Abstract
Bis-MPA dendron-coated free-base tetraphenylporphyrin and zinc-tetraphenyl-porphyrin (TPPH2 and TPPZn) were studied in comparison with simple porphyrins (H2P, ZnP) by theoretical simulation of their infrared, Raman and electronic absorption spectra, as well as fluorescense emission. Infrared and fluorescence spectra of the dendrimers were measured and interpreted along with time-resolved measurements of the fluorescence. The 0–1 emission band of the dendron substituted TPPZn was found to experience a “heavy substitution”-effect. The 0–1 vibronic emission signal is associated with a longer decay time (approx. 7 - 8 ns) than the 0-0 emission (approx. 1 - 1.5 ns). The former contributed with more relative emission yield for larger dendron substituents, in agreement with the appearance of steady-state emission spectra showing increased contribution from the 0–1 vibronic fluorescence band at 650 nm. No such substitution effect was observed in the electronic or vibrational spectra of the substituted free-base variant, TPPH2. Vibration spectra of the parent porphyrins (H2P, ZnP, TPPH2 and TPPZn) were calculated by density functional theory (DFT) using the B3LYP/6-31G** approximation and a detailed analysis of the most active vibration modes was made based on both literature and our own experimental data. Based on the results of theoretical calculations the wide vibronic bands in the visible region were assigned. The vibronic structure also gave a qualitative interpretation of bands in the electronic absorption spectra as well as in fluorescence emission depending on the size of dendrimer substitution. From the results of time-dependent DFT calculations it is suggested that the TPPZn-cored dendrimers indicate strong vibronic interaction and increased Jahn-Teller distortion of the prophyrin core for larger dendrimer generations. Specifically, this leads to the entirely different behaviour of the emission spectra upon substitution of the TPPH2 and TPPZn variants, which was also experimentally observed. Since TPPH2 is originally of lower symmetry the specific distortion upon dendron substitution is not expected to the same extent, which also was in agreement with the experimental findings.
1. Introduction
Porphyrins are important chromophores that play a crucial role in a number of biological processes such as photosynthesis, dioxygen transport and activation, and photodynamic cancer therapy [–]. The study of excited states of porphyrins is important for the understanding of their electronic structure in the context of various applications. Porphyrin photochemistry also provides insight into the dynamics of related biomolecules, such as the photosynthetic reaction centers in purple bacteria and green plants and heme-based metalloproteins such as hemoglobin and myoglobin. Much of this work has recently been focused on free-base and metalloporphyrin assemblies for light-harvesting purposes, porphyrin containing mimics of the photosynthetic reaction center, and electronic devices. The last decades have witnessed a vast number of experimental studies of porphyrins which have yielded very useful information about their electronic structure and optical spectra (see for example, [–,–]), but it has not always been possible to provide a well reasoned explanation of the results obtained [–]. Although the absorption and fluorescence spectra of many porphyrins are well-known [–], the vibronic band structures are not completely understood so far, apart for the fundamental free-base porphyrin that recently was interpreted on the basis of rigorous theoretical investigations [,].
Recently, the harmonic vibrational frequencies of a number of porphyrins (H2P, ZnP, MgP) and vibronic intensities in phosphorescence, in the first absorption (Qx) and fluorescence bands were investigated by density functional theory (DFT) [], also taking vibronic perturbations into account [,]. The transition probability was calculated by time-dependent DFT with Franck-Condon (FC) and Herzberg-Teller (HT) contributions to electric-dipole transition moments including the displacements along all active vibrational modes. Here, the HT mechanism was found much more important; only ag and b1g modes produce intense lines in free-base porphyrin fluorescence [], in agreement with polarization measurements [,]. Two weak wide bands observed in the gas phase absorption spectra of the H2P molecule at 626 and 576 nm could be interpreted as the 0-0 and 0–1 bands of the 1Ag → 1B3u transition, respectively. The 0–1 band with largest contributions from the ν10(ag) = 1,610 cm−1 and ν19(b1g) = 1,600 cm−1 modes [] was found to be in agreement with previous tentative assignments [,,]. Both bands were found to include asymmetric stretching vibrations of the methine bridges []. A number of fine structure bands, including combination of two vibrational quanta, were obtained and compared with available site-selected spectra from Shpolskii and noble-gas matrices. Both absorption and fluorescence spectra could be interpreted on the basis of the linear coupling model and a good applicability of the mirror-symmetry rule was established [].
