberts, F.D.; Dalla Serra, M.; Buxton, D.K.; Oppenheim, F.G.; Friden, P. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob. Agents Chemother. 2001, 45, 1367–1373. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, T.; Troxler, R.F.; Li, J.; Driscoll, J.; Oppenheim, F.G. Recombinant histatins: Functional domain duplication enhances candidacidal activity. Gene 1995, 161, 87–91. [Google Scholar] [CrossRef]
- Situ, H.; Tsai, H.; Bobek, L.A. Construction and characterization of human salivary histatin-5 multimers. J. Dent. Res. 1999, 78, 690–698. [Google Scholar] [CrossRef]
- Situ, H.; Balasubramanian, S.V.; Bobek, L.A. Role of alpha-helical conformation of histatin-5 in candidacidal activity examined by proline variants. Biochim. Biophys. Acta 2000, 1475, 377–382. [Google Scholar] [CrossRef]
- Melino, S.; Rufini, S.; Sette, M.; Morero, R.; Grottesi, A.; Paci, M.; Petruzzelli, R. Zn2+ ions selectively induce antimicrobial salivary peptide histatin-5 to fuse negatively charged vesicles. Identification and characterization of a zinc-binding motif present in the functional domain. Biochemistry 1999, 38, 9626–9633. [Google Scholar] [CrossRef]
- Rydengård, V.; Andersson Nordahl, E.; Schmidtchen, A. Zinc potentiates the antibacterial effects of histidine-rich peptides against Enterococcus faecalis. FEBS J. 2006, 273, 2399–2406. [Google Scholar] [CrossRef]
- Melino, S.; Gallo, M.; Trotta, E.; Mondello, F.; Paci, M.; Petruzzelli, R. Metal-binding and nuclease activity of an antimicrobial peptide analogue of the salivary histatin 5. Biochemistry 2006, 45, 15373–15383. [Google Scholar] [CrossRef]
- Wang, G. Structural studies of antimicrobial peptides provide insight into their mechanisms of action. In Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Wang, G., Ed.; CABI: Wallingford, UK, 2010; pp. 141–168. [Google Scholar]
- Xhindoli, D.; Pacor, S.; Guida, F.; Antcheva, N.; Tossi, A. Native oligomerization determines the mode of action and biological activities of human cathelicidin LL-37. Biochem. J. 2014, 457, 263–275. [Google Scholar] [CrossRef]
- Wang, G.; Elliott, M.; Cogen, A.L.; Ezell, E.L.; Gallo, R.L.; Hancock, R.E.W. Structure, dynamics, antimicrobial and immune modulatory activities of human LL-23 and its single residue variants mutated based on homologous primate cathelicidins. Biochemistry 2012, 51, 653–664. [Google Scholar] [CrossRef]
- Paulmann, M.; Arnold, T.; Linke, D.; Özdirekcan, S.; Kopp, A.; Gutsmann, T.; Kalbacher, H.; Wanke, I.; Schuenemann, V.J.; Habeck, M.; et al. Structure-activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat. J. Biol. Chem. 2012, 287, 8434–8443. [Google Scholar] [CrossRef]
- Song, C.; Weichbrodt, C.; Salnikov, E.S.; Dynowski, M.; Forsberg, B.O.; Bechinger, B.; Steinem, C.; de Groot, B.L.; Zachariae, U.; Zeth, K. Crystal structure and functional mechanism of a human antimicrobial membrane channel. Proc. Natl. Acad. Sci. USA 2013, 110, 4586–4591. [Google Scholar] [CrossRef]
- Jung, H.H.; Yang, S.T.; Sim, J.Y.; Lee, S.; Lee, J.Y.; Kim, H.H.; Shin, S.Y.; Kim, J.I. Analysis of the solution structure of the human antibiotic peptide dermcidin and its interaction with phospholipid vesicles. BMB Rep. 2010, 43, 362–368. [Google Scholar] [CrossRef]
