Norethindrone Acetate in the Medical Management of Adenomyosis
Abstract
:1. Introduction
2. Results
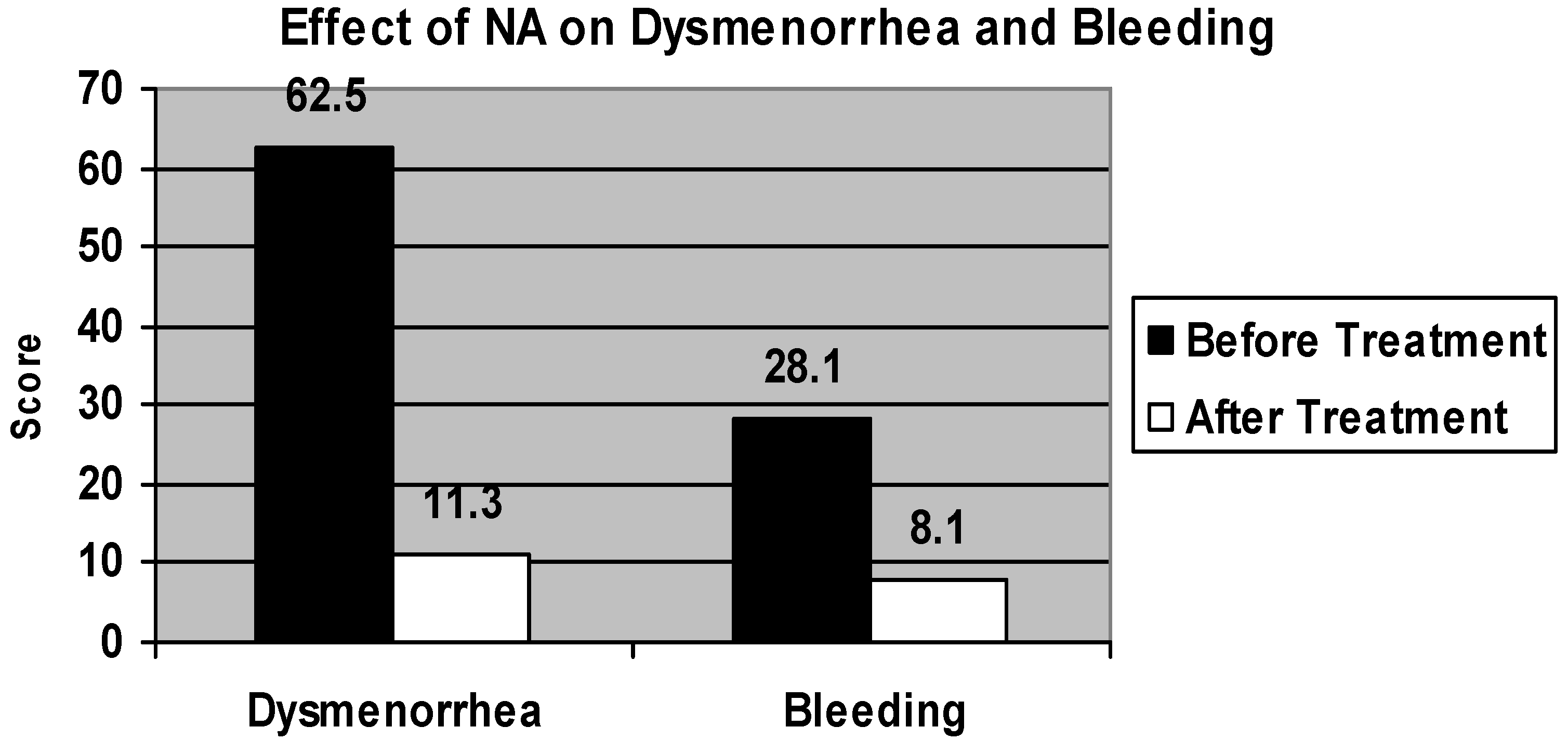
3. Discussion
4. Material and Methods
5. Conclusions
Conflict of Interest
References
- Rokitansky, K. Ueber uterus-neubildung (In German). Aerzte zu Wien Ztschr K Gesellsch 1860, 16, 577. [Google Scholar]
- Von Recklinghausen, F. Die Adenomyome und Cystadenome der Uterus und Tubenwandung: ihre Abkunst von Resent des Wolffschen Korpers (In German); Hirschwald: Berlin, Gemany, 1896. [Google Scholar]
- Englander, M.J. Uterine artery embolization for the treatment of adenomyosis. Semin. Intervent. Radiol. 2008, 25, 387–393. [Google Scholar] [CrossRef]
- Matalliotakis, I.M.; Katasikis, I.K.; Panidis, D.K. Adenomyosis: What is the impact on fertility. Curr. Opin. Obstet. Gynecol. 2005, 17, 261–264. [Google Scholar] [CrossRef]
- Devlieger, R.; D’Hooghe, T.; Timmerman, D. Uterine adenomyosis in the infertility clinic. Hum. Reprod. Update. 2003, 9, 139–147. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, H.Y.; Huang, C.H.; Twu, N.F.; Yen, M.S.; Wang, P.H.; Chou, T.Y.; Liu, Y.N.; Chao, K.C.; Yang, M.H. Oestrogen induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J. Pathol. 2010, 222, 261–270. [Google Scholar] [CrossRef]
- Panganamamula, U.R.; Harmanli, O.H.; Isik-Akbay, E.F.; Grotegut, C.A.; Dandolu, V.; Gaughan, J.P. Is prior uterine surgery a risk factor for adenomyosis? Obstet. Gynecol. 2004, 104, 1034–1038. [Google Scholar] [CrossRef]
- Katz, V.L. Comprehensive Gynecology, 5th ed; Mosby Elsevier: Philadelphia, PA, USA, 2007. [Google Scholar]
- Fedele, L.; Bianchi, S.; Dorta, M.; Arcaini, L.; Zanotti, F.; Carinelli, S. Transvaginal ultrasonography in the diagnosis of diffuse adenomyosis. Fertil. Steril. 1992, 58, 479. [Google Scholar]
- Fedele, L.; Bianchi, S.; Dorta, M.; Zanotti, F.; Brioschi, D.; Carinelli, S. Transvaginal ultrasonography in the differential diagnosis of adenomyoma versus leiomyoma. Am. J. Obstet. Gynecol. 1992, 167, 603–606. [Google Scholar]
- Atzori, E. Sonography for the diagnosis of adenomyosis. Ultrasound Obstet. Gynecol. 2003, 21, 626–627. [Google Scholar] [CrossRef]
- Brosen, J.J.; D’Souza, N.M.; Barker, F.G.; Paraschos, T.; Wintin, R.M.L. Endovaginal ultrasonography in the diagnosis of adenomyosis uteri: identifying the predictive characteristics. Am. J. Obstet. Gynecol. 1995, 102, 471–474. [Google Scholar]
- Atzori, E.; Tronci, C.; Sionis, L. Transvaginal ultrasound in the diagnosis of diffuse adenomyosis. Gynecol. Obstet. Invest. 1996, 42, 39–41. [Google Scholar] [CrossRef]