Dendritic encapsulated metalloporphyrins mimic efficiently a number of functions expressed in biological systems. These are hemoglobin- and myoglobin-like gas-binding ability, heme mono-oxygenase activity, electron-acceptor capacity in light-harvesting antenna systems, and shell-modulated redox potentials as found in cytochromes []. One very interesting property of the dendritic molecules is their ability to create a microenvironment inside. Such dendron coating can protect porphyrins from the surrounding environment [–]. The site isolation can be used for protecting an active pigment photo-center from de-excitation by oxygen [] or potentially even change monomolecular photophysical parameters, hence to some extent controlling the lifetimes of the excited states. Such controlled molecular photosystems could be of use for applications like optical power limiting devices [,] or in sensing applications []. For such and related purposes, porphyrins decorated with bis-MPA dendrons were prepared []. Specifically, Bis-MPA (2,2-bis(methyolol)propionic acid) repeating units were used as building block in the synthesis of dendron-coated meso-tetraphenyl porphyrins (TPP). They were further functionalized both as free-base porphyrin (TPPH2) and with a central zinc ion (TPPZn). Different sizes of molecules in terms of a systematic variation of the size of the dendrimer substituent were prepared, and their basic properties investigated []. For example, the hydrodynamic volume of the dendrimers could be determined from polarization anisotropy decay data, and it was established that the bis-MPA dendrimers are significantly smaller than the same generation Fréchet-type [] benzyl ether TPP dendrimer. The larger dendrimer substituents of the zinc ion case gave rise to entirely new features in the absorption and fluorescence spectra []: A broad shoulder at longer wavelengths was more prominent in the emission spectra of the larger dendrimers however, only in the case with the zinc ion in the center. The proto-porphyrin analogue did not show this size-effect. Since a large substituent could impose a larger “stress” to the molecule than a small one, we anticipate that this could also affect the porphyrin ring configuration and its associated vibronic structure to different extent.
Here, the results of more detailed photophysical studies are presented along with results of a detailed theoretical investigation of the vibronic structures relevant for the interpretation of the electronic spectra. The H2P molecule is in essence the heart of all porphyrins and calculations of its detailed vibronic structure [] were used as a guide-line for analysis of absorption and fluorescence spectra of meso-tetraphenyl derivatives and bis-MPA dendrimers grown on the basis of para-substituted tetraphenyl porphyrins. Specifically, we calculated the infrared (IR), absorption and non-resonance Raman spectra of the parent molecules HO-TPPH2, HO-TPPZn and by inference use the results to discuss results of dendrimers based on acetonide-2,2-bis(methoxy)propanoic (bis-MPA). The vibrational spectra are interpreted on the basis of density functional theory with the B3LYP functional [] and different basis sets together with our previous studies of vibrations in H2P and ZnP molecules [,]. IR and Raman spectra of free-base meso-tetraphenyl porphyrin (TPPH2) and TPPZn are also calculated and compared with published data [,,–]. Most previous IR and Raman spectroscopy studies of porphyrins were performed using substituted derivatives because of their high solubility and easier access. Detailed vibration spectra of the parent molecules, H2P and ZnP, have been experimentally and theoretically studied quite recently [,–] however, some old assignments of tetraphenyl derivatives [–,] are still controversial. We used DFT calculations for all these molecules in order to make a consistent interpretation of IR, Raman, electronic absorption and fluorescence spectra of bis-MPA dendrimers, and a model compound used in the calculations is shown in Figure 1, to be further discussed in the results and discussions section.
Figure 1.
Model structure for the dendron substituted tetraphenyl Zn-porphyrin (TPPZn) molecule used in the calculations.
2. Results and Discussion
2.1. General Appearance of Porphyrin Optical Absorption Spectra
As follows from Figure 2 in Vestberg et al. [], all optical absorption spectra of dendrimers are quite typical for porphyrins but include some additional features specific for the dendrimer substituted prophyrins. For the sake of discussion, representative steady state fluorescence excitation spectra for a number of TPPZn and TPPH2 dendrimers are shown in Figure 2. In order to interpret the dendrimer peculiarities one needs to comment on the common features of porphyrin chromophores. The first excited singlet state of the H2P molecule is 1B3u and the same “effective” symmetry can be used for the tetraphenyl derivative, since the electronic excitation is located mostly in the porphyrin ring (we use the common choice of axes []: the x-axis coincides with the N-H bonds, the z-axis is perpendicular to the plane of the molecule). This gives the Qx weak absorption band. For the H2P molecule it consists of two peaks, at 626 and 576 nm, which are interpreted [,,] as the 0-0 band of the 1Ag → 11B3u transition and the 1-0 band, respectively.
