Future Trends in the Pharmacogenomics of Brain Disorders and Dementia: Influence of APOE and CYP2D6 Variants
Abstract
:1. Introduction
1.1. Towards a Personalized Medicine in Neuropsychiatric Disorders
| Locus | Symbol | Aliases | Title |
|---|---|---|---|
| 1p21.3-p13.1 | SORT1 | Gp95, NT3 | sortilin |
| 1p31.3 | TM2D1 | BBP | TM2 domain containing 1 |
| 1p32 | ERI3 | PINT1; PRNPIP; MGC2683; FLJ22943 | ERI1 exoribonuclease family member 3 |
| 1p32.3 | ZFYVE9 | MADHIP, NSP, SARA, SMADIP | zinc finger, FYVE domain containing 9 |
| 1p33-p31.1 | DHCR24 | KIAA0018, Nbla03646, SELADIN1, seladin-1 | 24-dehydrocholesterol reductase |
| 1p34 | LRP8 | APOER2, HSZ75190, MCI1 | low density lipoprotein receptor-related protein 8, apolipoprotein e receptor |
| 1p36.1 | ECE1 | RP3-329E20.1, ECE | endothelin converting enzyme 1 |
| 1p36.13-q31.3 | APH1A | RP4-790G17.3, 6530402N02Rik, APH-1, APH-1A, CGI-78 | anterior pharynx defective 1 homolog A (C. elegans) |
| 1p36.22 | TARDBP | RP4-635E18.2, ALS10, TDP-43 | TAR DNA binding protein |
| 1p36.3 | MTHFR | 5,10-methylenetetrahydrofolate reductase (NADPH) | |
| 1q21 | S100A1 | S100, S100-alpha, S100A | S100 calcium-binding protein A1 |
| 1q21.2-q21.3 | LMNA | RP11-54H19.1, CDCD1, CDDC, CMD1A, CMT2B1, EMD2, FPL, FPLD, HGPS, IDC, LDP1, LFP, LGMD1B, LMN1, LMNC, PRO1 | lamin A/C |
| 1q21.3 | CHRNB2 | EFNL3, nAChRB2 | cholinergic receptor, nicotinic, beta 2 (neuronal) |
| 1q21-q23 | APCS | MGC88159, PTX2, SAP | amyloid P component, serum |
| 1q22-q23 | NCSTN | RP11-517F10.1, APH2, KIAA0253 | nicastrin |
| 1q25 | SOAT1 | RP11-215I23.1, ACACT, ACAT, ACAT1, RP11-215I23.2, SOAT, STAT | sterol O-acyltransferase 1 |
| 1q25.2-q25.3 | PTGS2 | COX-2, COX2, GRIPGHS, PGG/HS, PGHS-2, PHS-2, hCox-2 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
| 1q31-q32 | IL10 | CSIF, IL-10, IL10A, MGC126450, MGC126451, TGIF | interleukin 10 |
| 1q31-q42 | AD4 | AD3L, AD4, PS2, STM2 | presenilin 2 (alzheimer disease 4) |
| 1q32 | CR1 | C3BR, C4BR, CD35, KN | complement component (3b/4b) receptor 1 (Knops blood group) |
| 1q42-q43 | AGT | ANHU, FLJ92595, FLJ97926, SERPINA8 | angiotensinogen (serpin peptidase inhibitor, clade A, member 8) |
| 2p16.3 | RTN4 | ASY, NI220/250, NOGO, NOGO-A, NOGOC, NSP, NSP-CL, Nbla00271, Nbla10545, Nogo-B, Nogo-C, RTN-X, RTN4-A, RTN4-B1, RTN4-B2, RTN4-C | reticulon 4 |
| 2p25 | ADAM17 | ADAM18, CD156B, CSVP, MGC71942, TACE | ADAM metallopeptidase domain 17 |
| 2q14 | BIN1 | AMPH2, AMPHL, DKFZp547F068, MGC10367, SH3P9 | bridging integrator 1 |
| 2q14 | IL1A | IL-1A, IL1, IL1-ALPHA, IL1F1 | interleukin-1-Alpha |
| 2q21.1 | KCNIP3 | CSEN, DREAM, KCHIP3, MGC18289 | Kv channel interacting protein 3, calsenilin |
| 2q21.2 | LRP1B | LRP-DIT, LRPDIT | low density lipoprotein-related protein 1B (deleted in tumors) |
| 2q34 | CREB1 | CREB, MGC9284 | cAMP responsive element binding protein 1 |
| 3q25.1-q25.2 | CALLA, CD10, MME DKFZp686O16152, MGC126681, MGC126707, NEP | membrane metallo-endopeptidase | |
| 3q26.1-q26.2 | BCHE | CHE1, E1 | butyrylcholinesterase |
| 3q26.2-qter | APOD | apolipoprotein D | |
| 3q28 | SST | SMST | somatostatin |
| 4p14-p13 | APBB2 | DKFZp434E033, FE65L, FE65L1, MGC35575 | amyloid beta (A4) precursor protein-binding, family B, member 2 |
| 5q15 | CAST | BS-17, MGC9402 | calpastatin |
| 5q31 | APBB3 | FE65L2, MGC150555, MGC87674, SRA | amyloid beta (A4) precursor protein-binding, family B, member 3 |
| 5q35.3 | DBN1 | D0S117E, DKFZp434D064 | drebrin 1 |
| 6p12 | VEGFA | RP1-261G23.1, MGC70609, MVCD1, VEGF, VPF | vascular endothelial growth factor A |
| 6p21.3 | AGER | DAMA-358M23.4, MGC22357, RAGE | advanced glycosylation end product-specific receptor |
| 6p21.3 | HFE | HFE1, HH, HLA-H, MGC103790, MVCD7, dJ221C16.10.1 | hemochromatosis |
| 6p21.3 | HLA-A | DAQB-90C11.16, Aw-68, Aw-69, FLJ26655, HLAA | major histocompatibility complex, class I, A |
| 6p21.3 | TNF | DADB-70P7.1, DIF, TNF-alpha, TNFA, TNFSF2 | tumor necrosis factor (TNF superfamily, member 2) |
| 6p22.1 | PGDB1 | HUCEP-4, SCAND4, dJ874C20.4 | piggyBac transposable element derived 1 |
| 6p23 | ATXN1 | ATX1, D6S504E, SCA1 | ataxin 1 |
| 7p21 | IL6 | BSF2, HGF, HSF, IFNB2, IL-6 | interleukin 6 (interferon, beta 2) |
| 7q21.3 | PON1 | ESA, MVCD5, PON | paraoxonase 1 |
| 7q22 | RELN | PRO1598, RL | reelin |
| 7q36 | AD10 | Alzheimer disease-10 | |
| 7q36 | NOS3 | ECNOS, eNOS | nitric oxide synthase 3 (endothelial cell) |
| 7q36 | PAXIP1 | CAGF28, CAGF29, FLJ41049, PACIP1, PAXIP1L, PTIP, TNRC2 | PAX interacting (with transcription-activation domain) protein 1 |
| 8p21-p12 | CLU | AAG4, APOJ, CLI, KUB1, MGC24903, SGP-2, SGP2, SP-40, TRPM-2, TRPM2 | clusterin |
| 8p22 | CTSB | APPS, CPSB | cathepsin B |
| 9p24.1 | IL33 | C9orf26, DKFZp586H0523, DVS27, NF-HEV, NFEHEV, RP11-575C20.2 | interleukin 33 |
| 9q13-q21.1 | APBA1 | D9S411E, MINT1, X11, X11A, X11ALPHA | amyloid beta (A4) precursor protein-binding, family A, member 1 |
| 9q31.1 | GRIN3A | FLJ45414, NMDAR-L, NR3A | glutamate receptor, ionotropic, N-methyl-D-aspartate 3A |
| 9q33-q34.1 | HSPA5 | BIP, FLJ26106, GRP78, MIF2 | heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa) |
| 9q34.1 | DAPK1 | DAPK, DKFZp781I035 | death-associated protein kinase 1 |
| 10p13 | AD7 | Alzheimer disease 7 | |
| 10p15.2 | PITRM1 | RP11-298E9.1, KIAA1104, MGC138192, MGC141929, MP1, PreP, hMP1 | pitrilysin metallopeptidase 1 |
| 10q | AD6 | Alzheimer disease-6 | |
| 10q11.2 | ALOX5 | RP11-67C2.3, 5-LO, 5-LOX, 5LPG, LOG5, MGC163204 | arachidonate 5-lipoxygenase |
| 10q21 | TFAM | MtTF1, TCF6, TCF6L1, TCF6L2, TCF6L3, mtTFA | transcription factor A, mitochondrial |
| 10q23 | CH25H | C25H | cholesterol 25-hydroxylase |
| 10q23-q25 | IDE | RP11-366I13.1, FLJ35968, INSULYSIN | insulin-degrading enzyme |
| 10q23-q25 | SORCS1 | RP11-446H13.1, FLJ41758, FLJ43475, FLJ44957 | sortilin-related VPS10 domain containing receptor 1 |
| 10q23.32 | HECTD2 | FLJ16050 | HECT domain containing 2 |
| 10q24 | COX15 | COX15 homolog, cytochrome c oxidase assembly protein (yeast) | |
| 10q24 | PLAU | ATF, UPA, URK, u-PA | plasminogen activator, urokinase |
| 10q24.33 | CALHM1 | FAM26C, MGC39514, MGC39617 | calcium homeostasis modulator 1 |
| 10q24.33 | SH3PXD2A | FISH, SH3MD1 | SH3 and PX domains 2A |
| 10q26.3 | ADAM12 | RP11-295J3.5, MCMP, MCMPMltna, MLTN, MLTNA | ADAM metallopeptidase domain 12 |
| 11p13 | BDNF | MGC34632 | brain-derived neurotrophic factor |
| 11p15 | APBB1 | FE65, MGC:9072, RIR | amyloid beta (A4) precursor protein-binding, family B, member 1 (Fe65) |
| 11p15.1 | SAA1 | MGC111216, PIG4, SAA, TP53I4 | serum amyloid A1 |
| 11p15.5 | CTSD | CLN10, CPSD, MGC2311 | cathepsin D |
| 11q14 | PICALM | CALM, CLTH, LAP | phosphatidylinositol binding clathrin assembly protein |
| 11q14.1 | GAB2 | KIAA0571 | GRB2-associated binding protein 2 |
| 11q23.2-q23.3 | BACE1 | ASP2, BACE, FLJ90568, HSPC104, KIAA1149 | beta-site APP-cleaving enzyme 1 |
| 11q23.2-q24.2 | SORL1 | C11orf32, FLJ21930, FLJ39258, LR11, LRP9, SORLA, SorLA-1, gp250 | sortilin-related receptor, L(DLR class) A repeats-containing |
| 11q24 | APLP2 | APPH, APPL2, CDEBP | amyloid beta (A4) precursor-like protein 2 |
| 12p11.23-q13.12 | AD5 | Alzheimer disease 5 | |
| 12p12.3-p12.1 | IAPP | AMYLIN, DAP, IAP | islet amyloid polypeptide |
| 12p13.3-p12.3 | A2M | CPAMD5, DKFZp779B086, FWP007, S863-7 | alpha-2-macroglobulin |
