Probiotic Ferulic Acid Esterase Active Lactobacillus fermentum NCIMB 5221 APA Microcapsules for Oral Delivery: Preparation and in Vitro Characterization
Abstract
:1. Introduction
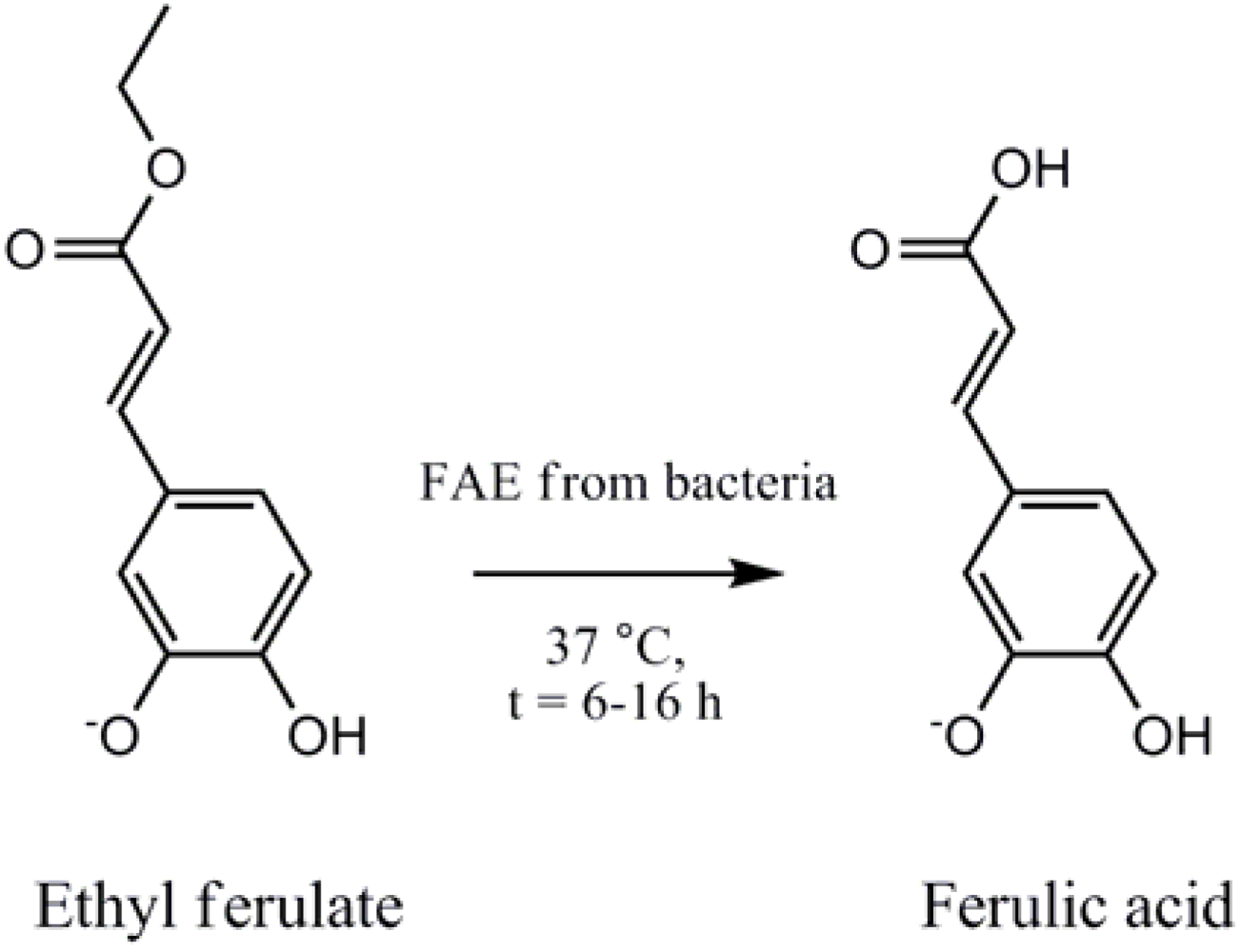
2. Experimental Methods and Materials
2.1. Bacterial Growth Media and Chemicals
2.2. Bacterial Strains and Culture Conditions
2.3. APA Microencapsulation
2.4. Ferulic Acid Esterase HPLC Assay To Measure FA Production
2.5. Simulated Gastrointestinal Conditions to Determine the Stability and Viability of Microencapsulated L. Fermentum NCIMB 5221 Delivered Orally
2.6. Statistical Analysis
3. Results and Discussion
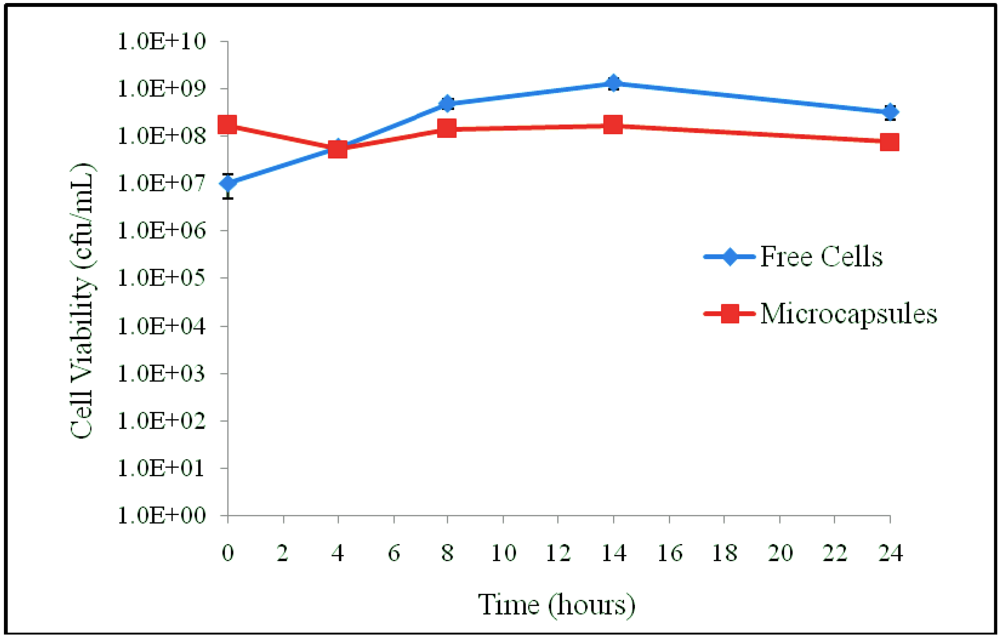
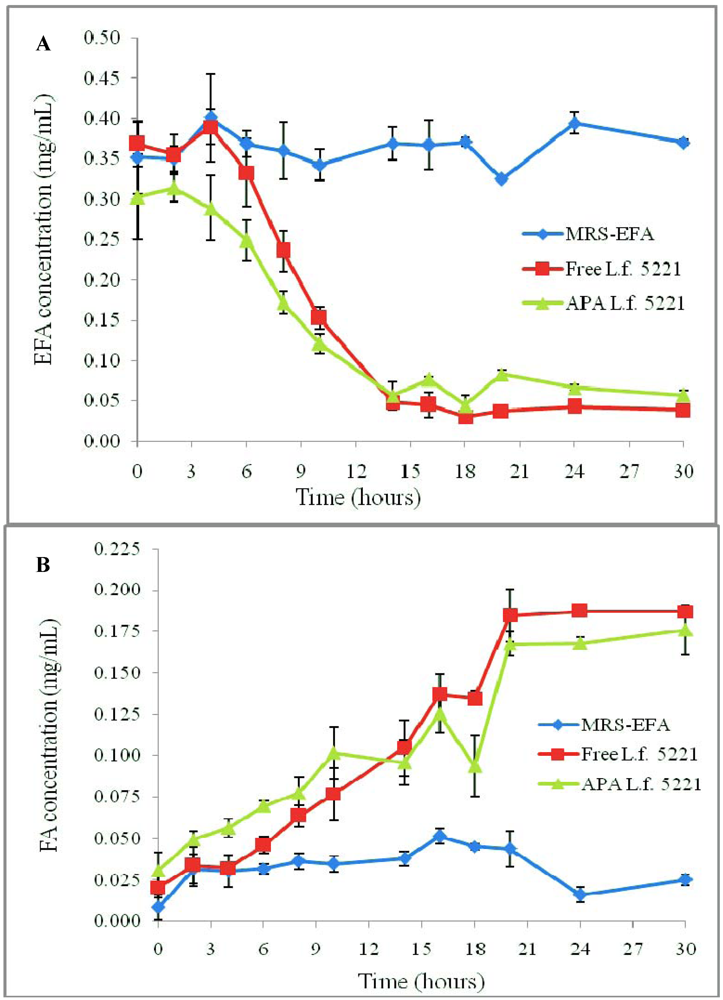
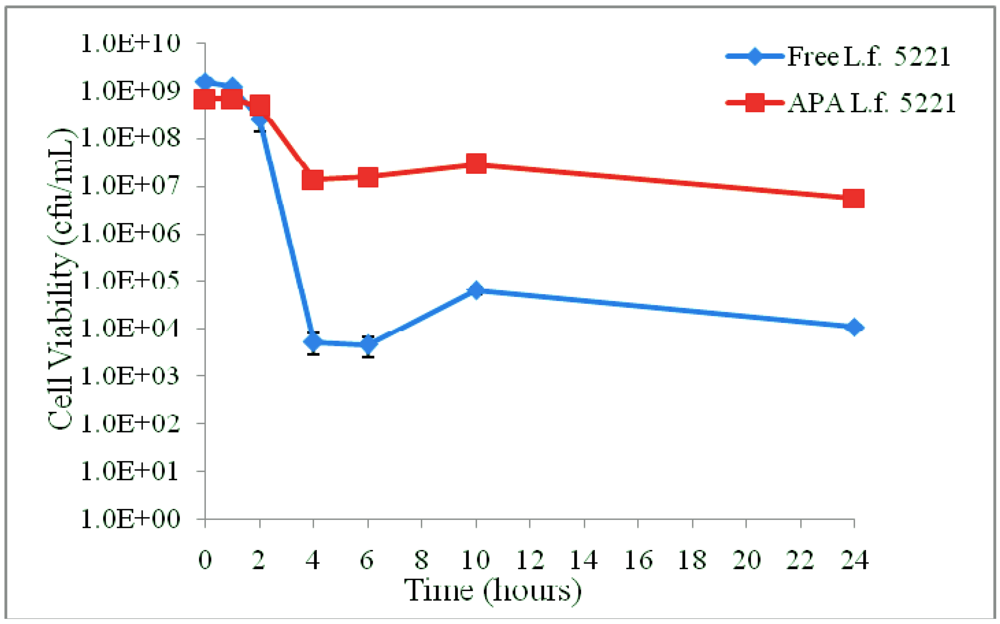
3.1. Results

| Time (hours) | GIT section | pH | Solutes | Viability (cfu/mL) | Viability (%) | ||
|---|---|---|---|---|---|---|---|
| Free | APA | Free | APA | ||||
| 0 | Stomach | 1.5 | Sodium chloride Peptic enzymes Glucose | 1.53 × 109 ± 9.02 × 107 | 6.77 × 108 ± 7.77 × 107 | 100.00 ± 0.059 | 100.00 ± 0.115 |
| 1 | 1.18 × 109 ± 2.04 × 108 | 6.83 × 108 ± 4.51 × 107 | 77.51 ± 13.365 | 100.99 ± 6.664 | |||
| 2 | 2.60 × 108 ± 1.22 × 108 | 4.73 × 108 ± 4.93 × 107 | 17.03 ± 7.969 | 69.95 ± 7.290 | |||
| 4 | Small / Large intestines | 6.8 | Potassium phosphate Pancreatic enzymes Bile Glucose | 5.33 × 103 ± 2.52 × 103 | 1.35 × 107 ± 1.12 × 106 | 0.0004 ± 0.0002 | 2.00 ± 0.165 |
| 6 | 4.67 × 103 ± 2.08 × 103 | 1.53 × 107 ± 1.15 × 106 | 0.0003 ± 0.0001 | 2.26 ± 0.170 | |||
| 10 | 6.37 × 104 ± 1.33 × 104 | 2.82 × 107 ± 1.23 × 106 | 0.0042 ± 0.0009 | 4.17 ± 0.182 | |||
| 24 | 1.10 × 104 ± 1.00 × 103 | 5.50 × 106 ± 1.00 × 105 | 0.0007 ± 0.0001 | 0.813 ± 0.015 | |||
3.2. Discussion
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; Gostissa, M.; Alt, F.W. DNA Repair, Genome Stability, and Aging. Cell 2005, 120, 497–512. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.-I.; Notas, G.; Nifli, A.-P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Canacer Res. 2003, 6, R63–R74. [Google Scholar]