- Koradi, R.; Billeter, M.; Wüthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef]
- Anderson, D.H.; Sawaya, M.R.; Cascio, D.; Ernst, W.; Modlin, R.; Krensky, A.; Eisenberg, D. Granulysin crystal structure and a structure-derived lytic mechanism. J. Mol. Biol. 2003, 325, 355–365. [Google Scholar]
- Peña, S.V.; Krensky, A.M. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin. Immunol. 1997, 9, 117–125. [Google Scholar] [CrossRef]
- Bruhn, H. A short guided tour through functional and structural features of saposin-like proteins. Biochem. J. 2005, 389, (Pt 2). 249–257. [Google Scholar]
- da Silva, A.P.; Unks, D.; Lyu, S.C.; Ma, J.; Zbozien-Pacamaj, R.; Chen, X.; Krensky, A.M.; Clayberger, C. In vitro and in vivo antimicrobial activity of granulysin-derived peptides against Vibrio cholerae. J. Antimicrob Chemother. 2008, 61, 1103–1109. [Google Scholar] [CrossRef]
- McInturff, J.E.; Wang, S.J.; Machleidt, T.; Lin, T.R.; Oren, A.; Hertz, C.J.; Krutzik, S.R.; Hart, S.; Zeh, K.; Anderson, D.H.; et al. Granulysin-derived peptides demonstrate antimicrobial and anti-inflammatory effects against Propionibacterium. acnes. J. Invest. Dermatol. 2005, 125, 256–263. [Google Scholar]
- Ericksen, B.; Wu, Z.; Lu, W.; Lehrer, R.I. Antibacterial activity and specificity of the six human α-defensins. Antimicrob. Agents Chemother. 2005, 49, 269–275. [Google Scholar] [CrossRef]
- Schibli, D.J.; Hunter, H.N.; Aseyev, V.; Starner, T.D.; Wiencek, J.M.; McCray PB, Jr.; Tack, B.F.; Vogel, H.J. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J. Biol. Chem. 2002, 277, 8279–8289. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Wu, Z.; Nuding, S.; Groscurth, S.; Marcinowski, M.; Beisner, J.; Buchner, J.; Schaller, M.; Stange, E.F.; Wehkamp, J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 2011, 469, 419–423. [Google Scholar] [CrossRef]
- Keizer, D.W.; Crump, M.P.; Lee, T.W.; Slupsky, C.M.; Clark-Lewis, I.; Sykes, B.D. Human CC chemokine I-309, structural consequences of the additional disulfide bond. Biochemistry 2000, 39, 6053–6059. [Google Scholar] [CrossRef]
- Blaszczyk, J.; Coillie, E.V.; Proost, P.; Damme, J.V.; Opdenakker, G.; Bujacz, G.D.; Wang, J.M.; Ji, X. Complete crystal structure of monocyte chemotactic protein-2, a CC chemokine that interacts with multiple receptors. Biochemistry 2000, 39, 14075–14081. [Google Scholar]
- Crump, M.P.; Rajarathnam, K.; Kim, K.S.; Clark-Lewis, I.; Sykes, B.D. Solution structure of eotaxin, a chemokine that selectively recruits eosinophils in allergic inflammation. J. Biol. Chem. 1998, 273, 22471–22479. [Google Scholar]
- Love, M.; Sandberg, J.L.; Ziarek, J.J.; Gerarden, K.P.; Rode, R.R.; Jensen, D.R.; McCaslin, D.R.; Peterson, F.C.; Veldkamp, C.T. Solution structure of CCL21 and identification of a putative CCR7 binding site. Biochemistry 2012, 51, 733–735. [Google Scholar]
- Jansma, A.L.; Kirkpatrick, J.P.; Hsu, A.R.; Handel, T.M.; Nietlispach, D. NMR analysis of the structure, dynamics, and unique oligomerization properties of the chemokine CCL27. J. Biol. Chem. 2010, 285, 14424–14437. [Google Scholar]
- Veldkamp, C.T.; Ziarek, J.J.; Su, J.; Basnet, H.; Lennertz, R.; Weiner, J.J.; Peterson, F.C.; Baker, J.E.; Volkman, B.F. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. 2009, 18, 1359–1369. [Google Scholar]