| 12q13-q14 | LRP1 | A2MR, APOER, APR, CD91, FLJ16451, IGFBP3R, LRP, MGC88725, TGFBR5 | low density lipoprotein-related protein 1 (alpha-2-macroglobulin receptor) |
| 13q34 | DAOA | G72, LG72, SG72 | D-amino acid oxidase activator |
| 14q24.3 | FOS | AP-1, C-FOS | FBJ murine osteosarcoma viral oncogene homolog |
| 14q24.3 | PSEN1 | AD3, FAD, PS1, S182 | presenilin-1 |
| 14q32 | RAGE | MOK, RAGE1 | renal tumor antigen |
| 14q32.1 | CYP46A1 | CP46, CYP46 | cytochrome P450, family 46, subfamily A, polypeptide 1 |
| 14q32.1 | SERPINA3 | AACT, ACT, GIG24, GIG25, MGC88254 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 |
| 15q21.1 | CYP19A1 | ARO, ARO1, CPV1, CYAR, CYP19, MGC104309, P-450AROM | cytochrome P450, family 19, subfamily A, polypeptide 1 |
| 15q22.2 | APH1B | APH-1B, DKFZp564D0372, FLJ33115, PRO1328, PSFL, TAAV688 | anterior pharynx defective 1 homolog B (C. elegans) |
| 15q11-q12 | APBA2 | D15S1518E, HsT16821, LIN-10, MGC99508, MGC:14091, MINT2, X11L | amyloid beta (A4) precursor protein-binding, family A, member 2 |
| 16p13.3 | UBE2I | C358B7.1, P18, UBC9 | ubiquitin-conjugating enzyme E2I (UBC9 homolog, yeast) |
| 16q21 | CETP | HDLCQ10 | cholesteryl ester transfer protein, plasma |
| 16q22 | NAE1 | A-116A10.1, APPBP1, HPP1, ula-1 | NEDD8 activating enzyme E1 subunit 1 |
| 17p12-p11.2 | COX10 | COX10 homolog, cytochrome c oxidase assembly protein, heme A: farnesyltransferase (yeast) | |
| 17p13 | MYH13 | MyHC-eo | myosin, heavy chain 13, skeletal muscle |
| 17p13.1 | TNK1 | MGC46193 | tyrosine kinase, non-receptor, 1 |
| 17q11.2 | BLMH | BH, BMH | bleomycin hydrolase |
| 17q11.2 | MIR144 | MIRN144 | microRNA 144 |
| 17q21.1 | MAPT | DDPAC, FLJ31424, FTDP-17, MAPTL, MGC138549, MSTD, MTBT1, MTBT2, PPND, TAU | microtubule-associated protein tau |
| 17q21.1 | STH | MAPTIT, MGC163191, MGC163193 | saitohin |
| 17q21.32 | GRN | GEP, GP88, PCDGF, PEPI, PGRN | granulin |
| 17q21-q22 | GPSC | gliosis, familial progressive subcortical | |
| 17q21-q23 | APPBP2 | HS.84084, KIAA0228, PAT1 | amyloid beta precursor protein (cytoplasmic tail) binding protein 2 |
| 17q23.1 | MPO | myeloperoxidase | |
| 17q23.3 | ACE | ACE1, CD143, DCP, DCP1, MGC26566, MVCD3 | angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 |
| 17q24.3 | BPTF | FAC1, FALZ, NURF301 | bromodomain PHD finger transcription factor |
| 18q12.1 | TTR | HsT2651, PALB, TBPA | transthyretin |
| 19p13 | PIN1 | DOD, UBL5 | peptidylprolyl cis/trans isomerase, NIMA-interacting 1 |
| 19p13.2 | AD9 | Alzheimer disease 9 | |
| 19p13.2-p13.1 | NOTCH3 | CADASIL, CASIL | Notch homolog 3 (Drosophila) |
| 19p13.3 | APBA3 | MGC:15815, X11L2, mint3 | amyloid beta (A4) precursor protein-binding, family A, member 3 |
| 19p13.3 | GRIN3B | NR3B | glutamate receptor, ionotropic, N-methyl-D-aspartate 3B |
| 19p13.3-p13.2 | ICAM | BB2, CD54, P3.58 | intercellular adhesion molecule 1 |
| 19q13 | TOMM40 | C19orf1, D19S1177E, PER-EC1, PEREC1, TOM40 | translocase of outer mitochondrial membrane 40 homolog (yeast) |
| 19q13.1 | APLP1 | APLP | amyloid beta (A4) precursor-like protein 1 |
| 19q13.12 | PEN2 | MDS033, MSTP064, PEN-2, PEN2 | presenilin enhancer 2 homolog (C. elegans) |
| 19q13.2 | APOE | AD2, LDLCQ5, LPG, MGC1571 | apolipoprotein E |
| 19q13.2 | APOC1 | apolipoprotein C-I | |
| 19q13.32 | BLOC1S3 | BLOS3, FLJ26641, FLJ26676, HPS8, RP | biogenesis of lysosomal organelles complex-1, subunit 3 |
| 19q13.32 | EXOC3L2 | FLJ36147, MGC16332, XTP7 | exocyst complex component 3-like 2 |
| 19q13.3 | MARK4 | FLJ90097, KIAA1860, MARKL1, Nbla00650 | MAP/microtubule affinity-regulating kinase 4 |
| 19q13.43 | GALP | galanin-like peptide | |
| 20p | AD8 | Alzheimer disease-8 | |
| 20p11.21 | CST3 | ARMD11, MGC117328 | cystatin C |
| 20p13 | PRNP | ASCR, CD230, CJD, GSS, MGC26679, PRIP, PrP, PrP27-30, PrP33-35C, PrPc, prion | prion protein |
| 20q13.31 | PCK1 | MGC22652, PEPCK-C, PEPCK1, PEPCKC | phosphoenolpyruvate carboxykinase 1 (soluble) |
| 21q21.3 | APP | AAA, ABETA, ABPP, AD1, APPI, CTFgamma, CVAP, PN2 | amyloid beta (A4) precursor protein |
| 21q22.3 | BACE2 | AEPLC, ALP56, ASP1, ASP21, BAE2, CDA13, CEAP1, DRAP | beta-site APP-cleaving enzyme 2 |
| 22q11.21 | RTN4R | NGR, NOGOR | reticulon 4 receptor |
| HN | humanin | ||
| 22q11.21 | COMT | catechol-O-methyltransferase |
2. Genomics of Dementia
2.1. Structural Genomics of Alzheimer’s Disease
2.2. Gene Interactions
2.3. Functional Genomics
3. Dementia Phenotype and Biomarkers
4. Therapeutic Strategies in Dementia
5. Pharmacogenomics
5.1. Pharmacogenetics of Psychotropic Drugs
6. CYPs in Dementia
6.1. Association of CYP2D6 Variants with Alzheimer’s Disease-Related Genes
6.2. CYP2D6-Related Biochemical and Hemodynamic Phenotypes in Alzheimer’s Disease
6.3. Influence of CYP2D6 Genotypes on Liver Transaminase Activity
7. CYP2D6-Related Therapeutic Response to a Multifactorial Treatment in Dementia
7.1. CYP Clustering in Alzheimer’s Disease
8. Pharmacogenomics of AD-Related Genes
8.1. APOE-Related Therapeutic Response to a Multifactorial Therapy in Alzheimer’s Disease
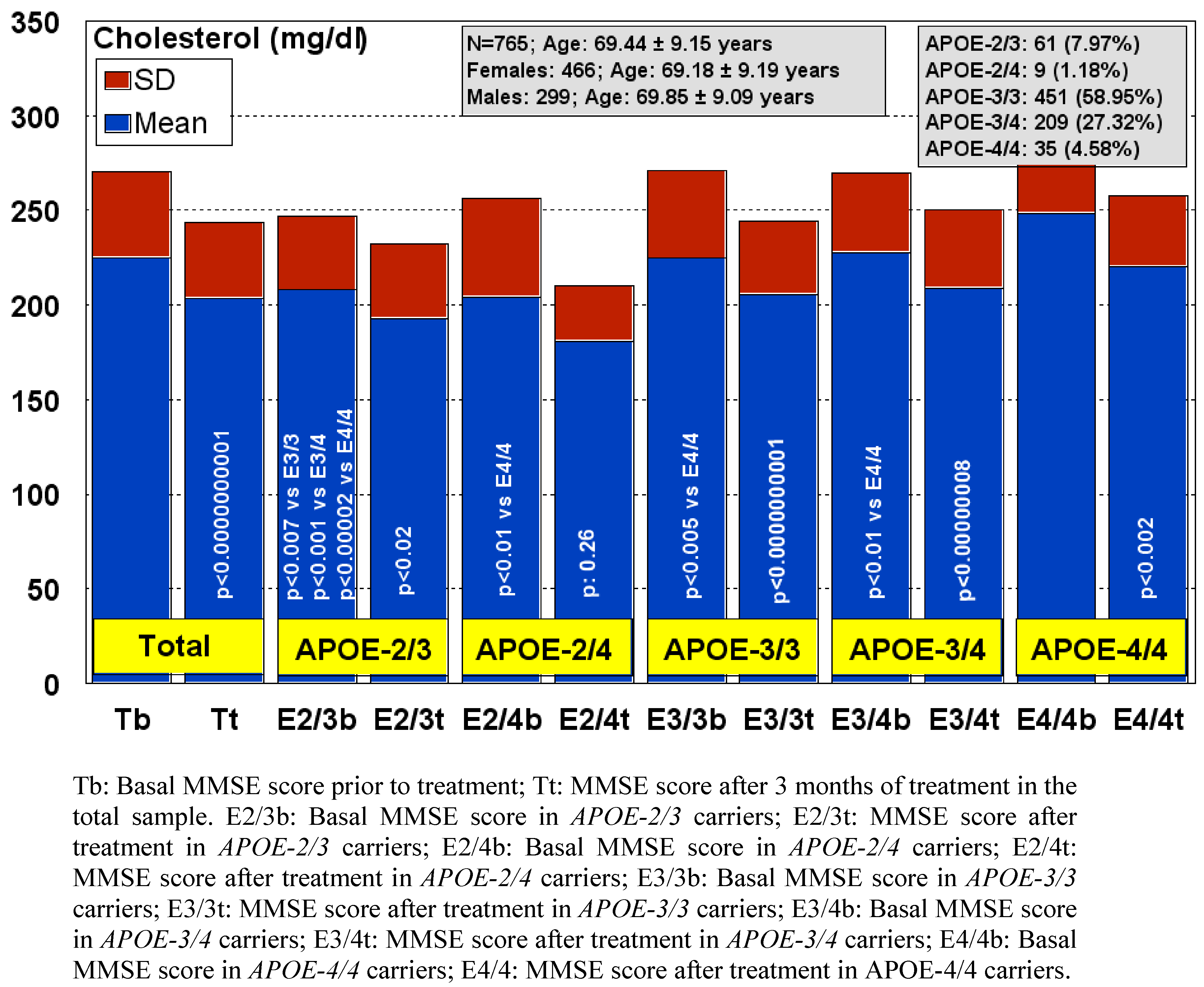
9. Practical Considerations
10. Future Trends
Acknowledgements
References
- Andlin-Sobocki, P.; Jönsson, B.; Wittchen, H.-U.; Olesen, J. Costs of disorders of the brain in Europe. Executive summary. Eur. J. Neurol. 2005, 12 (Suppl. 1), x–xi. [Google Scholar]
- Sousa, R.M.; Ferri, C.P.; Acosta, D.; Albanese, E.; Guerra, M.; Huang, Y.; Jacob, K.S.; Jotheeswaran, A.T.; Rodríguez, J.J.; Pichardo, G.R.; et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet 2009, 374, 1821–1830. [Google Scholar] [PubMed]
- Cacabelos, R. Psychogeriatric research. A conceptual introduction to geriatric neuroscience. Psychogeriatrics 2001, 1, 158–188. [Google Scholar] [CrossRef]
- Loveman, E.; Green, C.; Kirby, J.; Takeda, A.; Picot, J.; Payne, E.; Clegg, A. The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease. Health Technol. Assess. 2006, 10, 1–176. [Google Scholar]
- Cacabelos, R.; Álvarez, A.; Lombardi, V.; Fernández-Novoa, L.; Corzo, L.; Pérez, P.; Laredo, M.; Pichel, V.; Hernández, A.; Varela, M.; et al. Pharmacological treatment of Alzheimer disease: From psychotropic drugs and cholinesterase inhibitors to pharmacogenomics. Drugs Today 2000, 36, 415–499. [Google Scholar] [PubMed]
- Cacabelos, R. Pharmacogenomic biomarkers in neuropsychiatry: The path to personalized medicine in mental disorders. In The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes; Ritsner, M.S., Ed.; Springer: New York, NY, USA, 2009; Volume 4, pp. 3–63. [Google Scholar]
- Cacabelos, R. Pharmacogenomics and therapeutic strategies for dementia. Expert Rev. Mol. Diag. 2009, 9, 567–611. [Google Scholar]
- Cacabelos, R. Molecular genetics of Alzheimer’s disease and aging. Meth. Find. Exper. Clin. Pharmacol. 2005, 27 (Suppl. A), 1–573. [Google Scholar]
- Berry, N.; Jobanputra, V.; Pal, H. Molecular genetics of schizophrenia: a critical review. J Psychiatry Neurosci. 2003, 28, 415–429. [Google Scholar]
- Kato, T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin. Neurosci. 2007, 61, 3–19. [Google Scholar]
- Cacabelos, R. Pharmacogenomics in Alzheimer’s disease. Meth. Mol. Biol. 2008, 448, 213–357. [Google Scholar]
- Cacabelos, R. Pharmacogenomics for the treatment of dementia. Ann. Med. 2002, 34, 357–379. [Google Scholar]