- Reinhold, C.; Atri, M.; Mehio, A.; Zakarian, R.; Aldis, A.E.; Bret, P.M. Diffuse uterine adenomyosis: morphologic criteria and diagnostic accuracy of endovaginal sonography. Radiography 1995, 197, 609–614. [Google Scholar]
- Levgur, M. Diagnosis of adenomyosis. J. Reprod. Med. 2007, 52, 177–193. [Google Scholar]
- Champaneria, R.; Abedin, P.; Daniels, J.; Balogun, M.; Khan, K.S. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet. Gynecol. Scand. 2010, 89, 1374–1384. [Google Scholar] [CrossRef]
- Muneyyirci-Delale, O.; Karacan, M. Effect of Norethindrone acetate in the treatment of symptomatic endometriosis. Int. J. Fertil. 1998, 43, 24–27. [Google Scholar]
- Muneyyirci-Delale, O.; Jalou, S. Long-term treatment of symptomatic endometriosis with Norethindrone acetate. Clin. J. Women’s Health 2001, 1, 69–75. [Google Scholar] [CrossRef]
- Ferrero, S.; Camerini, G.; Ragni, N.; Venturini, P.L.; Biscaldi, E.; Remorgida, V. Norethisterone acetate in the treatment of colorectal endometriosis: A pilot study. Hum. Repro. Jan. 2010, 25, 94–100. [Google Scholar]
- Okada, H.; Okamoto, R.; Tsuzuki, T.; Tsuji, S.; Yasuda, K.; Kanzaki, H. Progestins inhibit estradiol-induced vascular endothelial growth factor and stromal cell-derived factor 1 in human endometrial stromal cells. Fertil. Steril. 2011, 96, 786–791. [Google Scholar] [CrossRef]
- Muneyyirci-Delale, O.; Jalou, S.; Rahman, M.; Nacharaju, V. Can we decrease breakthrough bleeding in patients with endometriosis on Norethindrone acetate? Int. J. Fertil. Women’s Med. 2003, 48, 32–36. [Google Scholar]
- Basak, S.; Saha, A. Adenomyosis: Still largely under-diagnosed. J. Obstet. Gynecol. 2009, 29, 533–535. [Google Scholar] [CrossRef]
- Bazot, M.; Cortez, A.; Darai, E.; Rouger, J.; Chopier, J.; Antoine, J.M.; Uzan, S. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum. Reprod. 2001, 16, 2427–2433. [Google Scholar]
- Dueholm, M.; Lundorf, E.; Hansen, E.S.; Sorensen, J.S.; Ledertoug, S.; Olesen, F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil. Steril. 2001, 76, 588–594. [Google Scholar] [CrossRef]
- Levgur, M. Therapeutic options for adenomyosis: a review. Arch Gynecol. Obstet. 2007, 276, 1–15. [Google Scholar] [CrossRef]
- McCausland, A.M.; McCausland, V.M. Long-term complications of endometrial ablation: cause, diagnosis, treatment and prevention. J. Minim. Invasive Gynecol. 2007, 14, 399–406. [Google Scholar] [CrossRef]
