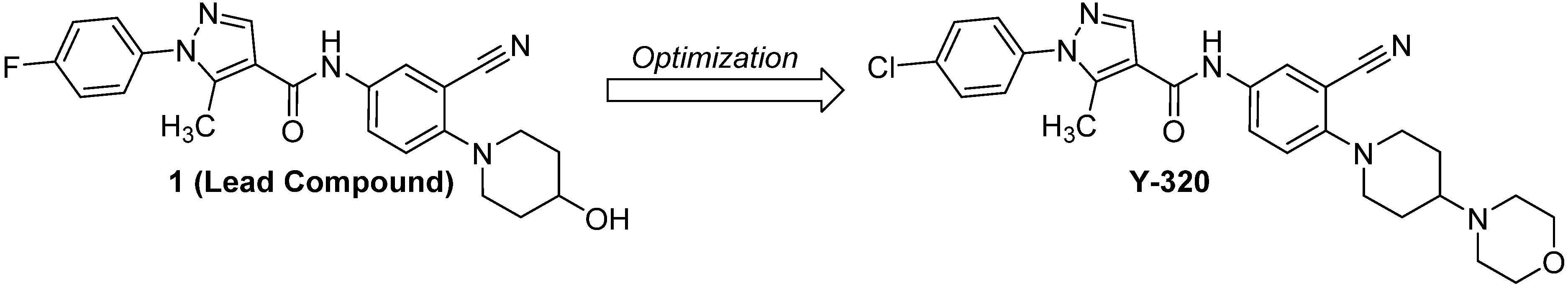
A New Phenylpyrazoleanilide, Y-320, Inhibits Interleukin 17 Production and Ameliorates Collagen-Induced Arthritis in Mice and Cynomolgus Monkeys
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemistry
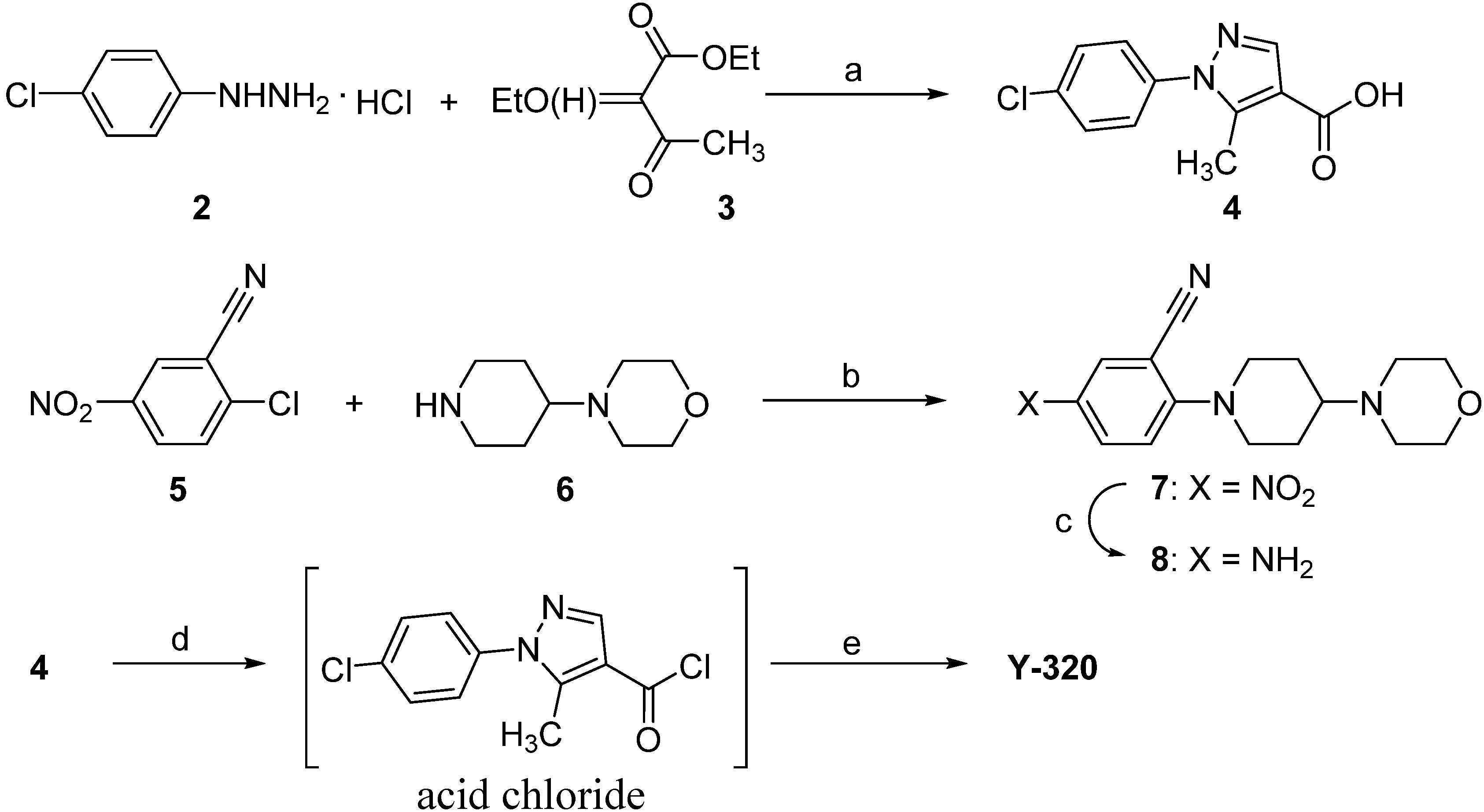
2.2. The General Procedure for the Synthesis of Y-320
2.3. Animals
2.4. IL-17 Production by CD4 T Cells Stimulated with IL-15
2.5. Generation of Th1 and Th17 Cells in Vitro
2.6. Phosphorylation of Janus Kinase (JAK) 1 and JAK3 by IL-15
2.7. CIA in DBA/1J Mice
2.8. CIA in Cynomolgus Monkeys
2.9. Real Time Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results and Discussion
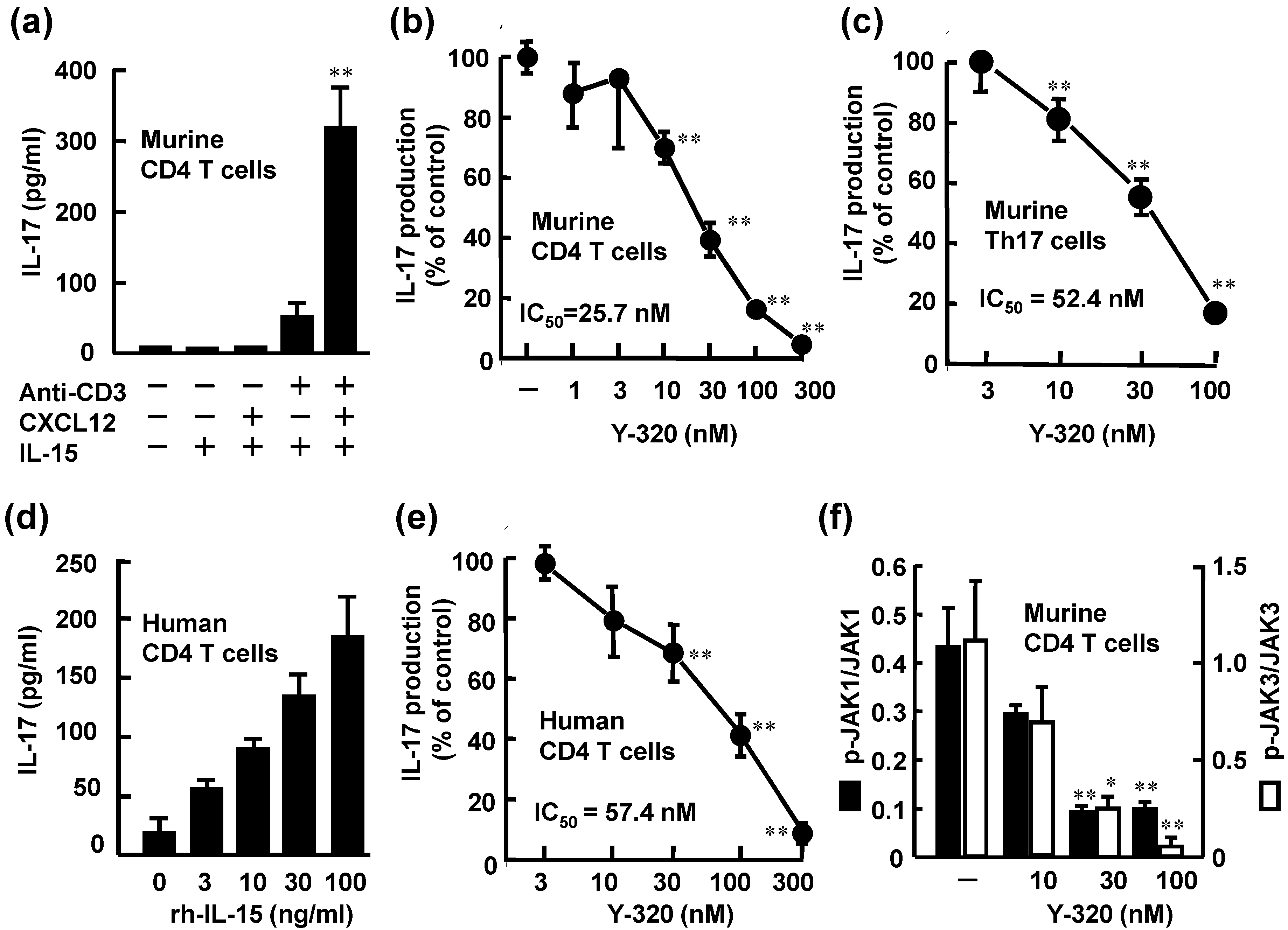
3.1. Y-320 Inhibits IL-17 Production by Murine and Human CD4 T Cells Stimulated with IL-15
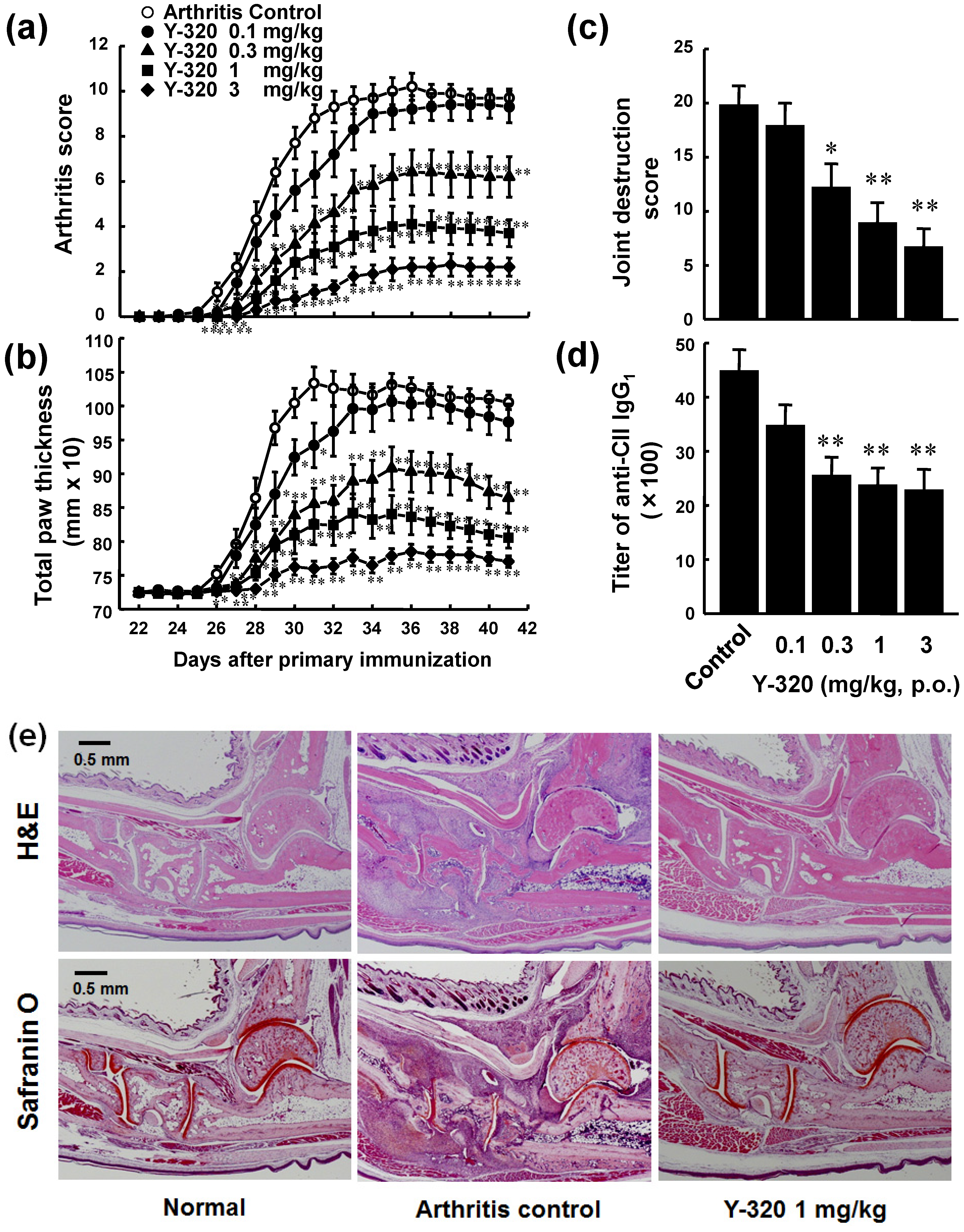
3.2. Y-320 Ameliorates CIA in Mice with a Reduction of IL-17 mRNA in Arthritic Joints

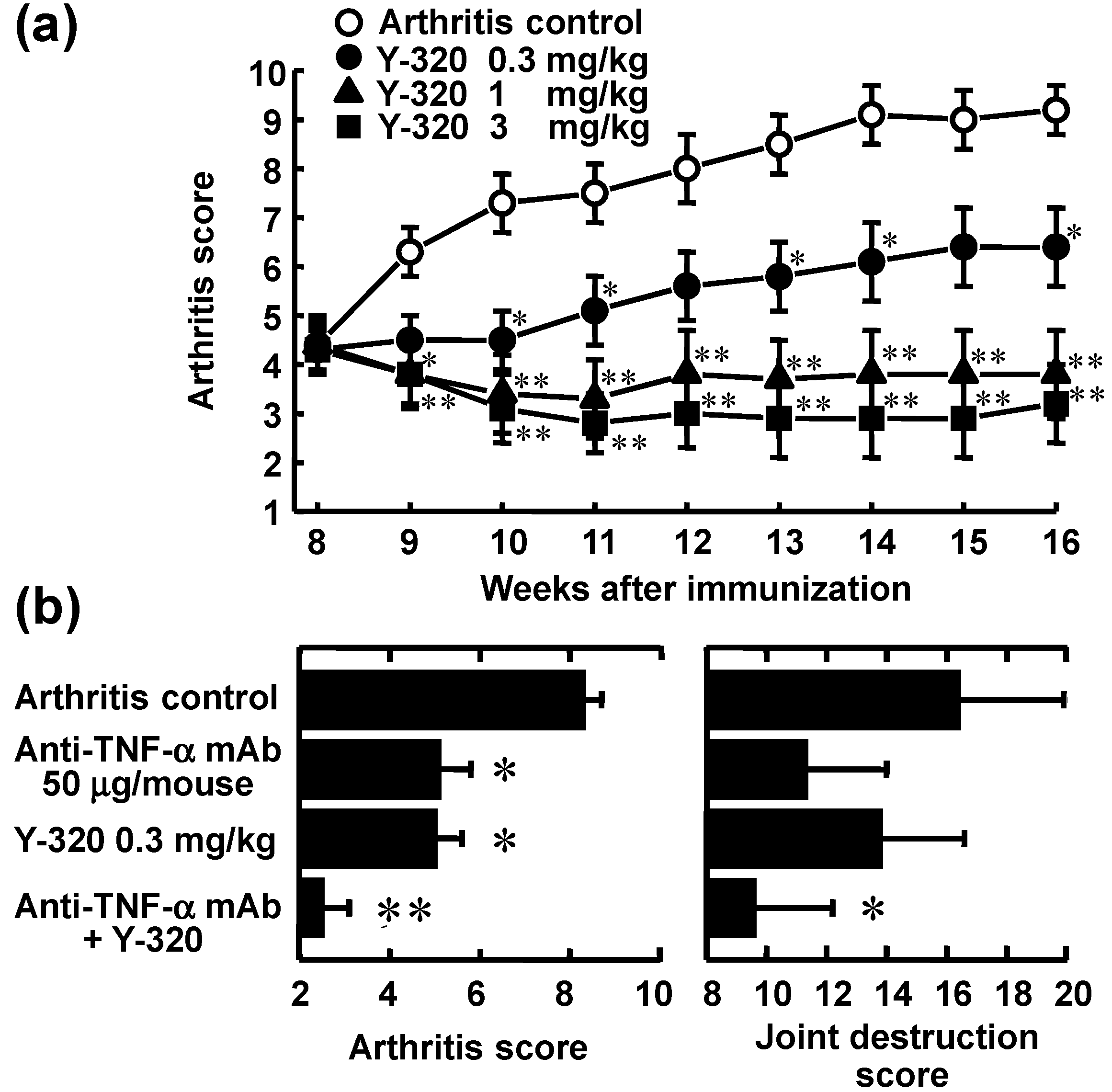
3.3. Y-320 Shows Therapeutic Effects and a Synergistic Effect in Combination with Anti-TNF-α mAb on Chronic-Progressing CIA in Mice
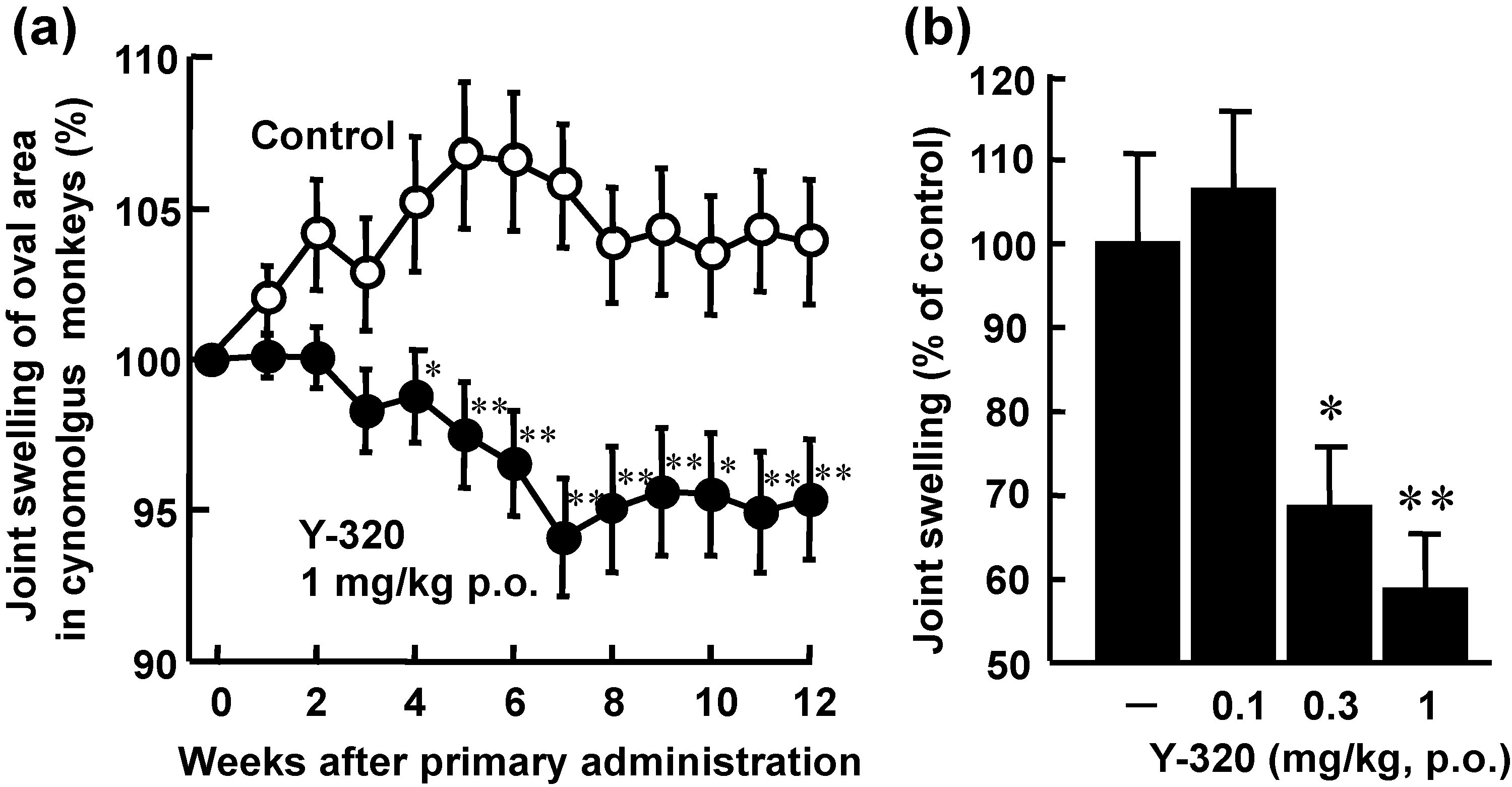
3.4. Y-320 Shows Therapeutic Effects on CIA in Cynomolgus Monkeys