- Hoover, D.M.; Boulegue, C.; Yang, D.; Oppenheim, J.J.; Tucker, K.; Lu, W.; Lubkowski, J. The structure of human macrophage inflammatory protein-3alpha/CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J. Biol. Chem. 2002, 277, 37647–37654. [Google Scholar]
- Barinka, C.; Prahl, A.; Lubkowski, J. Structure of human monocyte chemoattractant protein 4 (MCP-4/CCL13). Acta. Crystallogr. D Biol. Crystallogr. 2008, 64, (Pt 3). 273–278. [Google Scholar] [CrossRef]
- Kim, K.S.; Clark-Lewis, I.; Sykes, B.D. Solution structure of GRO/melanoma growth stimulatory activity determined by 1H-NMR spectroscopy. J. Biol. Chem. 1994, 269, 32909–32915. [Google Scholar]
- Swaminathan, G.J.; Holloway, D.E.; Colvin, R.A.; Campanella, G.K.; Papageorgiou, A.C.; Luster, A.D.; Acharya, K.R. Crystal structures of oligomeric forms of the IP-10/CXCL-10 chemokine. Structure 2003, 11, 521–532. [Google Scholar] [CrossRef]
- Yung, S.C.; Parenti, D.; Murphy, P.M. Host chemokines bind to Staphylococcus aureus and stimulate protein A release. J. Biol. Chem. 2011, 286, 5069–5077. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Kwakman, P.H.; Chan, D.I.; Liu, Z.; de Boer, L.; Zaat, S.A.; Vogel, H.J. Exploring platelet chemokine antimicrobial activity: Nuclear magnetic resonance backbone dynamics of NAP-2 and TC-1. Antimicrob. Agents Chemother. 2011, 55, 2074–2083. [Google Scholar] [CrossRef]
- Wang, G.; Epand, R.F.; Mishra, B.; Lushnikova, T.; Thomas, V.C.; Bayles, K.W.; Epand, R.M. Decoding the functional roles of cationic side chains of the major antimicrobial region of human cathelicidin LL-37. Antimicrob. Agents Chemother. 2012, 56, 845–856. [Google Scholar] [CrossRef]
- Lequin, O.; Thüring, H.; Robin, M.; Lallemand, J.Y. Three-dimensional solution structure of human angiogenin determined by 1H,15N-NMR spectroscopy--characterization of histidine protonation states and pKa values. Eur. J. Biochem. 1997, 250, 712–726. [Google Scholar]
- Wang, H.; Schwaderer, A.L.; Kline, J.; Spencer, J.D.; Kline, D.; Hains, D.S. Contribution of structural domains to the activity of ribonuclease 7 against uropathogenic bacteria. Antimicrob. Agents Chemother. 2013, 57, 766–774. [Google Scholar] [CrossRef]
- Torrent, M.; Pulido, D.; Valle, J.; Nogués, M.V.; Andreu, D.; Boix, E. Ribonucleases as a host-defence family: Evidence of evolutionarily conserved antimicrobial activity at the N-terminus. Biochem. J. 2013, 456, 99–108. [Google Scholar] [CrossRef]
- Boix, E.; Salazar, V.A.; Torrent, M.; Pulido, D.; Nogués, M.V.; Moussaoui, M. Structural determinants of the eosinophil cationic protein antimicrobial activity. Biol. Chem. 2012, 393, 801–815. [Google Scholar]
- Spencer, J.D.; Schwaderer, A.L.; Eichler, T.; Wang, H.; Kline, J.; Justice, S.S.; Cohen, D.M.; Hains, D.S. An endogenous ribonuclease inhibitor regulates the antimicrobial activity of ribonuclease 7 in the human urinary tract. Kidney Int. 2013, 85, 1179–1191. [Google Scholar]
- Lehotzky, R.E.; Partch, C.L.; Mukherjee, S.; Cash, H.L.; Goldman, W.E.; Gardner, K.H.; Hooper, L.V. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc. Natl. Acad. Sci. USA 2010, 107, 7722–7727. [Google Scholar]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.X.; et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2013, 505, 103–107. [Google Scholar] [CrossRef]