- Cacabelos, R. The application of functional genomics to Alzheimer’s disease. Pharmacogenomics 2003, 4, 597–621. [Google Scholar]
- Cacabelos, R. Pharmacogenomics and therapeutic prospects in Alzheimer’s disease. Exp. Opin. Pharmacother. 2005, 6, 1967–1987. [Google Scholar]
- Cacabelos, R. Pharmacogenomics, nutrigenomics and therapeutic optimization in Alzheimer’s disease. Aging Health 2005, 1, 303–438. [Google Scholar]
- Cacabelos, R.; Takeda, M. Pharmacogenomics, nutrigenomics and future therapeutics in Alzheimer’s disease. Drugs of the Future 2006, 31 (Suppl B), 5–146. [Google Scholar]
- Roses, A.D. Pharmacogenetics and drug development: the path to safer and more effective drugs. Nat. Rev. Genet. 2004, 5, 645–656. [Google Scholar]
- Sharp, A.J.; Cheng, Z.; Eichler, E.E. Structural variation of human genome. Annu. Rev. Genomics Hum. Genet. 2006, 7, 407–442. [Google Scholar]
- Crawford, D.C.; Akey, D.T.; Nickerson, D.A. The patterns of natural variation in human genes. Annu. Rev. Genomics Hum. Genet. 2005, 6, 287–312. [Google Scholar]
- NCBI. Available online: http://www.ncbi.nlm.nih.gov/OMIM/ (accessed on 29 September 2010).
- Alzheimer Research Forum. Available online: http://www.alzgene.org/ (accessed on 29 September 2010).
- National Human Genome Research Institute. HUGO Gene Nomenclature Committee. Available online: http://www.genenames.org (accessed on 29 September 2010).
- Selkoe, D.J.; Podlisny, M.B. Deciphering the genetic basis of Alzheimer’s disease. Annu. Rev. Genomics Hum. Genet. 2002, 3, 67–99. [Google Scholar]
- Suh, Y.-H.; Checler, F. Amyloid precursor protein, presenilins, and α-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Phamacol. Rev. 2002, 54, 469–525. [Google Scholar]
- Cacabelos, R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiat. Dis. Treat. 2007, 3, 303–333. [Google Scholar]
- Strittmatter, W.J.; Saunders, A.M.; Schmechel, D.; Pericak-Vance, M.; Enghild, J.; Salvesen, G.S.; Roses, A.D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allelein late-onset familial Alzheimer disease. Proc. Nat. Acad. Sci. USA 1993, 90, 1977–1981. [Google Scholar]
- Saunders, A.M.; Schmader, K.; Breitner, J.C.; Benson, M.D.; Brown, W.T.; Goldfarb, L.; Goldgaber, D.; Manwaring, M.G.; Szymanski, M.H.; McCown, N.; et al. Apolipoprotein E epsilon-4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet 1993, 342, 710–711. [Google Scholar] [PubMed]
- Saunders, A.M.; Strittmatter, W.J.; Schamechel, D.; St. George-Hyslop, M.D.; Pericak-Vance, M.A.; Joo, S.H.; Rosi, B.L.; Gusella, J.F.; Crapper-MacLachlan, D.R.; Alberts, M.J.; et al. Association of apolipoprotein E allele E4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43, 1467–1472. [Google Scholar] [PubMed]
- Corder, E.H.; Saunders, A.M.; Risch, N.J.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C., Jr.; Rimmler, J.B.; Locke, P.A.; Conneally, P.M.; Schmader, K.E.; et al. Gene dosage of apolipoprotein E type 4 allele and risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar]
- Corder, E.H.; Saunders, A.M.; Risch, N.J.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C., Jr.; Rimmler, J.B.; Locke, P.A.; Conneally, P.M.; Schmader, K.E.; et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature Genet. 1994, 7, 180–184. [Google Scholar]
- Schächter, F.; Faure-Delanef, L.; Guénot, F.; Rouger, H.; Froguel, P.; Lesueur-Ginot, L.; Cohen, D. Genetic association with human longevity at the APOE and ACE loci. Nature Genet. 1994, 6, 29–32. [Google Scholar]
- Roses, A.D. Pharmacogenetics and future drug development and delivery. Lancet 2000, 355, 1358–1361. [Google Scholar]
- Roses, A.D. Pharmacogenetics and the practice of medicine. Nature 2000, 405, 857–865. [Google Scholar]
- Filippini, N.; Ebmeier, K.P.; Macintosh, B.J.; Trachtenberg, A.J.; Frisoni, G.B.; Wilcock, G.K.; Beckmann, C.F.; Smith, S.M.; Matthews, P.M.; Mackay, C.E. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 2010. [Google Scholar]
- Rogaeva, E.; Meng, Y.; Lee, J.H.; Gu, Y.; Kawarai, T.; Zou, F.; Katayama, T.; Baldwin, C.T.; Cheng, R.; Hasegawa, H.; et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer’s disease. Nat. Genet. 2007, 39, 168–177. [Google Scholar] [PubMed]
- Meng, Y.; Lee, J.H.; Cheng, R.; St George-Hyslop, P.; Mayeux, R.; Farrer, L.A. Association between SORL1 and Alzheimer disease in a genome-wide study. Neuroreport 2007, 18, 1761–1764. [Google Scholar]
- Shibata, N.; Ohnuma, T.; Baba, H.; Higashi, S.; Nishioka, K.; Arai, H. Genetic association between SORL1 polymorphisms and Alzheimer’s disease in a Japanese population. Dement. Geriatr. Cogn. Disord. 2008, 26, 161–164. [Google Scholar]
- Rohe, M.; Synowitz, M.; Glass, R.; Paul, S.M.; Nykjaer, A.; Willnow, T.E. Brain-derived neurotrophic factor reduces amyloidogenic processing through control of SORLA gene expression. J. Neurosci. 2009, 29, 15472–15478. [Google Scholar]
- Fehér, A.; Juhász, A.; Rimanóczy, A.; Kálmán, J.; Janka, Z. Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis. Assoc. Disord. 2009, 23, 224–228. [Google Scholar]
- Wang, Y.; Rogers, P.M.; Stayrook, K.R.; Su, C.; Varga, G.; Shen, Q.; Nagpal, S.; Burris, T.P. The selective Alzheimer’s disease indicator-1 gene (Seladin-1/DHCR24) is a liver X receptor target gene. Mol. Pharmacol. 2008, 74, 1716–1721. [Google Scholar]
- Peri, A.; Serio, M. Neuroprotective effects of the Alzheimer’s disease gene seladin-1. J. Mol. Endocrinol. 2008, 41, 251–261. [Google Scholar]
- Sanders, A.E.; Wang, C.; Katz, M.; Derby, C.A.; Barzilai, N.; Ozelius, L.; Lipton, R.B. Association of a functional polymorphism in the cholesteryl ester transfer protein (CETP) gene with memory decline and incidence of dementia. JAMA 2010, 303, 150–158. [Google Scholar]
- Schaffer, B.A.; Bertram, L.; Miller, B.L.; Mullin, K.; Weintraub, S.; Johnson, N.; Bigio, E.H.; Mesulam, M.; Wiedau-Pazos, M.; Jackson, G.R.; et al. Association of GSK3B with Alzheimer’s disease and frontotemporal dementia. Arch. Neurol. 2008, 65, 1368–1374. [Google Scholar] [PubMed]
- Hsu, W.C.; Wang, H.K.; Lee, L.C.; Fung, H.C.; Lin, J.C.; Hsu, H.P.; Wu, Y.R.; Ro, L.S.; Hu, F.J.; Chang, Y.T.; et al. Promoter polymorphisms modulating HSPA5 expression may increase susceptibility to Taiwanese Alzheimer’s disease. J. Neural. Transm. 2008, 115, 1537–1543. [Google Scholar]
- Chen, Y.; Jia, L.; Wei, C.; Wang, F.; Lv, H.; Jia, J. Association between polymorphisms in the apolipoprotein D gene and sporadic Alzheimer’s disease. Brain Res. 2008, 1233, 196–202. [Google Scholar]
- Dreses-Werringloer, U.; Lambert, J.C.; Vingtdeux, V.; Zhao, H.; Vais, H.; Siebert, A.; Jain, A.; Koppel, J.; Rovelet-Lecrux, A.; Hannequin, D.; et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Aβ levels, and Alzheimer’s disease risk. Cell 2008, 133, 1149–1161. [Google Scholar]
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Gerrish, A.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; Dowzell, K.; Williams, A.; et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [PubMed]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar]
- Jun, G.; Naj, A.C.; Beecham, G.W.; Wang, L.S.; Buros, J.; Gallins, P.J.; Buxbaum, J.D.; Ertekin-Taner, N.; Fallin, M.D.; Friedland, R.; et al. Meta-analysis Confirms CR1, CLU, and PICALM as Alzheimer Disease Risk Loci and Reveals Interactions With APOE Genotypes. Arch. Neurol. 2010. [Google Scholar]