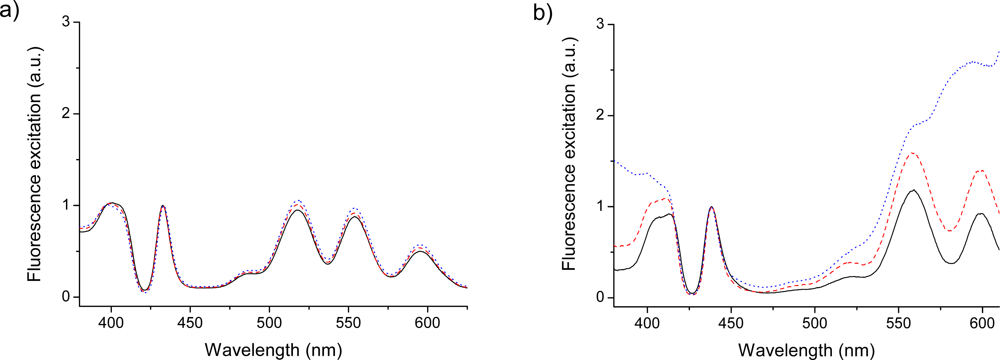
Figure 2.
Flourescence excitation spectra of the G0 (solid), G2 (dash) and G4 (dotted) variants of dendrimer capped TPP. (a) Acetonide-Gx-prop-TPPH2 for emission at 650 nm. (b) Acetonide-Gx-prop-TPPZn for emission at 660 nm.
As follows from DFT vibronic calculations [,] and from high-resolution Shpolskii spectra [,] both bands consist of a number of different vibration modes; thus the interval between two maxima has nothing in common with a particular single vibration. The latter band has the largest contributions from the ν10(ag) = 1610 cm−1 and ν19(b1g) = 1600 cm−1 modes (see notations of vibration modes in []). In TPPH2 and in all of its dendrimer variants these two peaks are at 652 (this band does not show up in the excitation spectra of Figure 2a) and 595 nm, thus indicating a red shift. The second weak Qy band of the H2P molecule also consists of two peaks at 510 nm (0-0) and 480 nm (1-0); the latter peak is more intense []. These are showing up at 518 and 554 nm in the excitation spectra (Figure 2a). Both 1-0 bands borrow intensity from the Soret band, which is produced by close lying 21B3u and 21B2u excited states. These Qx and Qy bands in free-base porphyrin are interpreted in terms of the well-known four orbitals model []. Our DFT calculation results supported this model and also reproduce the red and blue shifts in the derivatives (Table 1).

Table 1.
Electronic absorption spectrum of the free-base porphyrin, Zn-porphyrin, and their tetraphenyl derivatives. Excitation energy is in eV, oscillator strength (f) is given in parentheses. Experimental gas-phase data are from ref [].
The vibronic 1-0 transitions of the Q bands are more intense than the 0-0 transitions in the absorption spectra of all simple porphyrins [], but this is not the case for tetraphenyl derivatives. The main difference between emission properties of free-base porphyrins and Zn-porphyrins is connected with the longer radiative lifetime of the former []. In tetraphenyl derivatives (TPPH2 and TPPZn) and in dendrimers the 0-0 band is much more intense in fluorescence than the red-shifted 0–1 band []. The dendrimer variants of TPPZn also follow the general picture as long as the dendrimer substitutions are small. As reported for the optical absorption spectra and fluorescence the larger dendrimers (notably G4 and G5) gives entirely different spectra. This is also noted for the excitation spectra of the G4-dendrimer, as shown in Figure 2b. In order to understand these and other vibronic features in time-resolved fluorescence spectra of dendrimers it is necessary to first study their vibrational frequencies from the IR and Raman spectra.
2.2. IR Spectra of TPPH2, TPPZn and Porphyrin Dendrimers
Representative IR spectra of the free-base and Zn porphyrin dendrimers are displayed in Figures 3 and 4. IR spectra of tetraphenyl porphyrins have previously been studied in a number of works and the assignment of several IR bands has been proposed [–,,]. The band near 1,600 cm−1 was interpreted as a C-C vibration of the phenyl substituents; its shift upon deuteration supported this assignment []. The low-frequency region was studied by Kincaid and Nakamoto []; isotopes of different metal-ions revealed the modes at 400–470 cm−1 which include metal vibrations.