- De Leeuw, E.; Li, C.; Zeng, P.; Li, C.; Diepeveen-de Buin, M.; Lu, W.Y.; Breukink, E.; Lu, W. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett. 2010, 584, 1543–1548. [Google Scholar] [CrossRef]
- Böhling, A.; Hagge, S.O.; Roes, S.; Podschun, R.; Sahly, H.; Harder, J.; Schröder, J.M.; Grötzinger, J.; Seydel, U.; Gutsmann, T. Lipid-specific membrane activity of human beta-defensin-3. Biochemistry 2006, 45, 5663–5670. [Google Scholar] [CrossRef]
- Sass, V.; Schneider, T.; Wilmes, M.; Körner, C.; Tossi, A.; Novikova, N.; Shamova, O.; Sahl, H.G. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect. Immun. 2010, 78, 2793–2800. [Google Scholar] [CrossRef]
- Schmitt, P.; Wilmes, M.; Pugnière, M.; Aumelas, A.; Bachère, E.; Sahl, H.G.; Schneider, T.; Destoumieux-Garzón, D. Insight into invertebrate defensin mechanism of action: Oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. Biol. Chem. 2010, 285, 29208–29216. [Google Scholar] [CrossRef]
- Bierbaum, G.; Sahl, H.G. Lantibiotics: Mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 2009, 10, 2–18. [Google Scholar] [CrossRef]
- Schneider, T.; Kruse, T.; Wimmer, R.; Wiedemann, I.; Sass, V.; Pag, U.; Jansen, A.; Nielsen, A.K.; Mygind, P.H.; Raventós, D.S.; et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science 2010, 328, 1168–1172. [Google Scholar] [CrossRef]
- Derouaux, A.; Turk, S.; Olrichs, N.K.; Gobec, S.; Breukink, E.; Amoroso, A.; Offant, J.; Bostock, J.; Mariner, K.; Chopra, I.; et al. Small molecule inhibitors of peptidoglycan synthesis targeting the lipid II precursor. Biochem. Pharmacol. 2011, 81, 1098–1105. [Google Scholar] [CrossRef]
- Varney, K.M.; Bonvin, A.M.; Pazgier, M.; Malin, J.; Yu, W.; Ateh, E.; Oashi, T.; Lu, W.; Huang, J.; Diepeveen-de Buin, M.; et al. Turning Defense into Offense: Defensin Mimetics as Novel Antibiotics Targeting Lipid II. PLoS Pathog. 2013, 9, e1003732. [Google Scholar] [CrossRef]
- Poon, I.K.h.; Baxter, A.A.; Lay, F.T.; Mills, G.D.; Adda, C.G.; Payne, J.A.; Phan, T.K.; Ryan, G.F.; White, J.A.; Veneer, P.K.; et al. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. Elife 2014, 3, e01808. [Google Scholar] [CrossRef]
- Silva, P.M.; Gonçalves, S.; Santos, N.C. Defensins: Antifungal lessons from eukaryotes. Front. Microbiol. 2014, 5, 97. [Google Scholar]
- Sagaram, U.S.; El-Mounadi, K.; Buchko, G.W.; Berg, H.R.; Kaur, J.; Pandurangi, R.S.; Smith, T.J.; Shah, D.M. Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: identification of an RGFRRR motif governing fungal cell entry. PLoS One. 2013, 8, e82485. [Google Scholar] [CrossRef]
- Miki, T.; Holst, O.; Hardt, W.D. The bactericidal activity of the C-type lectin RegIIIβ against Gram-negative bacteria involves binding to lipid A. J. Biol. Chem. 2012, 287, 34844–34855. [Google Scholar] [CrossRef]
- Formanek, H. A three dimensional model of the digestion of peptidoglycan by lysozyme. Biophys. Struct. Mech. 1977, 4, 1–14. [Google Scholar] [CrossRef]
- Ma, G.; Greenwell-Wild, T.; Lei, K.; Jin, W.; Swisher, J.; Hardegen, N.; Wild, C.T.; Wahl, S.M. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J. Exp. Med. 2004, 200, 1337–1346. [Google Scholar] [CrossRef]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999, 341, (Pt. 3). 501–513. [Google Scholar]