- Froeling, V.; Scheurig-Muenkler, C.; Hamm, B.; Kroencke, T.J. Uterine artery embolisation to treat uterine adenomyosis with or without leiomyomata: Results of symptom control and health related quality of life 40 months after treatment. Cardiovasc. Interven. Radiol. 2012, 35, 523–529. [Google Scholar] [CrossRef]
- Braghato, A.M.; Caserta, N.; Bahamondes, L.; Petta, C.A. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception 2007, 76, 195–199. [Google Scholar] [CrossRef]
- Akira, S.; Mine, K.; Kuwabara, Y.; Takeshita, T. Efficacy of longterm low dose gonadotropin-releasing hormone agonist therapy (draw-back therapy) for adenomyosis. Med. Sci. Monit. 2009, 15, CR1–CR4. [Google Scholar]
- Akira, S.; Iwasaki, N.; Ichikawa, M.; Mine, K.; Kuwabara, Y.; Takeshita, T.; Tajima, H. Successful long-term management of adenomyosis associated with deep thrombosis by low dose gonadotropin-releasing hormone agonist therapy. Clin. Exp. Obstet. Gynecol. 2009, 36, 123–125. [Google Scholar]
- Zhang, X.; Yuan, H.; Deng, L.; Hu, F.; Ma, J.; Lin, J. Evaluation of the efficacy of a danazol-loaded intrauterine contraceptive device on adenomyosis in an ICR mouse model. Hum. Reprod. 2008, 23, 2024–2030. [Google Scholar] [CrossRef]
- Schweppe, K.W. The place of dydrogesterone in the treatment of endometriosis and adenomyosis. Maturitas 2009, 65, 23–27. [Google Scholar]
- Togashi, K.; Nishimura, K.; Itoh, K.; Fujisawa, I.; Noma, S.; Kanaoka, M.; Nakano, Y.; Itoh, H.; Ozasa, H.; Fujii, S.; et al. Adenomyosis: Diagnosis with MR imaging. Radiology 1998, 166, 111–114. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Muneyyirci-Delale, O.; Chandrareddy, A.; Mankame, S.; Osei-Tutu, N.; Gizycki, H.v. Norethindrone Acetate in the Medical Management of Adenomyosis. Pharmaceuticals 2012, 5, 1120-1127. https://doi.org/10.3390/ph5101120
Muneyyirci-Delale O, Chandrareddy A, Mankame S, Osei-Tutu N, Gizycki Hv. Norethindrone Acetate in the Medical Management of Adenomyosis. Pharmaceuticals. 2012; 5(10):1120-1127. https://doi.org/10.3390/ph5101120
Chicago/Turabian StyleMuneyyirci-Delale, Ozgul, Ashadeep Chandrareddy, Siddhi Mankame, Nanna Osei-Tutu, and Hans von Gizycki. 2012. "Norethindrone Acetate in the Medical Management of Adenomyosis" Pharmaceuticals 5, no. 10: 1120-1127. https://doi.org/10.3390/ph5101120
APA StyleMuneyyirci-Delale, O., Chandrareddy, A., Mankame, S., Osei-Tutu, N., & Gizycki, H. v. (2012). Norethindrone Acetate in the Medical Management of Adenomyosis. Pharmaceuticals, 5(10), 1120-1127. https://doi.org/10.3390/ph5101120