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Oppenheimer-Marks, N.; Brezinschek, R.I.; Mohamadzadeh, M.; Vita, R.; Lipsky, P.E. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse-human rheumatoid arthritis model in vivo. J. Clin. Investig. 1998, 101, 1261–1272. [Google Scholar] [CrossRef]
- Carroll, H.P.; Paunovic, V.; Gadina, M. Signalling, inflammation and arthritis: Crossed signals: The role of interleukin-15 and -18 in autoimmunity. Rheumatology 2008, 47, 1269–1277. [Google Scholar] [CrossRef]
- Grabstein, K.H.; Eisenman, J.; Shanebeck, K.; Rauch, C.; Srinivasan, S.; Fung, V.; Beers, C.; Richardson, J.; Schoenborn, M.A.; Ahdieh, M. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 1994, 264, 965–968. [Google Scholar]
- Giri, J.G.; Kumaki, S.; Ahdieh, M.; Friend, D.J.; Loomis, A.; Shanebeck, K.; DuBose, R.; Cosman, D.; Park, L.S.; Anderson, D.M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the chain of the IL-2 receptor. EMBO J. 1995, 14, 3654–3663. [Google Scholar]
- McInnes, I.B.; Al-Mughales, J.; Field, M.; Leung, B.P.; Huang, F.-P.; Dixon, R.; Sturrock, R.D.; Wilkinson, P.C.; Liew, F.Y. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 1996, 2, 175–182. [Google Scholar] [CrossRef]