- Lee, C.C.; Sun, Y.; Qian, S.; Huang, H.W. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys. J. 2011, 100, 1688–1696. [Google Scholar] [CrossRef]
- Maisetta, G.; Vitali, A.; Scorciapino, M.A.; Rinaldi, A.C.; Petruzzelli, R.; Brancatisano, F.L.; Esin, S.; Stringaro, A.; Colone, M.; Luzi, C.; et al. pH-dependent disruption of Escherichia coli ATCC 25922 and model membranes by the human antimicrobial peptides hepcidin 20 and 25. FEBS J. 2013, 280, 2842–2854. [Google Scholar] [CrossRef]
- Natividad, J.M.; Hayes, C.L.; Motta, J.P.; Jury, J.; Galipeau, H.J.; Philip, V.; Garcia-Rodenas, C.L.; Kiyama, H.; Bercik, P.; Verdu, E.F. Differential Induction of Antimicrobial REGIII by the Intestinal Microbiota and Bifidobacterium breve NCC2950. Appl. Environ. Microbiol. 2013, 79, 7745–7754. [Google Scholar] [CrossRef]
- Parker, M.W.; Feil, S.C. Pore-forming protein toxins: From structure to function. Prog. Biophys. Mol. Biol. 2005, 88, 91–142. [Google Scholar] [CrossRef]
- Birck, C.; Damian, L.; Marty-Detraves, C.; Lougarre, A.; Schulze-Briese, C.; Koehl, P.; Fournier, D.; Paquereau, L.; Samama, J.P. A new lectin family with structure similarity to actinoporins revealed by the crystal structure of Xerocomus chrysenteron lectin XCL. J. Mol. Biol. 2004, 344, 1409–1420. [Google Scholar] [CrossRef]
- Mechaly, A.E.; Bellomio, A.; Gil-Cartón, D.; Morante, K.; Valle, M.; González-Mañas, J.M.; Guérin, D.M. Structural insights into the oligomerization and architecture of eukaryotic membrane pore-forming toxins. Structure 2011, 19, 181–191. [Google Scholar] [CrossRef]
- Miller, K.W.; Evans, R.J.; Eisenberg, S.P.; Thompson, R.C. Secretory leukocyte protease inhibitor binding to mRNA and DNA as a possible cause of toxicity to Escherichia coli. J. Bacteriol. 1989, 171, 2166–2172. [Google Scholar]
- den Hertog, A.L.; van Marle, J.; van Veen, H.A.; Van't Hof, W.; Bolscher, J.G.; Veerman, E.C.; Nieuw Amerongen, A.V. Candidacidal effects of two antimicrobial peptides: Histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 2005, 388, 689–695. [Google Scholar] [CrossRef]
- Gyurko, C.; Lendenmann, U.; Troxler, R.F.; Oppenheim, F.G. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob. Agents Chemother. 2000, 44, 348–354. [Google Scholar] [CrossRef]
- Gyurko, C.; Lendenmann, U.; Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. Killing of Candida albicans by histatin 5: Cellular uptake and energy requirement. Antonie. Van Leeuwenhoek. 2001, 79, 297–309. [Google Scholar] [CrossRef]
- Li, X.S.; Sun, J.N.; Okamoto-Shibayama, K.; Edgerton, M. Candida albicans cell wall ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J. Biol. Chem. 2006, 281, 22453–22463. [Google Scholar]
- Helmerhorst, E.J.; Breeuwer, P.; Van’t Hof, W.; Walgreen-Weterings, E.; Amerongen, A.V.; Abee, T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 1999, 274, 7286–7291. [Google Scholar]
- Vylkova, S.; Jang, W.S.; Li, W.; Nayyar, N.; Edgerton, M. Histatin 5 initiates osmotic stress response in Candida albicans via activation of the Hog1 mitogen-activated protein kinase pathway. Eukaryot. Cell 2007, 6, 1876–1888. [Google Scholar] [CrossRef]
