Advances in Microbial Biofilm Prevention on Indwelling Medical Devices with Emphasis on Usage of Acoustic Energy
Abstract
:1. Introduction
2. Biofilms and Anti-Microbial Immunity
3. Chemical Approaches, To Biofilm Elimination
4. Biological Approaches to Bacterial Biofilm Eradication
5. Interference with Inter-Bacterial Signaling for Biofilm Formation
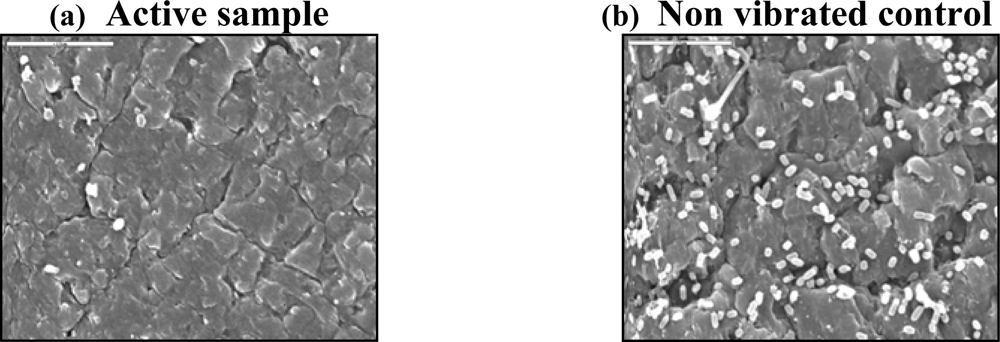
6. Uses of Acoustic Energy in Biofilm Prevention
- Abrogation of the two initial steps in biofilm formation: adhesion of planktonic microorganisms to surfaces and the ensuing firm attachment to substrates [57].
- Creation of stable cavitation in biofilms to enable more effective penetration and transport of antimicrobials and biocides through their exopolysaccharide encasings.
- Bypassing the obstacle of conditioning-films, which interfere with microbicidal coatings from reaching biofilms.
- Creation of microstreamings, which disrupt autoinducer gradients and abolish its signaling. Another byproduct of microstreamings is enhancement of the rate of cellular metabolism. Ultrasound is presumed to improve oxygen and nutrient transport to cells within biofilms and to planktonic cells [54,58], resulting in acceleration in the metabolic rates of the cells. The elevated metabolic rates may render bacterial cells more susceptible to antibiotics [58]. The hypothesis is that by invigorating the rate of bacterial cell metabolism ultrasound improves the efficiency of antibiotics.
- Infliction of mechanical damage to existing biofilms.
7. Complexity of High Frequency Acoustic Energy Effects on Biofilms and the Need for Improved Understanding of the Phenomenon
8. Fine Tuning the Acoustic Energy Properties is Required for Each Type of Indwelling Catheter
9. High Energy Ultrasound
10. Future Outlook and Conclusions
References
- Donlan, R.M. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis 2001, 33, 1387–1392. [Google Scholar]
- Maki, D.G.; Merme, L.A. Infections due to infusion therapy. In Hospital Infections, 4th Edtion; Bennet, J.V., Brachman, P.S., Eds.; Lippincott-Raven: Philadelphia, 1998; pp. 689–724. [Google Scholar]
- Raad, I. Intravascular-catheter-related infections. Lancet 1998, 351, 893–898. [Google Scholar]
- Maki, D.G.; Tambyah, P.A. Engineering out the risk of infection with urinary catheters. Emerging Infect. Dis 2001, 7, 1–6. [Google Scholar]
- Costerton, J.W. Bacterial biofilms: a common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar]
- Schachter, B. Slimy business—the biotechnology of biofilms. Nat. Biotechnol 2003, 21, 361–365. [Google Scholar]
- Branda, S.S.; Vik, A.S.; Friedman, L.; Kolter, R. Biofilms: the matrix revisited. Trends Microbiol 2005, 13. [Google Scholar]
- Parsek, M.R. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol 2003, 57, 677–701. [Google Scholar]
- Anwar, H.; Strap, J.L.; Costerton, J.W. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother 1992, 36, 1347–1351. [Google Scholar]
- Nichols, W.W.; Dorrington, S.M.; Slack, M.P.E.; Walmsley, H.L. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother 1988, 32, 518–523. [Google Scholar]
- Brown, M.R.W.; Allison, D.G.; Gilbert, P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother 1988, 22, 777–783. [Google Scholar]