- Chang, C.J.; Chiu, J.H.; Tseng, L.M.; Chang, C.H.; Chien, T.M.; Wu, C.W.; Lui, W.Y. Modulation of HER2 expression by ferulic acid on human breast cancer MCF7 cells. Eur. J. Clin. Investig. 2006, 36, 588–596. [Google Scholar] [CrossRef]
- Lee, Y. Role of NADPH oxidase-mediated generation of reactive oxygen species in the mechanism of apoptosis induced by phenolic acids in HepG2 human hepatoma cells. Arch. Pharm. Res. 2005, 28, 1183–1189. [Google Scholar] [CrossRef]
- Taniguchi, H.; Hosoda, A.; Tsuno, T.; Maruta, Y.; Nomura, E. Preparation of ferulic acid and its application for the synthesis of cancer chemopreventive agents. Anticancer Res. 1999, 19, 3757–3761. [Google Scholar]
- Lesca, P. Protective effects of ellagic acid and other plant phenols on benzo[a]pyrene-induced neoplasia in mice. Carcinogenesis 1983, 4, 1651–1653. [Google Scholar] [CrossRef]
- Tanaka, T.; Kojima, T.; Kawamori, T.; Wang, A.; Suzui, M.; Okamoto, K.; Mori, H. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis 1993, 14, 1321–1325. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar]
- Spencer, J.P.E.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Bhathena, J.; Martoni, C.; Kumar, A.; Urbanska, A.; Malhotra, M.; Prakash, S. Orally delivered microencapsulated live probiotic formulation lowers serum lipids in hypercholesterolemic hamsters. J. Med. Food 2009, 12, 310–319. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Coussa-Charley, M.; Al-Salami, H.; Jones, M.L.; Labbé, A.; Prakash, S. Lactobacillus fermentum NCIMB 5221 has a greater ferulic acid production compared to other ferulic acid esterase producing Lactobacilli. Int. J. Probiotics Prebiotics 2012, 7. in press. [Google Scholar]
- Prakash, S.; Tomaro-Duchesneau, C.; Saha, S.; Cantor, A. The gut microbiota and human health with an emphasis on the use of microencapsulated bacterial cells. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef]