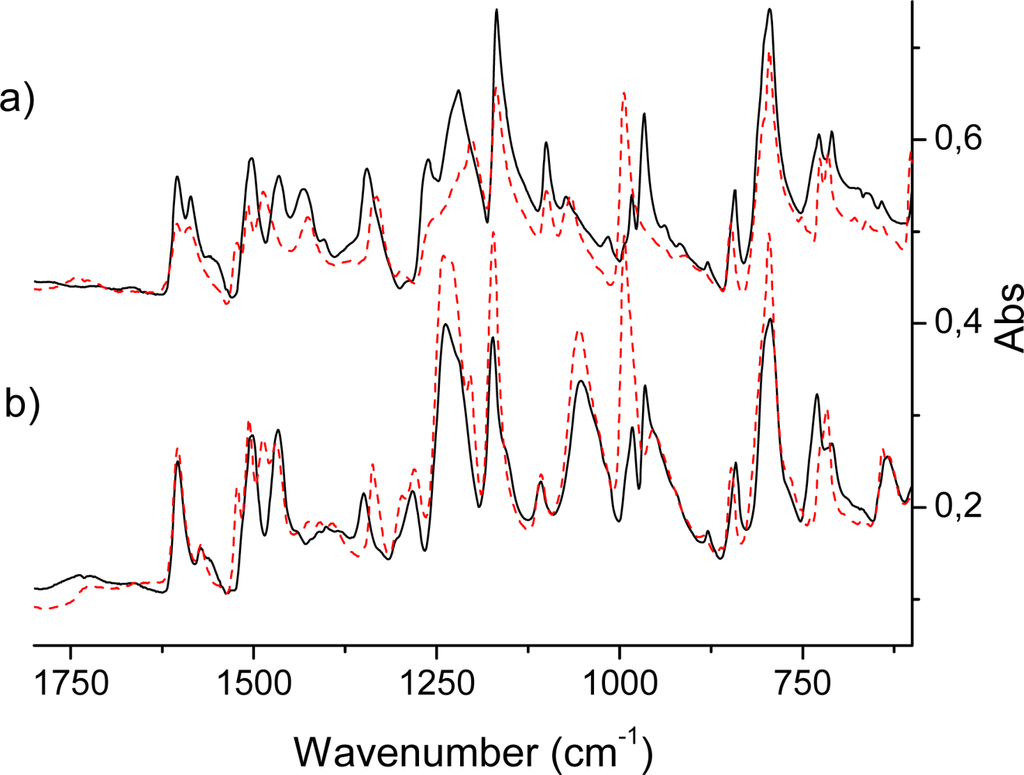
Figure 3.
(a) Infrared absorption spectra of the HO-TPPH2 (solid) and HO-TPPZn (dashed) molecules. (b) Infrared absorption spectra of the HO-prop-TPPH2 (solid) and HO-prop-TPPZn (dashed) molecules. N.b., the spectra of panel a) was added a constant (0.25) in order to make the plot.