- Wagener, J.; Schneider, J.J.; Baxmann, S.; Kalbacher, H.; Borelli, C.; Nuding, S.; Küchler, R.; Wehkamp, J.; Kaeser, M.D.; Mailänder-Sanchez, D.; et al. A peptide derived from the highly conserved protein Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is involved in tissue protection by different antifungal strategies and epithelial immunomodulation. J. Invest. Dermatol. 2013, 133, 144–153. [Google Scholar] [CrossRef]
- Aslam, R.; Atindehou, M.; Lavaux, T.; Haïkel, Y.; Schneider, F.; Metz-Boutigue, M.H. Chromogranin A-derived peptides are involved in innate immunity. Curr. Med. Chem. 2012, 19, 4115–4123. [Google Scholar] [CrossRef]
- Ochoa, M.T.; Stenger, S.; Sieling, P.A.; Thoma-Uszynski, S.; Sabet, S.; Cho, S.; Krensky, A.M.; Rollinghoff, M.; Nunes Sarno, E.; Burdick, A.E.; et al. T-cell release of granulysin contributes to host defense in leprosy. Nat. Med. 2001, 7, 174–179. [Google Scholar] [CrossRef]
- Stewart, S.E.; Kondos, S.C.; Matthews, A.Y.; D'Angelo, M.E.; Dunstone, M.A.; Whisstock, J.C.; Trapani, J.A.; Bird, P.I. The Perforin Pore Facilitates the Delivery of Cationic Cargos. J. Biol. Chem. 2014, 289, 9172–9181. [Google Scholar] [CrossRef]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef]
- Leung, T.F.; Ching, K.W.; Kong, A.P.; Wong, G.W.; Chan, J.C.; Hon, K.L. Circulating LL-37 is a biomarker for eczema severity in children. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 518–522. [Google Scholar] [CrossRef]
- Weber, G.; Chamorro, C.I.; Granath, F.; Liljegren, A.; Zreika, S.; Saidak, Z.; Sandstedt, B.; Rotstein, S.; Mentaverri, R.; Sánchez, F.; et al. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009, 11, R6. [Google Scholar] [CrossRef]
- Cheng, W.L.; Wang, C.S.; Huang, Y.H.; Liang, Y.; Lin, P.Y.; Hsueh, C.; Wu, Y.C.; Chen, W.J.; Yu, C.J.; Lin, S.R.; et al. Overexpression of a secretory leukocyte protease inhibitor in human gastric cancer. Int. J. Cancer 2008, 123, 1787–1796. [Google Scholar] [CrossRef]
- Emson, C.L.; Fitzmaurice, S.; Lindwall, G.; Li, K.W.; Hellerstein, M.K.; Maibach, H.I.; Liao, W.; Turner, S.M. A pilot study demonstrating a non-invasive method for the measurement of protein turnover in skin disorders: Application to psoriasis. Clin. Transl. Med. 2013, 2, 12. [Google Scholar] [CrossRef]
- Welling, M.M.; Paulusma-Annema, A.; Balter, H.S.; Pauwels, E.K.; Nibbering, P.H. Technetium-99m labeled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 2000, 27, 292–301. [Google Scholar] [CrossRef]
- Saeed, S.; Zafar, J.; Khan, B.; Akhtar, A.; Qurieshi, S.; Fatima, S.; Ahmad, N.; Irfanullah, J. Utility of 99mTc-labelled antimicrobial peptide ubiquicidin (29–41) in the diagnosis of diabetic foot infection. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 737–743. [Google Scholar] [CrossRef]
- Aryana, K.; Hootkani, A.; Sadeghi, R.; Davoudi, Y.; Naderinasab, M.; Erfani, M.; Ayati, N. (99m)Tc-labeled ubiquicidin scintigraphy: A promising method in hip prosthesis infection diagnosis. Nuklearmedizin 2012, 51, 133–139. [Google Scholar] [CrossRef]
- Wang, G. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; CABI: Wallingford, UK, 2010. [Google Scholar]