- Seshadri, S.; Fitzpatrick, A.L.; Ikram, M.A.; DeStefano, A.L.; Gudnason, V.; Boada, M.; Bis, J.C.; Smith, A.V.; Carassquillo, M.M.; Lambert, J.C.; et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 2010, 303, 1832–1840. [Google Scholar] [PubMed]
- Kramer, P.L.; Xu, H.; Woltjer, R.L.; Westaway, S.K.; Clark, D.; Erten-Lyons, D.; Kaye, J.A.; Welsh-Bohmer, K.A.; Troncoso, J.C.; Markesbery, W.R.; et al. Alzheimer disease pathology in cognitively healthy elderly: A genome-wide study. Neurobiol. Aging. 2010. [Google Scholar]
- Durakoglugil, M.S.; Chen, Y.; White, C.L.; Kavalali, E.T.; Herz, J. Reelin signaling antagonizes beta-amyloid at the synapse. Proc. Natl. Acad. Sci. U.S.A 2009, 106, 15938–15943. [Google Scholar]
- Roses, A.D.; Lutz, M.W.; Amrine-Madsen, H.; Saunders, A.M.; Crenshaw, D.G.; Sundseth, S.S.; Huentelman, M.J.; Welsh-Bohmer, K.A.; Reiman, E.M. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2009. [Google Scholar]
- Lutz, M.W.; Crenshaw, D.G.; Saunders, A.M.; Roses, A.D. Genetic variation at a single locus and age of onset for Alzheimer’s disease. Alzheimers Demen. 2010, 6, 125–131. [Google Scholar]
- Roses, A.D. An inherited variable poly-T repeat genotype in TOMM40 in Alzheimer disease. Arch. Neurol. 2010, 67, 536–541. [Google Scholar]
- Ohe, K.; Mayeda, A. HMGA1a trapping of U1 snRNP at an authentic 5' splice site induces aberrant exon skipping in sporadic Alzheimer’s disease. Mol. Cell. Biol. 2010, 30, 2220–2228. [Google Scholar]
- Kelley, B.J.; Haidar, W.; Boeve, B.F.; Baker, M.; Shiung, M.; Knopman, D.S.; Rademakers, R.; Hutton, M.; Adamson, J.; Kuntz, K.M.; Dickson, D.W.; Parisi, J.E.; Smith, G.E.; Petersen, R.C. Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch. Neurol. 2010, 67, 171–177. [Google Scholar]
- Yu, C.E.; Bird, T.D.; Bekris, L.M.; Montine, T.J.; Leverenz, J.B.; Steinbart, E.; Galloway, N.M.; Feldman, H.; Woltjer, R.; Miller, C.A.; et al. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch. Neurol. 2010, 67, 161–170. [Google Scholar] [PubMed]
- Smach, M.A.; Charfeddine, B.; Othman, L.B.; Lammouchi, T.; Ltaief, A.; Nafati, S.; Dridi, H.; Bennamou, S.; Limem, K. -1154G/A and -2578C/A polymorphisms of the vascular endothelial growth factor gene in Tunisian Alzheimer patients in relation to beta-amyloid (1-42) and total tau protein. Neurosci. Lett. 2010, 472, 139–142. [Google Scholar]
- Jin, Z.; Luxiang, C.; Huadong, Z.; Yanjiang, W.; Zhiqiang, X.; Hongyuan, C.; Lihua, H.; Xu, Y. Endothelin-converting enzyme-1 promoter polymorphisms and susceptibility to sporadic late-onset Alzheimer’s disease in a Chinese population. Dis. Markers 2009, 27, 211–215. [Google Scholar]
- Xue, S.; Jia, L.; Jia, J. Association between somatostatin gene polymorphisms and sporadic Alzheimer’s disease in Chinese population. Neurosci. Lett. 2009, 465, 181–183. [Google Scholar]
- Li, K.; Dai, D.; Zhao, B.; Yao, L.; Yao, S.; Wang, B.; Yang, Z. Association between the RAGE G82S polymorphism and Alzheimer’s disease. J. Neural. Transm. 2010, 117, 97–104. [Google Scholar]
- Takuma, K.; Fang, F.; Zhang, W.; Yan, S.; Fukuzaki, E.; Du, H.; Sosunov, A.; McKhann, G.; Funatsu, Y.; Nakamichi, N.; et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 20021–20026. [Google Scholar]
- Shibata, N.; Ohnuma, T.; Baba, H.; Arai, H. Genetic association analysis between TDP-43 polymorphisms and Alzheimer’s disease in a Japanese population. Dement. Geriatr. Cogn. Disord. 2009, 28, 325–329. [Google Scholar]
- Listì, F.; Caruso, C.; Lio, D.; Colonna-Romano, G.; Chiappelli, M.; Licastro, F.; Candore, G. Role of cyclooxygenase-2 and 5-lipoxygenase polymorphisms in Alzheimer’s disease in a population from northern Italy: implication for pharmacogenomics. J. Alzheimers Dis. 2010, 19, 551–557. [Google Scholar]
- Guerini, F.R.; Tinelli, C.; Calabrese, E.; Agliardi, C.; Zanzottera, M.; de Silvestri, A.; Franceschi, M.; Grimaldi, L.M.; Nemni, R.; Clerici, M. HLA-A*01 is associated with late onset of Alzheimer’s disease in Italian patients. Int. J. Immunopathol. Pharmaco. 2009, 22, 991–999. [Google Scholar]
- Vural, P.; Değirmencioğlu, S.; Parildar-Karpuzoğlu, H.; Doğru-Abbasoğlu, S.; Hanagasi, H.A.; Karadağ, B.; Gürvit, H.; Emre, M.; Uysal, M. The combinations of TNFalpha-308 and IL-6 -174 or IL-10 -1082 genes polymorphisms suggest an association with susceptibility to sporadic late-onset Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 396–401. [Google Scholar]
- Capurso, C.; Solfrizzi, V.; Colacicco, A.M.; D’Introno, A.; Frisardi, V.; Imbimbo, B.P.; Lorusso, M.; Vendemiale, G.; Denitto, M.; Santamato, A.; et al. Interleukin 6-174 G/C promoter and variable number of tandem repeats (VNTR) gene polymorphisms in sporadic Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 177–182. [Google Scholar]
- Yu, J.T.; Song, J.H.; Wang, N.D.; Wu, Z.C.; Zhang, Q.; Zhang, N.; Zhang, W.; Xuan, S.Y.; Tan, L. Implication of IL-33 gene polymorphism in Chinese patients with Alzheimer's disease. Neurobiol. Aging 2010. [Google Scholar]
- Butler, H.T.; Warden, D.R.; Hogervorst, E.; Ragoussis, J.; Smith, A.D.; Lehmann, D.J. Association of the aromatase gene with Alzheimer’s disease in women. Neurosci. Lett. 2010, 468, 202–206. [Google Scholar]
- Vepsäläinen, S.; Helisalmi, S.; Mannermaa, A.; Pirttilä, T.; Soininen, H.; Hiltunen, M. Combined risk effects of IDE and NEP gene variants on Alzheimer disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1268–1270. [Google Scholar]
- Zhong, L.; Dong-hai, Q.; Hong-ying, L.; Qing-feng, L. Analysis of the nicastrin promoter rs10752637 polymorphism and its association with Alzheimer’s disease. Eur. J. Neurosci. 2009, 30, 1831–1836. [Google Scholar]
- Laumet, G.; Petitprez, V.; Sillaire, A.; Ayral, A.M.; Hansmannel, F.; Chapuis, J.; Hannequin, D.; Pasquier, F.; Scarpini, E.; Galimberti, D.; et al. A study of the association between the ADAM12 and SH3PXD2A (SH3MD1) genes and Alzheimer’s disease. Neurosci. Lett. 2010, 468, 1–2. [Google Scholar]
- Leduc, V.; Théroux, L.; Dea, D.; Robitaille, Y.; Poirier, J. Involvement of paraoxonase 1 genetic variants in Alzheimer’s disease neuropathology. Eur. J. Neurosci. 2009, 30, 1823–1830. [Google Scholar]
- Liu, H.P.; Lin, W.Y.; Liu, S.H.; Wang, W.F.; Tsai, C.H.; Wu, B.T.; Wang, C.K.; Tsai, F.J. Genetic variation in N-methyl-D-aspartate receptor subunit NR3A but not NR3B influences susceptibility to Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009, 28, 521–527. [Google Scholar]
- di Maria, E.; Bonvicini, C.; Bonomini, C.; Alberici, A.; Zanetti, O.; Gennarelli, M. Genetic variation in the G720/G30 gene locus (DAOA) influences the occurrence of psychotic symptoms in patients with Alzheimer’s disease. J. Alzheimers Dis. 2009, 18, 953–960. [Google Scholar]
- Martínez, M.F.; Martín, X.E.; Alcelay, L.G.; Flores, J.C.; Valiente, J.M.; Juanbeltz, B.I.; Beldarraín, M.A.; López, J.M.; Gonzalez-Fernández, M.C.; Salazar, A.M.; et al. The COMT Val158 Met polymorphism as an associated risk factor for Alzheimer disease and mild cognitive impairment in APOE 4 carriers. BMC Neurosc. 2009, 10, 125. [Google Scholar] [CrossRef]
- Maruszak, A.; Safranow, K.; Gustaw, K.; Kijanowska-Haładyna, B.; Jakubowska, K.; Olszewska, M.; Styczyńska, M.; Berdyński, M.; Tysarowski, A.; Chlubek, D.; et al. PIN1 gene variants in Alzheimer’s disease. BMC Med. Genet. 2009, 10, 115. [Google Scholar]