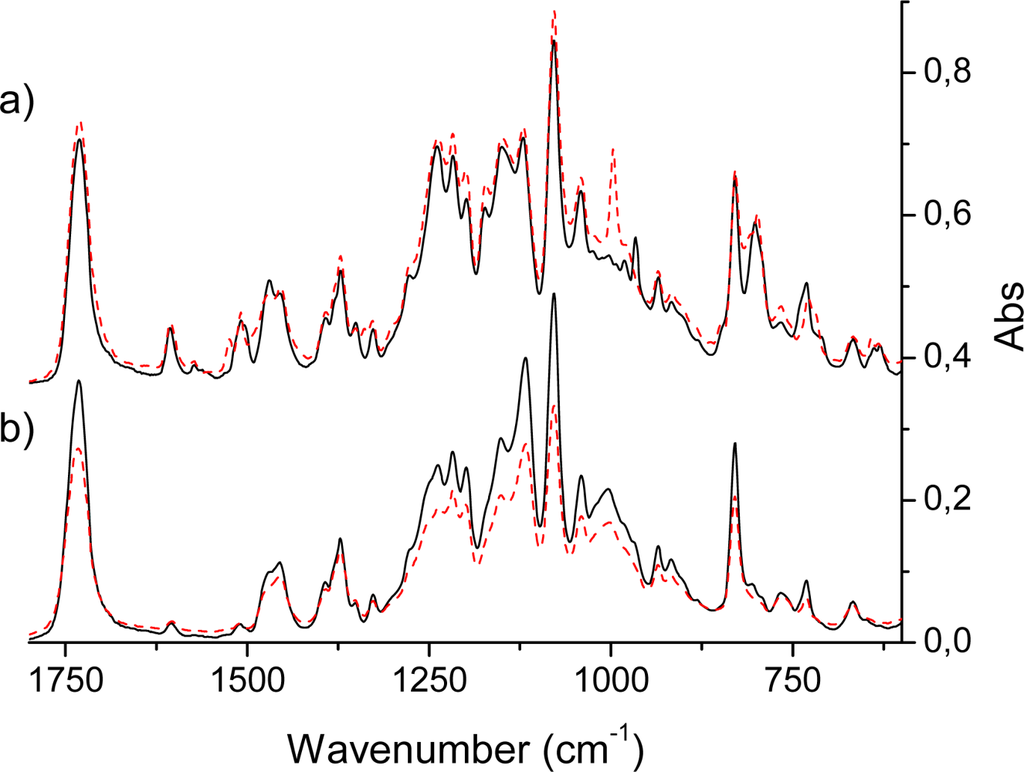
Figure 4.
(a) Infrared absorption spectra of Acetonide-G2-prop-TPPH2 (solid) and Acetonide-G2-prop-TPPZn (dashed) molecules. (b) Infrared absorption spectra of Acetonide-G5-prop-TPPH2 (solid) and Acetonide-G5-prop-TPPZn (dashed) molecules. N.b., the spectra of panel a) was added a constant (0.3) in order to make the plot.
In order to get a consistent description of all tetraphenyl derivatives at the DFT level we need to start to discuss the vibrational assignment of simple porphyrins. Firstly, we compared our B3LYP/6-31G** calculated IR absorption spectra of H2P and ZnP molecules, which previously were studied and interpreted by empirical force-field [,] and quantum scaled force-field calculations [,,]. The H2P molecule belongs to the D2h point group and has 108 vibrational modes, which can be separated into in-plane (73) and out-of-plane (35) modes. The former vibrations can be classified in the H2P molecule as belonging to 19 ag + 18 b1g + 18 b2u + 18 b3u modes. The b2u and b3u vibrations of the H2P molecule are transformed into degenerate eu modes in the ZnP molecule which belongs to the D4h point group. These modes are active in IR spectra together with the out-of-plane porphyrin ring vibrations of b1u (H2P) and a2u (ZnP) symmetry. The correlation of vibration modes in H2P and ZnP molecules is summarized in Table 2.

Table 2.
Correlation of vibrational symmetry between ZnP (D4h) and H2P (D2h) molecules.
The number of out-of-plane vibrational modes in H2P can be divided into the symmetry classes 8 b3g + 9 b2g + 8 au + 10 b1u. The former two symmetry types are allowed to occur in the Raman spectrum, but they are not very active as follows from our calculations and previous results [,,]. In ZnP there are 105 fundamental vibrations which have the following distribution in the symmetry classes belonging to the D4h point group, 71 in-plane vibrations: 18 eu + 9 a1g + 9 b1g + 8 a2g + 9 b2g, 34 out-of-plane vibrations: 8 eg + 3 a1u + 3 eu + 5 b1u + 4 b2u. (N.b., vibrations of eu and eg symmetry are doubly degenerate). The B3LYP DFT/6-31G** and /3-21G methods were employed in order to establish correlation between IR spectra of dendrimers and their generic ancestors. Though tetraphenyl derivatives and dendrimers are non-planar the use of the D2h and D4h symmetry point group notations is still useful, since the electronic features and force fields of the simple tetrapyrrole rings are mainly responsible for the UV and IR spectra of the dendrimers. Assignments of the most intense IR and Raman bands in the ancestors of dendrimers are presented in Tables 3–4, and Tables 5–6, respectively. Since the low-frequency part of the IR spectra was not available in our experimental data the comparison of the theoretical analysis will focus on the intense experimental absorption in the 600–1800 cm−1 region. Thus we have excluded C-H and N-H stretching with high frequencies (more than 3000 cm−1) from our Tables. Their spectral assignments are trivial [,,].