- Forde, E.; Humphreys, H.; Greene, C.M.; Fitzgerald-Hughes, D.; Devocelle, M. The potential of host defence peptide prodrugs as neutrophil elastase-dependent anti-infective agents for cystic fibrosis. Antimicrob. Agents. Chemother. 2013, 58, 978–985. [Google Scholar]
- Reynolds, N.L.; de Cecco, M.; Taylor, K.; Stanton, C.; Kilanowski, F.; Kalapothakis, J.; Seo, E.; Uhrin, D.; Campopiano, D.; Govan, J.; et al. Peptide fragments of a beta-defensin derivative with potent bactericidal activity. Antimicrob. Agents Chemother. 2010, 54, 1922–1929. [Google Scholar] [CrossRef]
- Roelandts, R. The history of phototherapy: Something new under the sun? J. Am. Acad. Dermatol. 2002, 46, 926–930. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Ala-Houhala, M.J.; Karppinen, T.; Vähävihu, K.; Kautiainen, H.; Dombrowski, Y.; Snellman, E.; Schauber, J.; Reunala, T. Narrow-band Ultraviolet B Treatment Boosts Serum 25-hydroxyvitamin D in Patients with Psoriasis on Oral Vitamin D Supplementation. Acta Derm. Venereol. 2014, 94, 146–151. [Google Scholar]
- Steinmann, J.; Halldórsson, S.; Agerberth, B.; Gudmundsson, G.H. Phenylbutyrate (PBA) induces antimicrobial peptide expression. Antimicrob. Agents Chemother. 2009, 53, 5127–5133. [Google Scholar] [CrossRef]
- Gan, Y.; Cui, X.; Ma, T.; Liu, Y.; Li, A.; Huang, M. Paeoniflorin Upregulates β-Defensin-2 Expression in Human Bronchial Epithelial Cell Through the p38 MAPK, ERK, and NF-κB Signaling Pathways. Inflammation. 2014, in press. [Google Scholar]
- Kim, B.J.; Rho, Y.K.; Lee, H.I.; Jeong, M.S.; Li, K.; Seo, S.J.; Kim, M.N.; Hong, C.K. The effect of calcipotriol on the expression of human beta defensin-2 and LL-37 in cultured human keratinocytes. Clin. Dev. Immunol. 2009, 2009, 645898. [Google Scholar]
- Sunkara, L.T.; Zeng, X.; Curtis, A.R.; Zhang, G. Cyclic AMP synergizes with butyrate in promoting β-defensin9 expression in chickens. Mol. Immunol. 2014, 57, 171–180. [Google Scholar] [CrossRef]
- Cederlund, A.; Nylén, F.; Miraglia, E.; Bergman, P.; Gudmundsson, G.H.; Agerberth, B. Label-Free Quantitative Mass Spectrometry Reveals Novel Pathways Involved in LL-37 Expression. J. Innate Immun. 2014, 6, 365–376. [Google Scholar]
- Liu, Q.; Liu, J.; Roschmann, K.I.; van Egmond, D.; Golebski, K.; Fokkens, W.J.; Wang, D.; van Drunen, C.M. Histone deacetylase inhibitors up-regulate LL-37 expression independent of toll-like receptor mediated signalling in airway epithelial cells. J. Inflamm. (Lond) 2013, 10, 15. [Google Scholar] [CrossRef]
- Strandberg, K.L.; Richards, S.M.; Gunn, J.S. Cathelicidin antimicrobial peptide expression is not induced or required for bacterial clearance during salmonella enterica infection of human monocyte-derived macrophages. Infect. Immun. 2012, 80, 3930–3938. [Google Scholar] [CrossRef]
- Yin, Q.; Xu, X.; Lin, Y.; Lv, J.; Zhao, L.; He, R. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: A possible role for chemerin. Autoimmunity. 2013, 47, 185–192. [Google Scholar]
- Karlsson, J.; Carlsson, G.; Larne, O.; Andersson, M.; Pütsep, K. Vitamin D3 induces pro-LL-37 expression in myeloid precursors from patients with severe congenital neutropenia. J. Leukoc. Biol. 2008, 84, 1279–1286. [Google Scholar] [CrossRef]