- Mahenthiralingam, E.; Campbell, M.E.; Speert, D.P. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun 1994, 62, 596–605. [Google Scholar]
- Kristian, S.A.; Birkenstock, T.A.; Sauder, U.; Mack, D.; Götz, F.; Landmann, R. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J. Infect. Dis 2008, 197, 1028–1035. [Google Scholar]
- Walker, T.S.; Tomlin, K.L.; Worthen, G.S.; Poch, K.R.; Lieber, J.G.; Saavedra, M.T.; Fessler, M.B.; Malcolm, K.C.; Vasil, M.L.; Nick, J.A. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect. Immun 2005, 73, 3693–3701. [Google Scholar]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect. Immun 2006, 74, 6118–6123. [Google Scholar]
- Riley, D.K. A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. The Amer. J. Medicine 1995, 98, 349–356. [Google Scholar]
- Kollef, M.H.; Afessa, B.; Anzueto, A.; Veremakis, C.; Kerr, K.M.; Margolis, B.D.; Craven, D.E.; Roberts, P.R.; Arroliga, A.C.; Hubmayr, R.D.; Restrepo, M.I.; Auger, W.R.; Schinner, R. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. J. Amer. Med. Assoc 2008, 300, 805–813. [Google Scholar]
- Shah, C.B.; Mittelman, M.W.; Costerton, J.W.; Parenteau, S.; Pelak, M.; Arsenault, R.; Mermel, L.A. Antimicrobial activity of a novel catheter lock solution. Antimicrob. Agents Chemother 2002, 46, 1674–1679. [Google Scholar]
- Krzywda, E.A.; Andris, D.A.; Edminston, C.E.; Quebbeman, E.J. Treatment of Hickman catheter sepsis using antibiotic lock technique. Infect. Control Hosp. Epidemiol 1995, 16, 596–598. [Google Scholar]
- Krishnasami, Z.; Carlton, D.; Bimbo, L.; Taylor, M.E.; Balkovetz, D.F.; Baker, J.; Allon, M. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int 2002, 61, 1136–1142. [Google Scholar]
- Raad, I.; Chatzinikolaou, I.; Chaiban, G.; Hanna, H.; Hachem, R.; Dvorak, T.; Cook, G.; Costerton, W. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob. Agents Chemother 2003, 47, 3580–3585. [Google Scholar]
- Cateau, E.; Rodier, M.H.; Imbert, C. In vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J. Antimicrob Chemother 2008, 62, 153–155. [Google Scholar]
- van Heerden, J.; Turner, M.; Hoffmann, D.; Moolman, J. Antimicrobial coating agents: can biofilm formation on a breast implant be prevented? J. Plast Reconstr Aesthet Surg 2008, in press.. [Google Scholar]
- Doig, P.; Todd, T.; Sastry, P.A.; Lee, K.K.; Hodges, R.S.; Paranchych, W.; Irvin, R.T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect. Immun 1988, 56, 1641–1646. [Google Scholar]
- Hultgren, S.J.; Lindberg, F.; Magnusson, G.; Kihlberg, J.; Tennent, J.M.; Normark, S. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc. Natl. Acad. Sci. U.S.A 1989, 86, 4357–4361. [Google Scholar]
- Yilmaz, O.; Watanabe, K.; Lamont, R.J. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol 2002, 4, 305–314. [Google Scholar]
- Merino, S.; Rubires, X.; Aguilar, A.; Tomas, J.M. The role of flagella and motility in the adherence and invasion to fish cell lines by Aeromonas hydrophila serogroup O:34 strains. FEMS Microbiol. Lett 1997, 151, 213–217. [Google Scholar]