Table 3.
The part of the infra-red spectrum of free-base porphyrin in the region 760 – 1,750 cm−1. “Int.” is the IR absorption intensity (km/mol), “νi“ the wavenumber (cm−1).

Table 4.
The part of the infra-red spectrum of Zn-porphyrin calculated employing the B3LYP DFT method. “Int.” is the IR absorption intensity (km/mol), “νi“ the wavenumber (cm−1).

Table 5.
The most important Raman fequencies of free-base porphyrin. “R” is the Raman scattering activity (Å4/amu), “νi“ the wavenumber (cm−1).

Table 6.
The most important Raman frequencies of ZnP. “R” is the Raman scattering activity (Å4/amu), “νi“ the wavenumber (cm−1).
IR active intense out-of-plane porphyrin ring vibrations
An intense IR absorption starts to grow at 700 cm−1 and gives the first very strong band at 785 cm−1 in the H2P molecule [,,,] by excitation of the vibrational mode ν43 (following our throughout numeration of Table 3) of b1u symmetry. This is an out-of-plane wagging vibration of the N-H and C-H bonds in the protonated pyrrole rings with weak involvement of the Cm-H bonds (Cm are the methylene-bridge carbons). Because of the substitution in the TPPH2 and HO-TPPH2 molecules this mode is slightly shifted being mixed with the phenyl C-H bending. For the tetraphenyl derivatives in the same region there are also four close-lying intense lines determined by pure out-of-plane symmetric C-H bending in phenyl rings (δCHPh). By overlap with the porphyrin mode ν43 they give one of the most intense lines at 790 cm−1 in the TPPH2 and HO-TPPH2 molecules (Figure 3). In the ZnP molecule this vibration corresponds to the ν38 mode of a2u symmetry (Table 4). It consists of C-H out-of-plane wagging for Cβ-H bonds and includes also the Cm-H wagging vibrations (out-of-phase to the former). Since the nature of this mode is rather different in the H2P and ZnP molecules (no N-H bond in the latter) the frequency of the ν38 mode is shifted in ZnP to 765 cm−1 and the corresponding intensity decreased (Table 4). The experimental frequency shift for this mode, nH2P-nZnP = 20 cm−1, can be compared with the calculated one (29 cm−1). In TPPZn [] and HO-TPPZn (Figure 3) this line is also overlapped by four intense δCHPh bands with frequencies 795 and 797 cm−1, respectively; resulting in a larger shift compared to ZnP (in comparison with free-base variants) because of the stronger involvement of the Cm-H wagging vibrations. It is also more strongly mixed with the C-H bending vibrations of the phenyl rings. In the HO-prop variants (Figure 3b) the intensity ratio for the free-base and Zn porphyrins is reversed in agreement with DFT/3-21G calculations. In dendrimers this IR band is more shifted to 802 cm−1 and a new close lying intense band 829 cm−1 occurs (Figure 4). From the 3-21G calculation of the acetonide-G1-TPPZn dendrimer (Figure 1) the latter band is connected with a few CH2 modes of acetonide groups. The band 802 cm−1 is the former ν43 b1u mode of free-base porphyrin ring (Table 3) mixed with the δCHPh vibrations and with the C-O-C bending in the acetonide groups. Its intensity is diminished for the dendrimer model in agreement with calculation; there are also a number of close lying new (acetonide) lines. A similar behaviour of this IR band was observed for Zn-containing dendrimers (Figure 4).
The other intense line in the IR spectrum of the H2P molecule [,] at 852 cm−1 also belongs to the out-of-plane vibration of the b1u symmetry (ν48 in Table 3, mostly the Cm-H bending) that slightly involves the Cβ-H bond vibration. Since this mode is strongly affected by meso-tetrathenyl substitution of the porphyrin ring, it is less intense and shifted to 842 cm−1 in TPPH2 and HO-TPPH2 molecules (Figure 3). The shift and intensity reduction are supported by our calculations. In ZnP this mode appears similar. It corresponds to the ν49 vibration of a2u symmetry (Table 4). Its measured frequency (849 cm−1) is almost the same as for H2P. The calculated frequencies and intensities are also very similar (Table 3 and Table 4).