- Poncelet, D. Microencapsulation: Fundamentals, methods and applications. In Surface Chemistry in Biomedical and Environmental Science; Blitz, J.P., Gun'ko, V.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 23–34. [Google Scholar]
- Chang, T.; Prakash, S. Procedures for microencapsulation of enzymes, cells and genetically engineered microorganisms. Mol. Biotechnol. 2001, 17, 249–260. [Google Scholar] [CrossRef]
- Mastihuba, V.; Kremnicky, L.; Mastihubova, M.; Willett, J.L.; Cote, G.L. A spectrophotometric assay for feruloyl esterases. Anal. Biochem. 2002, 309, 96–101. [Google Scholar]
- U.S. Pharmacopeia. Test Solutions. 2010.
- Prakash, S.; Rodes, L.; Coussa-Charley, M.; Tomaro-Duchesneau, C. Gut microbiota: Next frontier in understanding human health and development of biotherapeutics. Biol. Targets Ther. 2011, 2011, 71–86. [Google Scholar]
- FAO and WHO, Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Food and Agricultural Organization of the United Nations: Cordoba, Argentina; 1–4 October 2001.
- Branton, W.B.; Jones, M.L.; Tomaro-Duchesneau, C.; Martoni, C.J.; Prakash, S. In vitro characterization and safety of the probiotic strain Lactobacillus reuteri Cardioviva NCIMB 30242. Int. J. Probiotics Prebiotics 2011, 6, 1–12. [Google Scholar]
- Wolvers, D.; Antoine, J.M.; Myllyluoma, E.; Schrezenmeir, J.; Szajewska, H.; Rijkers, G.T. Guidance for substantiating the evidence for beneficial effects of probiotics: Prevention and management of infections by probiotics. J. Nutr. 2010, 140, 698S–712S. [Google Scholar] [CrossRef]
- Jankovic, I.; Sybesma, W.; Phothirath, P.; Ananta, E.; Mercenier, A. Application of probiotics in food products – challenges and new approaches. Curr. Opin. Biotechnol. 2010, 21, 175–181. [Google Scholar] [CrossRef]
- Floch, M.H. Probiotic therapy for ulcerative colitis. J. Clin. Gastroenterol. 2010, 44, 237–238. [Google Scholar] [CrossRef]