- McInnes, I.B.; Leung, B.P.; Sturrock, R.D.; Field, M.; Liew, F.Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat. Med. 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Ziolkowska, M.; Koc, A.; Luszczykiewicz, G.; Ksiezopolska-Pietrzak, K.; Klimczak, E.; Chwalinska-Sadowska, H.; Maslinski, W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporine A-sensitive mechanism. J. Immunol. 2000, 164, 2832–2838. [Google Scholar]
- Ruchatz, H.; Leung, B.P.; Wei, X.; McInnes, I.B.; Liew, F.Y. Soluble IL-15 receptor α-chain administration prevents murine collagen-induced arthritis: A role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 1998, 160, 5654–5660. [Google Scholar]
- Ferrari-Lacraz, S.; Zanelli, E.; Neuberg, M.; Donskoy, E.; Kim, Y.S.; Zheng, X.X.; Hancock, W.W.; Maslinski, W.; Li, X.C.; Strom, T.B.; et al. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J. Immunol. 2004, 173, 5818–5826. [Google Scholar]
- Baslund, B.; Tvede, N.; Danneskiold-Samsoe, B.; Larsson, P.; Panayi, G.; Petersen, J.; Petersen, L.J.; Beurskens, F.J.M.; Schuurman, J.; van de Winkel, J.G.J.; et al. Targeting interleukin-15 in patients with rheumatoid arthritis. A proof-of-concept study. Arthritis Rheum. 2005, 52, 2686–2692. [Google Scholar] [CrossRef]
- Halvorsen, E.H.; Strønen, E.; Hammer, H.B.; Goll, G.L.; Sollid, L.M.; Molberg, Ø. Interleukin-15 induces interleukin-17 production by synovial T cell lines from patients with rheumatoid arthritis. Scand. J. Immunol. 2011, 73, 243–249. [Google Scholar] [CrossRef]