- Kellett, K.A.; Hooper, N.M. Prion protein and Alzheimer disease. Prion 2009, 3, 190–194. [Google Scholar]
- Lloyd, S.E.; Rossor, M.; Fox, N.; Mead, S.; Collinge, J. HECTD2, a candidate susceptibility gene for Alzheimer’s disease on 10q. BMC Med. Genet. 2009, 10, 90. [Google Scholar]
- Ahn, K.; Song, J.H.; Kim, D.K.; Park, M.H.; Jo, S.A.; Koh, Y.H. Ubc9 gene polymorphisms and late-onset Alzheimer’s disease in the Korean population: a genetic association study. Neurosci. Lett. 2009, 465, 272–275. [Google Scholar]
- Persengiev, S.; Kondova, I.; Otting, N.; Koeppen, A.H.; Bontrop, R.E. Genome-wide analysis of miRNA expression reveals a potential role for miR-144 in brain aging and spinocerebellar ataxia pathogenesis. Neurobiol. Aging 2010. [Google Scholar]
- Bettens, K.; Brouwers, N.; van Miegroet, H.; Gil, A.; Engelborghs, S.; de Deyn, P.P.; Vandenberghe, R.; van Broeckhoven, C.; Sleegers, K. Follow-up study of susceptibility Loci for Alzheimer’s disease and onset age identified by genome-wide association. J. Alzheimers Dis. 2010, 19, 1169–1175. [Google Scholar]
- Donkin, J.J.; Stukas, S.; Hirsch-Reinshagen, V.; Namjoshi, D.; Wilkinson, A.; May, S.; Chan, J.; Fan, J.; Collins, J.; Wellington, C.L. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver-X-receptor agonist GW3965 on object recognition memory and amyloid burden in APP/PS1 mice. J. Biol. Chem. 2010. [Google Scholar]
- Kuerban, B.; Shibata, N.; Komatsu, M.; Ohnuma, T.; Arai, H. Genetic Association between PLTP Gene Polymorphisms and Alzheimer's Disease in a Japanese Population. Dement. Geriatr. Cogn. Disord. 2010, 30, 78–82. [Google Scholar]
- Bertram, L.; Tanzi, R.E. Genome-wide association studies in Alzheimer’s disease. Hum. Mol. Genet. 2009, 18, 137–145. [Google Scholar]
- Lin, M.T.; Simon, D.K.; Ahn, C.H.; Kim, L.M.; Beal, M.F. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum. Mol. Genet. 2002, 11, 133–145. [Google Scholar]
- Pinho, C.M.; Björk, B.F.; Alikhani, N.; Bäckman, H.G.; Eneqvist, T.; Fratiglioni, L.; Glaser, E.; Graff, C. Genetic and biochemical studies of SNPs of the mitochondrial A beta-degrading protease, hPreP. Neurosci. Lett. 2010, 469, 204–208. [Google Scholar]
- Vitali, M.; Venturelli, E.; Galimberti, D.; Benerini Gatta, L.; Scarpini, E.; Finazzi, D. Analysis of the genes coding for subunit 10 and 15 of cytochrome c oxidase in Alzheimer’s disease. J. Neural. Transm. 2009, 116, 1635–1641. [Google Scholar]
- Takasaki, S. Mitochondrial haplogroups associated with Japanese Alzheimer’s patients. J. Bioenerg. Biomembr. 2009, 41, 407–410. [Google Scholar]
- Santoro, A.; Balbi, V.; Balducci, E.; Pirazzini, C.; Rosini, F.; Tavano, F.; Achilli, A.; Siviero, P.; Minicuci, N.; Bellavista, E.; et al. Evidence for sub-haplogroup h5 of mitochondrial DNA as a risk factor for late onset Alzheimer's disease. PLoS One 2010, 5, e12037. [Google Scholar]
- Zhang, M.; Poplawski, M.; Yen, K.; Cheng, H.; Bloss, E.; Zhu, X.; Patel, H.; Mobbs, C.V. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol. 2009, 7, e1000245. [Google Scholar]
- Lukens, J.N.; van Deerlin, V.; Clark, C.M.; Xie, S.X.; Johnson, F.B. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimers Dement. 2009, 5, 463–469. [Google Scholar]
- Zekry, D.; Herrmann, F.R.; Irminger-Finger, I.; Graf, C.; Genet, C.; Vitale, A.M.; Michel, J.P.; Gold, G.; Krause, K.H. Telomere length and ApoE polymorphism in mild cognitive impairment, degenerative and vascular dementia. J. Neurol. Sci. 2010. [Google Scholar]
- Anderson, C.N.G.; Grant, S.G.N. High throughput protein expression screening in the nervous system-needs and limitations. J. Physiol. 2006, 575.2, 367–372. [Google Scholar]
- Xu, X.; Zhan, M.; Duan, W.; Prabhu, V.; Brenneman, R.; Wood, W.; Firman, J.; Li, H.; Zhang, P.; Ibe, C.; et al. Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol. 2007, 8, 234. [Google Scholar]
- Mastrangelo, M.A.; Bowers, W.J. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008, 9, 81. [Google Scholar]
- Rodríguez, J.J.; Jones, V.C.; Tabuchi, M.; Allan, S.M.; Knight, E.M.; LaFerla, F.M.; Oddo, S.; Verkhratsky, A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One 2008, 3, e2935. [Google Scholar]
- Cacabelos, R. Pharmacogenomics and therapeutic prospect in dementia. Eur Arch Psychiatry Clin. Neurosci. 2008, 258 (Suppl. 1), 28–47. [Google Scholar]
- Cacabelos, R. Pharmacogenetic basis for therapeutic optimization in Alzheimer’s disease. Mol. Diag. Ther. 2007, 11, 385–405. [Google Scholar]
- Cacabelos, R.; Llovo, R.; Fraile, C.; Fernández-Novoa, L. Pharmacogenetic aspects of therapy with cholinesterase inhibitors: the role of CYP2D6 in Alzheimer’s disease pharmacogenetics. Curr. Alzheimer Res. 2007, 4, 479–500. [Google Scholar]
- Cacabelos, R. Molecular pathology and pharmacogenomics in Alzheimer’s disease: polygenic-related effects of multifactorial treatments on cognition, anxiety, and depression. Meth. Find. Exper. Clin. Pharmacol. 2007, 29 (Suppl. B), 1–91. [Google Scholar]
- Cacabelos, R.; Fernández-Novoa, L.; Pichel, V.; Lombardi, V.; Kubota, Y.; Takeda, M. Pharmacogenomic studies with a combination therapy in Alzheimer’s disease. In Molecular Neurobiology of Alzheimer Disease and Related Disorders; Takeda, M., Tanaka, T., Cacabelos, R., Eds.; Karger: Basel, Switzerland, 2004; pp. 94–107. [Google Scholar]
- Cacabelos, R. Pharmacogenomics in Alzheimer’s disease. In Pharmacogenomics and Personalized Medicine; Cohen, N., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 317–368. [Google Scholar]
- Thomann, P.A.; Roth, A.S.; Dos Santos, V.; Toro, P.; Essig, M.; Schröder, J. Apolipoprotein E polymorphism and brain morphology in mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2008, 26, 300–305. [Google Scholar]
- Sando, S.B.; Melquist, S.; Cannon, A.; Hutton, M.L.; Sletvold, O.; Saltvedt, I.; White, L.R.; Lydersen, S.; Aasly, J.O. APOEε4 lowers age at onset and is a high risk factor for Alzheimer’s disease; A case control study from central Norway. BMC Neurology 2008, 8, 9. [Google Scholar]
- Thambisetty, M.; Beason-Held, L.; An, Y.; Kraut, M.A.; Resnick, S.M. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch. Neurol. 2010, 67, 93–98. [Google Scholar]
- Cacabelos, R.; Fernández-Novoa, L.; Corzo, L.; Pichel, V.; Lombardi, V.; Kubota, Y. Genomics and phenotypic profiles in dementia: Implications for pharmacological treatment. Meth. Find. Exper. Clin. Pharmacol. 2004, 26, 421–444. [Google Scholar]
- Cacabelos, R.; Fernández-Novoa, L.; Lombardi, V.; Corzo, L.; Pichel, V.; Kubota, Y. Cerebrovascular risk factors in Alzheimer’s disease: Brain hemodynamics and pharmacogenomic implications. Neurol. Res. 2003, 25, 567–580. [Google Scholar]
- Roher, A.E.; Maarouf, C.L.; Sue, L.I.; Hu, Y.; Wilson, J.; Beach, T.G. Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer’s disease. Biomarkers 2009, 14, 493–501. [Google Scholar]
- Giacobini, E. Cholinesterases in human brain: the effect of cholinesterase inhibitors on Alzheimer’s disease and related disorders. In The Brain Cholinergic System in Health and Disease; Giacobini, E., Pepeu, G., Eds.; Informa Healthcare: Oxon, UK, 2006; pp. 235–264. [Google Scholar]
- Reisberg, B.; Doody, R.; Stoffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar]