- Azghani, A.O.; Idell, S.; Bains, M.; Hancock, R.E.W. Pseudomonas aeruginosa outer membrane protein F is an adhesin in bacterial binding to lung epithelial cells in culture. Microb. Pathog 2002, 33, 109–114. [Google Scholar]
- Busscher, H.J.; Weerkamp, A.H. Specific and non-specific interactions in bacterial adhesion to solid substrate. FEMS Microbiol. Rev 1987, 46, 165–174. [Google Scholar]
- Saiman, L.; Prince, A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Invest 1993, 92, 1875–1880. [Google Scholar]
- Buts, L.; Bouckaert, J.; De Genst, E.; Loris, R.; Oscarson, S.; Lahmann, M.; Messens, J.; Brosens, E.; Wyns, L.; De Greve, H. The fimbrial adhesin F17-G of enterotoxigenic Escherichia coli has an immunoglobulin-like lectin domain that binds N-acetylglucosamine. Mol. Microbiol 2003, 49, 705–715. [Google Scholar]
- Tong, H.H.; Liu, X.; Chen, Y.P.; James, M.; DeMaria, T. Effect of neuraminidase on receptor-mediated adherence of Streptococcus pneumoniae to chinchilla tracheal epithelium. Acta Otolaryngol 2002, 122, 413–419. [Google Scholar]
- Simon, P.M.; Goode, P.L.; Mobasseri, A.; Zopf, D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect. Immun 1996, 65, 750–757. [Google Scholar]
- Schumm, K.; Lam, TB. Types of urethral catheters for management of short-term voiding problems in hospitalized adults: a short version cochrane review. Neurourol Urodyn 2008, 27, 738–746. [Google Scholar]
- Erickson, B.A.; Navai, N.; Patil, M.; Chang, A.; Gonzalez, C.M. A prospective, randomized trial evaluating the use of hydrogel coated latex versus all silicone urethral catheters after urethral reconstructive surgery. J Urol 2008, 179, 203–206. [Google Scholar]
- Tenke, P.; Riedl, C.R.; Jones, G.L.; Williams, G.J.; Stickler, D.; Nagy, E. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int. J. Antimicrob. Agents 2004, 23, S67–S74. [Google Scholar]
- Lewis, K.; Klibanov, A.M. Trends Biotechnol 2005, 23, 343–348.
- Gottenbos, B; van der Mei, HC; Klatter, F; Nieuwenhuis, P; Busscher, HJ. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar]
- Trautner, B.W.; Darouich, R.O. Catheter associated infections pathogenesis affect prevention. Arch. Intern. Med 2004, 164, 842–850. [Google Scholar]
- Cox, A.J.; Millington, R.S.; Hukins, D.W.L.; Sutton, T.M. Resistance of catheters coated with a modified hydrogel to encrustation during an in vitro test. Urol. Res 1989, 17, 353–356. [Google Scholar]
- Deretic, V.; Schurr, M.J.; Boucher, J.C. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factor. J. Bacteriol 1994, 176, 2773–2780. [Google Scholar]
- Stewart, P.S. Biofilm Accumulation Model that Predicts Antibiotic Resistance of Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother 1994, 38, 1052–1058. [Google Scholar]
- Stewart, P.S. Theoretical Aspects of Antibiotic Diffusion into Microbial Biofilms. Antimicrob. Agents Chem 1996, 40, 2517–2522. [Google Scholar]
- Azeredo, J.; Sutherland, I.W. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol 2008, 9, 261–266. [Google Scholar]
- Beckmann, C.; Brittnacher, M.; Ernst, R.; Mayer-Hamblett, N.; Miller, S.I.; Burns, J.L. Use of phage display to identify potential Pseudomonas aeruginosa gene products relevant to early cystic fibrosis airway infections. Infect. Immun 2005, 73, 444–452. [Google Scholar]