- Kolls, J.K.; Linden, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ghilardi, N.; Xie, M.H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914. [Google Scholar] [CrossRef]
- Nakae, S.; Saijo, S.; Horai, R.; Sudo, K.; Mori, S.; Iwakura, Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA 2003, 100, 5986–5990. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Steinman, L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007, 13, 139–145. [Google Scholar] [CrossRef]
- Fossiez, F.; Djossou, O.; Chomarat, P.; Flores-Romo, L.; it-Yahia, S.; Maat, C.; Pin, J.J.; Garrone, P.; Garcia, E.; Saeland, S.; et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef]
- Nakae, S.; Nambu, A.; Sudo, K.; Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003, 171, 6173–6177. [Google Scholar]
- Miossec, P. IL-17 in rheumatoid arthritis: A new target for treatment or just another cytokine? Joint Bone Spine 2004, 71, 87–90. [Google Scholar] [CrossRef]
- Lubberts, E.; Schwarzenberger, P.; Huang, W.; Schurr, J.R.; Peschon, J.J.; van den Berg, W.B.; Kolls, J.K. Requirement of IL-17 receptor signaling in radiation-resistant cells in the joint for full progression of destructive synovitis. J. Immunol. 2005, 175, 3360–3368. [Google Scholar]
- Ushio, H.; Ishibuchi, S.; Adachi, K.; Oshita, K.; Chiba, K. Phenylpyrazoleanilides as a potent inhibitor of IL-15 dependent T cell proliferation. Part 1: A new class of orally available immunomodulators. Lett. Drug Des. Discov. 2008, 5, 111–115. [Google Scholar] [CrossRef]
- Ushio, H.; Adachi, K.; Oshita, K.; Chiba, K. Phenylpyrazoleanilides as potent inhibitor of IL-15 dependent T cell proliferation. Part 2: Discovery of a new drug candidate, Y-320. Lett. Drug Des. Discov. 2008, 5, 292–296. [Google Scholar] [CrossRef]
- Doleschall, G.; Seres, P. Isoxazole-Oxazole Conversion by Beckmann Rearrangement. J. Chem. Soc. Perkin Trans. I 1988, 7, 1875–1879. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Flavell, R.A. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 2007, 26, 579–591. [Google Scholar] [CrossRef]
- Maeda, Y.; Seki, N.; Sato, N.; Sugahara, K.; Chiba, K. Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int. Immunol. 2010, 22, 515–525. [Google Scholar] [CrossRef]
- Chiba, K.; Kataoka, H.; Seki, N.; Shimano, K.; Koyama, M.; Fukunari, A.; Sugahara, K.; Sugita, T. Fingolimod (FTY720), sphingosine 1-phosphate receptor modulator, shows superior efficacy as compared with interferon-β in mouse experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2011, 11, 366–372. [Google Scholar] [CrossRef]