- Schenk, D.B.; Seubert, P.; Grundman, M.; Black, R. Aβ immunotherapy: lessons learned for potential treatment of Alzheimer’s disease. Neurodegener. Dis. 2005, 2, 255–260. [Google Scholar]
- Wisniewski, T.; Boutajangout, A. Vaccination as a therapeutic approach to Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 17–31. [Google Scholar]
- de Strooper, B.; Vassar, R.; Golde, T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar]
- Shelton, C.C.; Zhu, L.; Chau, D.; Yang, L.; Wang, R.; Djaballah, H.; Zheng, H.; Li, Y.M. Modulation of gamma-secretase specificity using small molecule allosteric inhibitors. Proc. Natl. Acad. Sci. USA 2009, 106, 20228–20233. [Google Scholar]
- Lambracht-Washington, D.; Qu, B.X.; Fu, M.; Eagar, T.N.; Stüve, O.; Rosenberg, R.N. DNA beta-amyloid(1-42) trimer immunization for Alzheimer disease in a wild-type mouse model. JAMA 2009, 302, 1796–1802. [Google Scholar]
- Lang, F.; Görlach, A. Heterocyclic indazole derivatives as SGK1 inhibitors, WO2008138448. Expert Opin. Ther. Pat. 2010, 20, 129–135. [Google Scholar]
- Sala Frigerio, C.; Kukar, T.L.; Fauq, A.; Engel, P.C.; Golde, T.E.; Walsh, D.M. An NSAID-like compound, FT-9, preferentially inhibits gamma-secretase cleavage of the amyloid precursor protein compared to its effect on amyloid precursor-like protein 1. Biochemistry 2009, 48, 10894–10904. [Google Scholar] [PubMed]
- Boado, R.J.; Lu, J.Z.; Hui, E.K.; Pardridge, W.M. IgG-single chain Fv fusion protein therapeutic for Alzheimer’s disease: Expression in CHO cells and pharmacokinetics and brain delivery in the rhesus monkey. Biotechnol. Bioeng. 2010, 105, 627–635. [Google Scholar]
- Adachi, H.; Katsuno, M.; Waza, M.; Minamiyama, M.; Tanaka, F.; Sobue, G. Heat shock proteins in neurodegenerative diseases: pathogenic roles and therapeutic implications. Int. J. Hyperthermia 2009, 25, 647–654. [Google Scholar]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar]
- Kalinin, S.; Richardson, J.C.; Feinstein, D.L. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 431–437. [Google Scholar]
- Roshan, R.; Ghosh, T.; Scaria, V.; Pillai, B. MicroRNAs: novel therapeutic targets in neurodegenerative diseases. Drug Discov. Today 2009, 14, 1123–1129. [Google Scholar]
- Maxwell, M.M. RNAi applications in therapy development for neurodegenerative disease. Curr. Pharm. Des. 2009, 15, 3977–3991. [Google Scholar]
- Verrils, N.M. Clinical proteomics: Present and future prospects. Clin. Biochem. Rev. 2006, 27, 99–116. [Google Scholar] [PubMed]
- Doshi, J.A.; Shaffer, T.; Briesacher, B.A. National estimates of medication use in nursing homes: Findings from 1997 Medicare Current Beneficiary Survey and the 1996 Medical Expenditure Survey. J. Am. Geriatr. Soc. 2005, 53, 438–443. [Google Scholar]
- Percudani, M.; Barbui, C.; Fortino, I.; Petrovich, L. Antidepressant drugs prescribing among elderly subjects: a population-based study. Int. J. Geriat. Psychiat. 2005, 20, 113–118. [Google Scholar]
- Fialová, D.; Topinková, E.; Gambassi, G.; Finne-Soveri, H.; Jónsson, P.V.; Carpenter, I.; Schroll, M.; Onder, G.; Sørbye, L.W.; Wagner, C.; et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA 2005, 293, 1348–1358. [Google Scholar] [PubMed]
- Simon, S.R.; Chan, K.A.; Soumerai, S.B.; Wagner, A.K.; Andrade, S.E.; Feldstein, A.C.; Lafata, J.E.; Davis, R.L.; Gurwitz, J.H. Potentially inappropriate medication use by elderly persons in U.S. Health Maintenance Organizations, 2000-2001. J. Am. Geriatr. Soc. 2005, 53, 227–232. [Google Scholar] [PubMed]
- Weinshilboum, R. Inheritance and drug response. N. Engl. J. Med. 2003, 348, 529–537. [Google Scholar]
- Zhou, S.F.; Di, Y.M.; Chan, E.; Du, Y.M.; Chow, V.D.; Xue, C.C.; Lai, X.; Wang, J.C.; Li, C.G.; Tian, M.; Duan, W. Clinical pharmacogenetics and potential application in personalized medicine. Curr. Drug Metab. 2008, 9, 738–784. [Google Scholar]
- Malhotra, A.K.; Lencz, T.; Correll, C.U.; Kane, J.M. Genomics and the future of pharmacotherapy in psychiatry. Int. Rev. Psychiatry 2007, 19, 523–530. [Google Scholar]
- Nnadi, C.U.; Malhorta, A.K. Individualizing antipsychotic drug therapy in schizophrenia: the promise of pharmacogenetics. Curr. Psychiatry Rep. 2007, 9, 313–318. [Google Scholar]
- Foster, A.; Miller, D.; Buckley, P.F. Pharmacogenetics and schizophrenia. Psychiatr. Clin. North Am. 2007, 30, 417–435. [Google Scholar]
- Arranz, M.J.; de Leon, J. Pharmacogenetics and pharmacogenomics of schizophrenia: A review of last decade of research. Mol. Psychiatry 2007, 12, 707–747. [Google Scholar]
- Mizutani, T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab. Rev. 2003, 35, 99–106. [Google Scholar]
- Xie, H.G.; Prasad, H.G.; Kim, R.B.; Stein, C.M. CYP2C9 allelic variants: ethnic ditribution and functional significance. Adv. Drug Deliv. Rev. 2002, 54, 1257–1270. [Google Scholar]
- Isaza, C.A.; Henao, J.; López, A.M.; Cacabelos, R. Isolation, sequence and genotyping of the drug metabolizer CYP2D6 gene in the Colombian population. Meth. Find. Exp. Clin. Pharmacol. 2000, 22, 695–705. [Google Scholar]
- Ozawa, S.; Soyama, A.; Saeki, M.; Fukushima-Uesaka, H.; Itoda, M.; Koyano, S.; Sai, K.; Ohno, Y.; Saito, Y.; Sawada, J. Ethnic differences in genetic polymorphisms of CYP2D6, CYP2C19, CYP3As and MDR1/ABCB1. Drug Metab. Pharmacokin. 2004, 19, 83–95. [Google Scholar]
- Xie, H.G.; Kim, R.B.; Wood, A.J.; Stein, C.M. Molecular basis of ethnic differences in drug disposition and response. Annu. Rev. Pharm. Toxicol. 2001, 41, 815–850. [Google Scholar]
- Madan, A.; Graham, R.A.; Carroll, K.M.; Mudra, D.R.; Burton, L.A.; Krueger, L.A.; Downey, A.D.; Czerwinski, M.; Forster, J.; Ribadeneira, M.D.; et al. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab. Dispos. 2003, 31, 421–431. [Google Scholar]
- Hedlund, E.; Gustafsson, J.A.; Warner, M. Cytochrome P450 in the brain: a review. Curr. Drug Metabol. 2001, 2, 245–263. [Google Scholar]
- Funae, Y.; Kishimoto, W.; Cho, T.; Niwa, T.; Hiroi, T. CYP2D in the brain. Drug Metab. Pharmacokin. 2003, 18, 337–349. [Google Scholar]
- Scordo, M.G.; Spina, E. Cytochrome P450 polymorphisms and response to antipsychotic therapy. Pharmacogenomics 2002, 3, 201–218. [Google Scholar]
- Ingelman-Sundberg, M.; Sim, S.C.; Gomez, A.; Rodríguez-Antona, C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 2007, 116, 496–526. [Google Scholar]
- Kobylecki, C.J.; Jakobsen, K.D.; Hansen, T.; Jakobsen, I.V.; Rasmussen, H.B.; Werge, T. CYP2D6 genotype predicts antipsychotic side effects in schizophrenia inpatients: a retrospective matched case-control study. Neuropsychobiology 2009, 59, 222–226. [Google Scholar]
- Kang, R.H.; Jung, S.M.; Kim, K.A.; Lee, D.K.; Cho, H.K.; Jung, B.J.; Kim, Y.K.; Kim, S.H.; Han, C.; Lee, M.S.; Park, J.Y. Effects of CYP2D6 and CYP3A5 genotypes on the plasma concentrations of risperidone and 9-hydroxyrisperidone in Korean schizophrenic patients. J. Clin. Psychopharmacol. 2009, 29, 272–277. [Google Scholar]
- Yagihashi, T.; Mizuno, M.; Chino, B.; Sato, Y.; Sakuma, K.; Takebayashi, T.; Takao, T.; Kosaki, K. Effects of the CYP2D6*10 alleles and co-medication with CYP2D6-dependent drugs on risperidone metabolism in patients with schizophrenia. Hum. Psychopharmacol. 2009, 24, 301–308. [Google Scholar]
- Fleeman, N.; McLeod, C.; Bagust, A.; Beale, S.; Boland, A.; Dundar, Y.; Jorgensen, A.; Payne, K.; Pirmohamed, M.; Pushpakom, S.; et al. The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation. Health Technol. Assess. 2010, 14, 1–182. [Google Scholar]