- Al-Salami, H.; Butt, G.; Fawcett, J.; Tucker, I.; Golocorbin-Kon, S.; Mikov, M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 101–106. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Altermann, E.; Hoover-Fitzula, R.L.; Cano, R.J.; Klaenhammer, T.R. Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance. Appl. Environ. Microbiol. 2004, 70, 5315–5322. [Google Scholar]
- Guglielmetti, S.; de Noni, I.; Caracciolo, F.; Molinari, F.; Parini, C.; Mora, D. Bacterial cinnamoyl esterase activity screening for the production of a novel functional food product. Appl. Environ. Microbiol. 2008, 74, 1284–1288. [Google Scholar] [CrossRef]
- Mates, J.M.; Perez-Gomez, C.; de Castro, I.N.; Asenjo, M.; Marquez, J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int. J. Biochem. Cell Biol. 2002, 34, 439–458. [Google Scholar] [CrossRef]
- Martoni, C.; Bhathena, J.; Urbanska, A.M.; Prakash, S. Microencapsulated bile salt hydrolase producing Lactobacillus reuteri for oral targeted delivery in the gastrointestinal tract. Appl. Microbiol. Biotechnol. 2008, 81, 225–233. [Google Scholar] [CrossRef]
- Christopher, M.; Jasmine, B.; Mitchell Lawrence, J.; Aleksandra Malgorzata, U.; Hongmei, C.; Satya, P. Investigation of microencapsulated BSH active Lactobacillus in the simulated human GI tract. J. Biomed. Biotechnol. 2007. [Google Scholar] [CrossRef]
- Tanaka, H.; Doesburg, K.; Iwasaki, T.; Mierau, I. Screening of lactic acid bacteria for bile salt hydrolase activity. J. Dairy Sci. 1999, 82, 2530–2535. [Google Scholar] [CrossRef]
- Pfeiler, E.A.; Klaenhammer, T.R. Role of transporter proteins in bile tolerance of Lactobacillus acidophilus. Appl. Environ. Microbiol. 2009, 75, 6013–6016. [Google Scholar]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Moonsan, P.; Yibchok-anun, S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 7838–7844. [Google Scholar] [CrossRef]