- Anderson, D.M.; Kumaki, S.; Ahdieh, M.; Bertles, J.; Tometsko, M.; Loomis, A.; Giri, J.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; et al. Functional characterization of the human interleukin-15 receptor a chain and close linkage of IL-15RA and IL-2RA genes. J. Biol. Chem. 1995, 270, 29862–29869. [Google Scholar] [CrossRef]
- Kumaki, S.; Ishii, N.; Minegishi, M.; Tsuchiya, S.; Cosman, D.; Sugamura, K.; Konno, T. Functional role of interleukin-4 (IL-4) and IL-17 in the development of X-linked severe combines immunodeficiency. Blood 1999, 93, 607–612. [Google Scholar]
- Paska, W.; McDonald, K.J.; Croft, M. Studies on type II collagen induced arthritis in mice. Agents Actions 1986, 18, 413–420. [Google Scholar] [CrossRef]
- Nemoto, K.; Mae, T.; Abe, F.; Takeuchi, T. Successful treatment with a novel immunosuppressive agent, deoxyspergualin, in type II collagen-induced arthritis in mice. “Immunomodulating Drugs”. Ann. N. Y. Acad. Sci. 1993, 685, 148–154. [Google Scholar] [CrossRef]
- Kamada, H.; Nakagami, K. Effect of mizoribine on collagen-induced arthritis in mice. Jpn. J. Pharmacol. 1996, 70, 169–175. [Google Scholar] [CrossRef]