- Buchanan, R.W.; Freedman, R.; Javitt, D.C.; Abi-Dargham, A.; Lieberman, J.A. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr. Bull. 2007, 33, 1120–1130. [Google Scholar]
- Tamminga, C.A. The neurobiology of cognition in schizophrenia. J. Clin. Psychiatry 2006, 67, e11. [Google Scholar]
- Malhotra, A.K.; Murphy, G.M., Jr.; Kennedy, J.L. Pharmacogenetics of psychotropic drug response. Am. J. Psychiatry 2004, 161, 780–796. [Google Scholar]
- de León, J. The future (or lack of future) of personalized prescription in psychiatry. Pharmacol. Res. 2009, 59, 81–89. [Google Scholar]
- Need, A.C.; Keefe, R.S.; Ge, D.; Grossman, I.; Dickson, S.; McEvoy, J.P.; Goldstein, D.B. Pharmacogenetics of antipsychotic response in the CATIE trial: a candidate gene analysis. Eur. J. Hum. Genet. 2009, 17, 946–957. [Google Scholar]
- Ikeda, M.; Tomita, Y.; Mouri, A.; Koga, M.; Okochi, T.; Yoshimura, R.; Yamanouchi, Y.; Kinoshita, Y.; Hashimoto, R.; Williams, H.J.; et al. Identification of Novel Candidate Genes for Treatment Response to Risperidone and Susceptibility for Schizophrenia: Integrated Analysis Among Pharmacogenomics, Mouse Expression, and Genetic Case-Control Association Approaches. Biol. Psychiatry 2010, 67, 263–269. [Google Scholar]
- Iwahashi, K.; Murayama, O.; Aoki, J.; Watanabe, M.; Ishigouoka, J. Influence of serotonin (5-HT) 2A-receptor and transporter (5HTT) gene polymorphism upon the effect of olanzapine. Nihon Shinkei Seishin Yakurigaku Zasshi 2009, 29, 141–144. [Google Scholar]
- Wei, Z.; Wang, L.; Xuan, J.; Che, R.; Du, J.; Qin, S.; Xing, Y.; Gu, B.; Yang, L.; Li, H.; et al. Association analysis of serotonin receptor 7 gene (HTR7) and risperidone response in Chinese schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 547–551. [Google Scholar]
- Chen, S.F.; Shen, Y.C.; Chen, C.H. Effects of the DRD3 Ser9Gly polymorphism on aripiprazole efficacy in schizophrenic patients as modified by clinical factors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 470–474. [Google Scholar]
- Chen, S.F.; Shen, Y.C.; Chen, C.H. HTR2A A-1438G/T102C polymorphisms predict negative symptoms performance upon aripiprazole treatment in schizophrenic patients. Psychopharmacology (Berlin) 2009, 205, 285–292. [Google Scholar]
- Park, S.W.; Lee, J.G.; Ha, E.K.; Choi, S.M.; Cho, H.Y.; Seo, M.K.; Kim, Y.H. Differential effects of aripiprazole and haloperidol on BDNF-mediated signal changes in SH-SY5Y cells. Eur. Neuropsychopharmacol. 2009, 19, 356–362. [Google Scholar]
- Zuo, L.; Luo, X.; Krystal, J.H.; Cramer, J.; Charney, D.S.; Gelernter, J. The efficacies of clozapine and haloperidol in refractory schizophrenia are related to DTNBP1 variation. Pharmacogenet. Genomics 2009, 19, 437–446. [Google Scholar]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats. Schizophr. Res. 2009, 111, 138–152. [Google Scholar]
- Guidotti, A.; Dong, E.; Kundakovic, M.; Satta, R.; Grayson, D.R.; Costa, E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol. Sci. 2009, 30, 55–60. [Google Scholar]
- Cashman, J.R.; Zhang, J.; Nelson, M.R.; Braun, A. Analysis of flavin-containing monooxygenase 3 genotype data in populations administered the anti-schizophrenia agent olanzapine. Drug Metab. Lett. 2008, 2, 100–114. [Google Scholar]
- Gupta, M.; Bhatnagar, P.; Grover, S.; Kaur, H.; Baghel, R.; Bhasin, Y.; Chauhan, C.; Verma, B.; Manduva, V.; Mukherjee, O.; et al. Association studies of catechol-O-methyltransferase (COMT) gene with schizophrenia and response to antipsychotic treatment. Pharmacogenomics 2009, 10, 385–397. [Google Scholar]
- Herken, H.; Erdal, M.; Aydin, N.; Sengul, C.; Karadag, F.; Barlas, O.; Akin, F. The association of olanzapine-induced weight gain with peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism in patients with schizophrenia. DNA Cell Biol. 2009, 28, 515–519. [Google Scholar]
- Ujike, H.; Nomura, A.; Morita, Y.; Morio, A.; Okahisa, Y.; Kotaka, T.; Kodama, M.; Ishihara, T.; Kuroda, S. Multiple genetic factors in olanzapine-induced weight gain in schizophrenia patients: a cohort study. J. Clin. Psychiatry 2008, 69, 1416–1422. [Google Scholar]
- Basile, V.S.; Masellis, M.; McIntyre, R.S.; Meltzer, H.Y.; Lieberman, J.A.; Kennedy, J.L. Genetic dissection of atypical antipsychotic-induced weight gain: novel preliminary data on the pharmacogenetic puzzle. J. Clin. Psychiatry 2001, 62 (Suppl 23), 45–66. [Google Scholar]
- Gunes, A.; Melkersson, K.I.; Scordo, M.G.; Dahl, M.L. Association between HTR2C and HTR2A polymorphisms and metabolic abnormalities in patients treated with olanzapine or clozapine. J. Clin. Psychopharmacol. 2009, 29, 65–68. [Google Scholar]
- Gregoor, J.G.; van der Weide, J.; Mulder, H.; Cohen, D.; van Megen, H.J.; Egberts, A.C.; Heerdink, E.R. Polymorphisms of the LEP- and LEPR gene and obesity in patients using antipsychotic medication. J. Clin. Psychopharmacol. 2009, 29, 21–25. [Google Scholar]
- Mulder, H.; Cohen, D.; Scheffer, H.; Gispen-de Wied, C.; Arends, J.; Wilmink, F.W.; Franke, B.; Egberts, A.C. HTR2C gene polymorphisms and the metabolic syndrome in patients with schizophrenia: a replication study. J. Clin. Psychopharmacol. 2009, 29, 16–20. [Google Scholar]
- Kwon, J.S.; Joo, Y.H.; Nam, H.J.; Lim, M.; Cho, E.Y.; Jung, M.H.; Choi, J.S.; Kim, B.; Kang, D.H.; Oh, S.; et al. Association of the glutamate transporter gene SLC1A1 with atypical antipsychotics-induced obsessive-compulsive symptoms. Arch. Gen. Psychiatry 2009, 66, 1233–1241. [Google Scholar]
- Volpi, S.; Potkin, S.G.; Malhotra, A.K.; Licamele, L.; Lavedan, C. Applicability of a genetic signature for enhanced iloperidone efficacy in the treatment of schizophrenia. J. Clin. Psychiatry 2009, 70, 801–809. [Google Scholar]
- Choi, K.H.; Higgs, B.W.; Weis, S.; Song, J.; Llenos, I.C.; Dulay, J.R.; Yolken, R.H.; Webster, M.J. Effects of typical and atypical antipsychotic drugs on gene expression profiles in the liver of schizophrenia subjects. BMC Psychiatry 2009, 9, 57. [Google Scholar]
- Chana, G.; Lucero, G.; Salaria, S.; Lozach, J.; Du, P.; Woelk, C.; Everall, I. Upregulation of NRG-1 and VAMP-1 in human brain aggregates exposed to clozapine. Schizophr. Res. 2009, 113, 273–276. [Google Scholar]
- Ji, B.; La, Y.; Gao, L.; Zhu, H.; Tian, N.; Zhang, M.; Yang, Y.; Zhao, X.; Tang, R.; Ma, G.; et al. A comparative proteomics analysis of rat mitochondria from the cerebral cortex and hippocampus in response to antipsychotic medications. J. Proteome Res. 2009, 8, 3633–3641. [Google Scholar]
- Ma, D.; Chan, M.K.; Lockstone, H.E.; Pietsch, S.R.; Jones, D.N.; Cilia, J.; Hill, M.D.; Robbins, M.J.; Benzel, I.M.; Umrania, Y.; et al. Antipsychotic treatment alters protein expression associated with presynaptic function and nervous system development in rat frontal cortex. J. Proteome Res. 2009, 8, 3284–3297. [Google Scholar] [PubMed]
- Dell’Aversano, C.; Tomasetti, C.; Iasevoli, F.; de Bartolomeis, A. Antipsychotic and antidepressant co-treatment: effects on transcripts of inducible postsynaptic density genes possibly implicated in behavioural disorders. Brain Res. Bull. 2009, 79, 123–129. [Google Scholar]
- World Guide for Drug Use and Pharmacogenomics; Cacabelos, R. (Ed.) EuroEspes Publishing: Coruña, Spain, 2010; In Press.