- Martineau, A.R.; Wilkinson, K.A.; Newton, S.M.; Floto, R.A.; Norman, A.W.; Skolimowska, K.; Davidson, R.N.; Sørensen, O.E.; Kampmann, B.; Griffiths, C.J.; et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: The role of cathelicidin LL-37. J. Immunol. 2007, 178, 7190–7198. [Google Scholar] [CrossRef]
- Cederlund, A.; Kai-Larsen, Y.; Printz, G.; Yoshio, H.; Alvelius, G.; Lagercrantz, H.; Strömberg, R.; Jörnvall, H.; Gudmundsson, G.H.; Agerberth, B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS One 2013, 8, e53876. [Google Scholar]
- Jiang, W.; Sunkara, L.T.; Zeng, X.; Deng, Z.; Myers, S.M.; Zhang, G. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides 2013, 50C, 129–138. [Google Scholar]
- Zeng, X.; Sunkara, L.T.; Jiang, W.; Bible, M.; Carter, S.; Ma, X.; Qiao, S.; Zhang, G. Inductionof porcine host defense peptidegene expression by short-chain fatty acids and their analogs. PLoS One 2013, 8, e72922. [Google Scholar]
- Sherman, H.; Chapnik, N.; Froy, O. Albumin and amino acids upregulate the expression of human beta-defensin 1. Mol. Immunol. 2006, 43, 1617–1623. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Rao, M.; Zasloff, M.; Anderson, G.M. An essential amino acid induces epithelial beta-defensin expression. Proc. Natl. Acad. Sci. USA 2000, 97, 12723–12728. [Google Scholar] [CrossRef]
- Rivas-Santiago, C.E.; Rivas-Santiago, B.; León, D.A.; Castañeda-Delgado, J.; Hernández Pando, R. Induction of β-defensins by l-isoleucine as novel immunotherapy in experimental murine tuberculosis. Clin. Exp. Immunol. 2011, 164, 80–89. [Google Scholar] [CrossRef]
- Pernet, I.; Reymermier, C.; Guezennec, A.; Branka, J.E.; Guesnet, J.; Perrier, E.; Dezutter-Dambuyant, C.; Schmitt, D.; Viac, J. Calcium triggers beta-defensin (hBD-2 and hBD-3) and chemokine macrophage inflammatory protein-3 alpha (MIP-3alpha/CCL20) expression in monolayers of activated human keratinocytes. Exp. Dermatol. 2003, 12, 755–760. [Google Scholar] [CrossRef]
- Talukder, P.; Satho, T.; Irie, K.; Sharmin, T.; Hamady, D.; Nakashima, Y.; Kashige, N.; Miake, F. Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int. Immunopharmacol. 2011, 11, 141–144. [Google Scholar] [CrossRef]
- Schauber, J.; Iffland, K.; Frisch, S.; Kudlich, T.; Schmausser, B.; Eck, M.; Menzel, T.; Gostner, A.; Lührs, H.; Scheppach, W. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol. Immunol. 2004, 41, 847–854. [Google Scholar] [CrossRef]
- Thivierge, K.; Cotton, S.; Schaefer, D.A.; Riggs, M.W.; To, J.; Lund, M.E.; Robinson, M.W.; Dalton, J.P.; Donnelly, S.M. Cathelicidin-like helminth defence molecules (HDMs): Absence of cytotoxic, anti-microbial and anti-protozoan activities imply a specific adaptation to immune modulation. PLoS Negl. Trop. Dis. 2013, 7, e2307. [Google Scholar]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Peschel, A. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 2002, 10, 179–186. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, G. Human Antimicrobial Peptides and Proteins. Pharmaceuticals 2014, 7, 545-594. https://doi.org/10.3390/ph7050545
Wang G. Human Antimicrobial Peptides and Proteins. Pharmaceuticals. 2014; 7(5):545-594. https://doi.org/10.3390/ph7050545
Chicago/Turabian StyleWang, Guangshun. 2014. "Human Antimicrobial Peptides and Proteins" Pharmaceuticals 7, no. 5: 545-594. https://doi.org/10.3390/ph7050545