- Williams, R.O.; Mauri, C.; Mason, L.J.; M-Mutafchieva, L.; Ross, S.E.; Feldmann, M.; Maini, R.N. Therapeutic actions of cyclosporine and anti-tumor necrosis factor α in collagen-induced arthritis and the effect of combination therapy. Arthritis Rheum. 1998, 41, 1806–1812. [Google Scholar] [CrossRef]
- Williams, R.O.; M-Mutafchieva, L.; Feldmann, M.; Maini, R.N. Evaluation of TNF-α and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-α/anti-CD4 therapy. J. Immunol. 2000, 165, 7240–7245. [Google Scholar]
- M-Mutafchieva, L.; Williams, R.O.; Mauri, C.; Mason, L.J.; Walmsley, M.J.; Taylor, P.C.; Feldmann, M.; Maini, R.N. A comparative study into the mechanisms of action of anti-tumor necrosis factor α, anti-CD4, and combined anti-tumor necrosis factor α/anti-CD4 treatment in early collagen-induced arthritis. Arthritis Rheum. 2000, 43, 638–644. [Google Scholar] [CrossRef]
- Yoo, T.J.; Kim, S.-Y.; Stuart, J.M.; Floyd, R.A.; Olson, G.A.; Cremer, M.A.; Kang, A.H. Induction of arthritis in monkeys by immunization with type II collagen. J. Exp. Med. 1988, 168, 777–782. [Google Scholar] [CrossRef]
- Terato, K.; Arai, H.; Shimozuru, Y.; Fukuda, T.; Tanaka, H.; Watanabe, H.; Nagai, Y.; Fujimoto, K.; Okubo, F.; Cho, F.; et al. Sex-linked differences in susceptibility of cynomolgus monkeys to type II collagen-induced arthritis. Arthritis Rheum. 1989, 32, 748–758. [Google Scholar] [CrossRef]