- Hughes, K.A.; Sutherland, I.W.; Clark, J.; Jones, M.V. Bacteriophage and associated polysaccharide depolymerases--novel tools for study of bacterial biofilms. J. Appl. Microbiol 1998, 85, 583–590. [Google Scholar]
- Brozel, V.S.; Strydom, G.M.; Cloete, T.E. A method for the study of de novo protein synthesis in Pseudomonas aeruginosa after attachment. Biofouling 1995, 8, 195–210. [Google Scholar]
- Davies, D.G.; Chakrabarty, A.M.; Geesey, G.G. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl. Environ. Microbiol 1993, 59, 1181–1186. [Google Scholar]
- Sauer, K.; Camper, A.K. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol 2001, 183, 6579–6589. [Google Scholar]
- Li, Y.H.; Tang, N.; Aspiras, M.B.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol 2002, 184, 2699–2708. [Google Scholar]
- Holden, M.T.; Ram Chhabra, S.; de Nys, R.; Stead, P.; Bainton, N.J.; Hill, P.J.; Manefield, M.; Kumar, N.; Labatte, M.; England, D.; et al. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol. Microbiol 1999, 33, 1254–1266. [Google Scholar]
- Waters, C.M.; Bassler, B.L. Quorum sensing: cell-to-cell communication in bacteria. Ann. Rev. Cell Dev. Biol 2005, 21, 319–346. [Google Scholar]
- Jayaraman, A.; Wood, T.K. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annual Rev. Biomed. Eng 2008, 10, 145–167. [Google Scholar]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol 2008, 125, 204–208. [Google Scholar]
- Qian, Z.; Sagers, R.D.; Pitt, W.G. Investigation of the mechanism of the bioacoustic effect. J. Biomed. Mater. Res 1999, 44, 198–205. [Google Scholar]
- An, Y.H.; Dickinson, R.B.; Doyle, R.J. Mechanisms of bacterial adhesion and pathogenesis of implant and tissue infections. In Handbook of Bacterial Adhesion: Principles, Methods and Applications; An, Y.H., Friedman, R.J., Eds.; Humana Press: Totowa, N.J., 2000; pp. 1–27. [Google Scholar]
- Pitt, W.G.; Ross, S.A. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Prog 2003, 19, 1038–1044. [Google Scholar]
- Carmen, C.; Nelson, J.L.; Beckstead, B.L.; Runyan, C.M.; Robison, R.A.; Schaalje, G.B.; Pitt, W.G. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J. Infect. Chemother 2004, 10, 193–199. [Google Scholar]
- Qian, Z.; Stoodley, P.; Pitt, W.G. Effect of low intensity ultrasound upon biofilm structure from confocal scanning laser microscopy observation. Biomaterials 1996, 17, 1975–1980. [Google Scholar]
- Carmen, J.C.; Roeder, B.L.; Nelson, J.L.; Robison Ogilvie, R.L.; Robison, R.A.; Schaalje, G.B.; Pitt, W.G. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control 2005, 33, 78–82. [Google Scholar]
- Rediske, A.M.; Roeder, B.L.; Brown, M.K. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob. Agents Chemother 1999, 43, 1211–1214. [Google Scholar]
- Rediske, A.M.; Roeder, B.L.; Nelson, J.L. Pulsed ultrasound enhances the killing of E. coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob. Agents Chemother 2000, 44, 771–772. [Google Scholar]
- Yoshimura, F.; Nikaido, H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J. Bacteriol 1982, 152, 636–642. [Google Scholar]