- Home Page of the Human Cytochrome P450 (CYP) Allele Nomenclature Committee. Available online: http://www.cypalleles.ki.se (accessed on 29 September 2010).
- PharmGKB. Available online: http://www.pharmgkb.org (accessed on 29 September 2010).
- Sachse, C.; Brockmoller, J.; Bauer, S.; Roots, I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am. J. Hum. Genet. 1997, 60, 284–295. [Google Scholar]
- Weinshilboum, R.M.; Wang, L. Pharmacogenetics and pharmacogenomics: Development, science, and translation. Annu. Rev. Genomics Hum. Genet. 2006, 7, 223–245. [Google Scholar]
- Pilotto, A.; Franceschi, M.; D’Onofrio, G.; Bizzarro, A.; Mangialasche, F.; Cascavilla, L.; Paris, F.; Matera, M.G.; Pilotto, A.; Daniele, A.; et al. Effect of a CYP2D6 polymorphism on the efficacy of donepezil in patients with Alzheimer disease. Neurology 2009, 73, 761–767. [Google Scholar]
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K.E. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar]
- Mannens, G.S.; Snel, C.A.; Hendrickx, J.; Verhaeghe, T.; Le Jeune, L.; Bode, W.; van Beijsterveldt, L.; Lavrijsen, K.; Leempoels, J.; van Osselaer, N.; et al. The metabolism and excretion of galantamine in rats, dogs, and humans. Drug Metab. Dispos. 2002, 30, 553–563. [Google Scholar]
- Egger, S.S.; Bachmann, A.; Hubmann, N.; Schlienger, R.G.; Krähenbühl, S. Prevalence of potentially inappropriate medication use in elderly patients. Comparison between general medicine and geriatric wards. Drugs Aging 2006, 23, 823–837. [Google Scholar]
- Roses, A.D. The medical and economic roles of pipeline pharmacogenetics: Alzheimer’s disease as a model of efficacy and HLA-B(*)5701 as a model of safety. Neuropsychopharmacology 2009, 34, 6–17. [Google Scholar]
- Roses, A.D.; Saunders, A.M.; Huang, Y.; Strum, J.; Weisgraber, K.H.; Mahley, R.W. Complex disease-associated pharmacogenetics: drug efficacy, drug safety, and confirmation of a pathogenic hypothesis (Alzheimer’s disease). Pharmacogenomics J. 2007, 7, 10–28. [Google Scholar]
- Risner, M.E.; Saunders, A.M.; Altman, J.F.; Ormandy, G.C.; Craft, S.; Foley, I.M.; Zvartau-Hind, M.E.; Hosford, D.A.; Roses, A.D. Rosiglitazone in Alzheimer’s Disease Study Group. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006, 6, 246–254. [Google Scholar] [PubMed]
- Lombardi, V.R.M.; Cacabelos, R. E-SAR-94010: a marine fish extract obtained by advanced biotechnological methods. Drugs Future 1999, 24, 167–176. [Google Scholar]
- Cacabelos, R.; Vallejo, A.I.; Lombardi, V.; Fernández-Novoa, L.; Pichel, V. E-SAR-94010 (LipoEsar®): A pleiotropic lipoprotein compound with powerful anti-atheromatous and lipid lowering effects. CNS Drug Rev. 2004, 10, 200–201. [Google Scholar]
- Cacabelos, R. Pharmacogenomics and Nutraceuticals. Scientific Progress and Pharmaceutical Development; Ebiotec Foundation: Coruña, Spain, 2004. [Google Scholar]
- Philips, K.A.; van Bebber, S.L. Measuring the value of pharmacogenomics. Nat. Rev. Drug Discovery 2005, 4, 500–509. [Google Scholar]
- Veenstra, D.L.; Higashi, M.K. Assessing the cost-effectiveness of pharmacogenomics. AAPS PharmSci. 2000, 2, 80–90. [Google Scholar]
- Sink, K.M.; Holden, K.F.; Yaffe, K. Pharmacological treatment of neuropsychiatric symptoms of dementia. A review of the evidence. JAMA 2005, 293, 596–608. [Google Scholar]
- van Steen, K.; McQueen, M.B.; Herbert, A.; Raby, B.; Lyon, H.; Demeo, D.L.; Murphy, A.; Su, J.; Datta, S.; Rosenow, C.; et al. Genomic screening and replication using the same data set in family-based association testing. Nat. Genet. 2005, 37, 683–691. [Google Scholar] [PubMed]
- Eichelbaum, M.; Ingelman-Sundberg, M.; Evans, W.E. Pharmacogenomics and individualized drug therapy. Annu. Rev. Med. 2006, 57, 119–137. [Google Scholar]
- Sowell, R.A.; Owen, J.B.; Butterfield, D.A. Proteomics in animal models of Alzheimer’s and Parkinson’s disease. Ageing Res. Rev. 2009, 8, 1–17. [Google Scholar]
- Khachaturian, Z.S.; Petersen, R.C.; Gauthier, S.; Buckholtz, N.; Corey-Bloom, J.P.; Evans, B.; Fillit, H.; Foster, N.; Greenberg, B.; Grundman, M.; et al. A roadmap for the prevention of dementia: The inaugural Leon Thal Symposium. Alzheimers Dement. 2008, 4, 156–163. [Google Scholar] [PubMed]
- Cacabelos, R. Role of nutrition in the prevention of Alzheimer’s disease. Aging Health 2005, 1, 359–362. [Google Scholar]
- Need, A.C.; Motulsky, A.G.; Goldstein, D.B. Priorities and standards in pharmacogenetic research. Nat. Genet. 2005, 37, 671–681. [Google Scholar]
- Johnson, A.D.; Wang, S.; Sadee, W. Polymorphisms affecting gene regulation and mRNA processing: broad implications for pharmacogenetics. Pharmacol. Ther. 2005, 106, 19–38. [Google Scholar]
- Ishikawa, T.; Onishi, Y.; Hirano, H.; Oosumi, K.; Nagakura, M.; Tarui, S. Pharmacogenomics of drug transporters: a new approach to functional analysis of the genetic polymorphisms of ABCB1 (P-glycoprotein/MDR1). Biol. Pharm. Bull. 2004, 27, 939–948. [Google Scholar]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab. Pharmacokin. 2008, 23, 22–44. [Google Scholar]
- Wilcock, D.M.; Colton, C.A. Anti-Aß immunotherapy in Alzheimer’s disease: relevance of transgenic mouse studies to clinical trials. J. Alzheimer Dis. 2008, 15, 555–569. [Google Scholar]
- Agrawal, N.; Dasaradhi, P.V.; Mohammed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar]
- Spänkuch, B.; Strebhardt, K. RNA interference-based gene silencing in mice: the development of a novel therapeutical strategy. Curr. Pharm. Des. 2005, 11, 3405–3419. [Google Scholar]
- Leung, R.K.; Whittaker, P.A. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol. Ther. 2005, 107, 222–239. [Google Scholar]
- Ying, S.Y.; Lin, S.L. Intron-mediated RNA interference and microRNA biogenesis. Methods Mol. Biol. 2009, 487, 387–413. [Google Scholar]
- González-Alegre, P. Therapeutic RNA interference for neurodegenerative diseases: From promise to progress. Pharmacol. Ther. 2007, 114, 34–55. [Google Scholar]
- Aagaard, L.; Rossi, J.J. RNA therapeutics: principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Bergmans, B.; Papadopoulou, A.S.; Delacourte, A.; de Strooper, B. MicroRNA regulation of Alzheimer’s amyloid precursor protein expression. Neurobiol. Dis. 2009, 33, 422–428. [Google Scholar]
- Feng, Z.; Zhao, G.; Lei, Y. Neural stem cells and Alzheimer’s disease: challenges and hope. Am. J. Alzheimers Dis. Other Demen. 2009, 24, 52–57. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cacabelos, R.; Fernández-Novoa, L.; Martínez-Bouza, R.; McKay, A.; Carril, J.C.; Lombardi, V.; Corzo, L.; Carrera, I.; Tellado, I.; Nebril, L.; et al. Future Trends in the Pharmacogenomics of Brain Disorders and Dementia: Influence of APOE and CYP2D6 Variants. Pharmaceuticals 2010, 3, 3040-3100. https://doi.org/10.3390/ph3103040
Cacabelos R, Fernández-Novoa L, Martínez-Bouza R, McKay A, Carril JC, Lombardi V, Corzo L, Carrera I, Tellado I, Nebril L, et al. Future Trends in the Pharmacogenomics of Brain Disorders and Dementia: Influence of APOE and CYP2D6 Variants. Pharmaceuticals. 2010; 3(10):3040-3100. https://doi.org/10.3390/ph3103040
Chicago/Turabian StyleCacabelos, Ramón, Lucía Fernández-Novoa, Rocío Martínez-Bouza, Adam McKay, Juan C. Carril, Valter Lombardi, Lola Corzo, Iván Carrera, Iván Tellado, Laura Nebril, and et al. 2010. "Future Trends in the Pharmacogenomics of Brain Disorders and Dementia: Influence of APOE and CYP2D6 Variants" Pharmaceuticals 3, no. 10: 3040-3100. https://doi.org/10.3390/ph3103040
APA StyleCacabelos, R., Fernández-Novoa, L., Martínez-Bouza, R., McKay, A., Carril, J. C., Lombardi, V., Corzo, L., Carrera, I., Tellado, I., Nebril, L., Alcaraz, M., Rodríguez, S., Casas, Á., Couceiro, V., & Álvarez, A. (2010). Future Trends in the Pharmacogenomics of Brain Disorders and Dementia: Influence of APOE and CYP2D6 Variants. Pharmaceuticals, 3(10), 3040-3100. https://doi.org/10.3390/ph3103040