- Sri Balasubashini, M.; Rukkumani, R.; Menon, V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003, 40, 118–122. [Google Scholar] [CrossRef]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Menon, V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 2004, 18, 310–314. [Google Scholar] [CrossRef]
- Atsuyo, F.; Hideyuki, S.; Asako, D.; Kunihisa, O.; Shohei, M.; Hiroto, F.; Masahiro, N.; Taisei, N.; Takuo, T.; Hisaji, T.; et al. Ferulic acid prevents pathological and functional abnormalities of the kidney in Otsuka Long-Evans Tokushima Fatty diabetic rats. Diabetes Res. Clin. Pract. 2008, 79, 11–17. [Google Scholar] [CrossRef]
- Bhathena, J.; Kulamarva, A.; Martoni, C.; Urbanska, A.M.; Prakash, S. Preparation and in vitro analysis of microencapsulated live Lactobacillus fermentum 11976 for augmentation of feruloyl esterase in the gastrointestinal tract. Biotechnol. Appl. Biochem. 2008, 50, 1–9. [Google Scholar] [CrossRef]
- Kailasapathy, K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT - Food Sci. Technol. 2006, 39, 1221–1227. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Coussa-Charley, M.; Kahouli, I.; Jones, M.L.; Labbé, A.; Prakash, S. Probiotic Ferulic Acid Esterase Active Lactobacillus fermentum NCIMB 5221 APA Microcapsules for Oral Delivery: Preparation and in Vitro Characterization. Pharmaceuticals 2012, 5, 236-248. https://doi.org/10.3390/ph5020236
Tomaro-Duchesneau C, Saha S, Malhotra M, Coussa-Charley M, Kahouli I, Jones ML, Labbé A, Prakash S. Probiotic Ferulic Acid Esterase Active Lactobacillus fermentum NCIMB 5221 APA Microcapsules for Oral Delivery: Preparation and in Vitro Characterization. Pharmaceuticals. 2012; 5(2):236-248. https://doi.org/10.3390/ph5020236
Chicago/Turabian StyleTomaro-Duchesneau, Catherine, Shyamali Saha, Meenakshi Malhotra, Michael Coussa-Charley, Imen Kahouli, Mitchell L. Jones, Alain Labbé, and Satya Prakash. 2012. "Probiotic Ferulic Acid Esterase Active Lactobacillus fermentum NCIMB 5221 APA Microcapsules for Oral Delivery: Preparation and in Vitro Characterization" Pharmaceuticals 5, no. 2: 236-248. https://doi.org/10.3390/ph5020236