- Nikaido, H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother 1989, 33, 1831–1836. [Google Scholar]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev 1985, 49, 1–32. [Google Scholar]
- Hancock, R. Aminoglycoside uptake and mode of action---with special reference to streptomycin and gentamicin. II. Effects of aminoglycosides on cells. J. Antimicrob. Chemother 1981, 8, 429–445. [Google Scholar]
- Ensing, G.T.; Roeder, B.L.; Nelson, J.L.; Van Horn, J.R.; Van der Mei, H.C.; Busscher, H.J.; Pitt, W.G. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J. Applied Microbiol 2005, 99, 443–448. [Google Scholar]
- Ensing, G.T.; Neut, D.; Horn, J.R.; Mei, H.C.; Busscher, H.J. The combination of ultrasound with antibiotics released from bone cement decreases the viability of planktonic and biofilm bacteria: an in vitro study with clinical strains. J. Antimicrob. Chemother 2006, 58, 1287–1290. [Google Scholar]
- Sanderson, P.J. Infection in orthopaedic implants. J. Hosp Infect 1991, 18, 367–375. [Google Scholar]
- Carmen, J.C.; Roeder, B.L.; Nelson, J.L.; Beckstead, B.L.; Runyan, C.M.; Schaalje, G.B.; Robison, R.A.; Pitt, W.G. Ultrasonically enhanced vancomycin activity against staphylococcus epidermidis biofilms in vivo. J. Biomater. Appl 2004, 18, 237–245. [Google Scholar]
- Oulahal-Lagsir, N.; Martial-Gros, A.; Bonneau, M.; Blum, L.J. “Escherichia coli-milk” biofilm removal from stainless steel surfaces: synergism between ultrasonic waves and enzymes. Biofouling 2003, 19, 159–168. [Google Scholar]
- Zips, A.; Schaule, G.; Flemming, H.C. Ultrasound as a means of detaching biofilms. Biofouling 1990, 2, 323–333. [Google Scholar]
- Mott, I.E.C.; Stickler, D.J.; Coakley, W.T.; Bott, T.R. The removal of bacterial biofilm from water-filled tubes using axially propagated ultrasound. J. Applied Microbiol 1998, 84, 509–514. [Google Scholar]
- Hazan, Z.; Zumeris, J.; Jacob, H.; Raskin, H.; Kratysh, G.; Vishnia, M.; Dror, N.; Barliya, T.; Mandel, M.; Lavie, G. Effective prevention of microbial biofilm formation on medical devices by low-energy surface acoustic waves. Antimicrob. Agents Chemother 2006, 50, 4144–4152. [Google Scholar]
- Leighton, T.G. The Acoustic Bubble; Academic Press: New York, 1997; pp. 526–528. [Google Scholar]
- Pitt, W.G. Removal of oral biofilm by sonic phenomena. Am. J. Dent 2005, 18, 345–352. [Google Scholar]
- Parini, M.R.; Pitt, W.G. Removal of oral biofilms by bubbles: the effect of bubble impingement angle and sonic waves. J. Am. Dent. Assoc 2005, 136, 1688–1693. [Google Scholar]
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dror, N.; Mandel, M.; Hazan, Z.; Lavie, G. Advances in Microbial Biofilm Prevention on Indwelling Medical Devices with Emphasis on Usage of Acoustic Energy. Sensors 2009, 9, 2538-2554. https://doi.org/10.3390/s90402538
Dror N, Mandel M, Hazan Z, Lavie G. Advances in Microbial Biofilm Prevention on Indwelling Medical Devices with Emphasis on Usage of Acoustic Energy. Sensors. 2009; 9(4):2538-2554. https://doi.org/10.3390/s90402538
Chicago/Turabian StyleDror, Naama, Mathilda Mandel, Zadik Hazan, and Gad Lavie. 2009. "Advances in Microbial Biofilm Prevention on Indwelling Medical Devices with Emphasis on Usage of Acoustic Energy" Sensors 9, no. 4: 2538-2554. https://doi.org/10.3390/s90402538