- Nanki, T.; Hayashida, K.; El-Gabalawy, H.S.; Suson, S.; Shi, K.; Girschick, H.J.; Yavuz, S.; Lipsky, P.E. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J. Immunol. 2000, 165, 6590–6598. [Google Scholar]
- Nanki, T.; Lipsky, P.E. Stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J. Immunol. 2000, 164, 5010–5014. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ushio, H.; Ishibuchi, S.; Oshita, K.; Seki, N.; Kataoka, H.; Sugahara, K.; Adachi, K.; Chiba, K. A New Phenylpyrazoleanilide, Y-320, Inhibits Interleukin 17 Production and Ameliorates Collagen-Induced Arthritis in Mice and Cynomolgus Monkeys. Pharmaceuticals 2014, 7, 1-17. https://doi.org/10.3390/ph7010001
Ushio H, Ishibuchi S, Oshita K, Seki N, Kataoka H, Sugahara K, Adachi K, Chiba K. A New Phenylpyrazoleanilide, Y-320, Inhibits Interleukin 17 Production and Ameliorates Collagen-Induced Arthritis in Mice and Cynomolgus Monkeys. Pharmaceuticals. 2014; 7(1):1-17. https://doi.org/10.3390/ph7010001
Chicago/Turabian StyleUshio, Hiroyuki, Seigo Ishibuchi, Koichi Oshita, Noriyasu Seki, Hirotoshi Kataoka, Kunio Sugahara, Kunitomo Adachi, and Kenji Chiba. 2014. "A New Phenylpyrazoleanilide, Y-320, Inhibits Interleukin 17 Production and Ameliorates Collagen-Induced Arthritis in Mice and Cynomolgus Monkeys" Pharmaceuticals 7, no. 1: 1-17. https://doi.org/10.3390/ph7010001