The b1u out-of-plane vibrations of H2P correlate with the a2u and b2u symmetry of the ZnP molecule of the D4h point group (Table 2) and only the former vibrations are IR active. Although the optimized TPPZn and HO-TPPZn molecular structures are nonplanar, we can use correlation with the D4h symmetry since some intense vibrations are determined by characteristic modes of the porphyrin ring. Since the ν48 is one of the dominating out-of-plane vibrations of the Cm-H bonds, it is quite natural that the corresponding intensity is strongly reduced upon tetraphenyl substitution (Figure 3). This mode is transformed in TPPZn in such a way that it includes out-of-plane vibrations of phenyl rings. In dendrimer substituted TPPs this vibration is quenched; the corresponding out-of-plane vibrations is shifted to the low-frequency region (the vibrations of light H atoms are transformed into out-of-plane movement of the massive and bulky substituent). It should be noted that the out-of-plane vibrations were not considered in empirical force-field calculations [,] and we need to use our throughout numeration of all modes, as presented in Tables 3 and 4. The presented B3LYP/6-31G** calculations are in good agreement with the scaled results of Pulay et al. [,,] with respect to intensity and polarization of IR and Raman spectra (Tables 3–6). Correlation with the AM1 results is strightforfard and obvious.
IR active in-plane porphyrin ring vibrations
There are no b1u (a2u) out-of-plane vibrations in the H2P (ZnP) molecules with frequencies larger than 854 cm−1 (Tables 3 and 4). The same was found in calculations of the porphyrin ring out-of-plane vibrations in the tetraphenyl derivatives. Thus, the remainder of the porphyrin IR spectrum is caused by in-plane vibrations, for which assignment and numeration of Li and Zhierski [] are available. (Here, this numeration is denoted by a prime symbol ν′i in order to avoid confusion with our complete numeration including also out-of-plane vibrations.) Analyses of the in-plane vibrations were also reported in a large number of experimental studies [–,,,]. We introduce the discussion of the vibrational structure with the ZnP analysis since it is more convenient to consider first the degenerate eu vibrations, and then to discuss the corresponding vibrations of lower symmetry that occur in e.g., dendrimer capped porphyrins.
The most prominent features in the IR spectrum of the ZnP molecule [] occur at 993 and 1,052 cm−1 and originate from the eu vibrations (ν54,55 and ν62,63, respectively, Table 4). The former mode includes the out-of-phase Cα-Cβ stretching vibrations in the opposite-lying pyrrole rings with the corresponding Zn-N asymmetric stretches. This is the most intense (doubly degenerate) vibrational transition of the IR spectrum of ZnP (Table 4) in agreement with experiment []. In the notation of Ref. [] this is the ν′47 mode, described as a pyrrole breathing mode. The results of our DFT calculations are not in complete agreement with the interpretation of Ref. []. Our results also contradict the assignment of the 993 cm−1 mode suggested in Ref. []. The weaker line at 1,052 cm−1 originates from Cβ-H deformations being out-of-phase in the opposite-lying pyrrole rings. Both these modes (993 and 1,052 cm−1) are mixed with the phenyl vibrations in TPPZn. Because of the specific character of the ν54,55 vibrations, the Cm-Ph stretches are silent in TPPZn and the frequency 993 cm−1 is hardly shifted in the tetraphenyl derivatives, TPPZn [], HO-TPPZn and HO-prop-TPPZn (Figure 3). The mode at 993 cm−1 of ZnP is split in the TPPZn molecule into two close lying frequencies (992 and 994 cm−1 in our scaled DFT calculation), both having relatively high intensity (61 and 65 km/mole). They also include C-C vibrations of the phenyl rings. In the dendrimers, intensity of these very characteristic vibrations of the porphyrin ring are significantly changed. In the acetonide-G2-prop-TPPZn dendrimer the line at 993 cm−1 is not the most intense and slightly shifted to 996.8 cm−1. Its intensity is reduced by 25%, since the bulky acetonide groups withdraw electron density from the porphyrin ring and quench the dipole moment derivative along this Cα-Cβ stretching vibration. In the acetonide-G5-prop-TPPZn dendrimer, the intensity of this line is reduced much more (by 67%) for similar reasons; the band is broadened because of mixing with acetonide vibrations and the maximum is shifted to 1002 cm−1. At the same time the weaker line 1,052 cm−1 of ZnP changes considerably upon tetraphenyl substitution, since these modes ν62,63, directly involve Cm-H bending modes. In the the TPPZn and HO-TPPZn molecules this frequency shifts to 1,069 and 1,067 cm−1, respectively. In HO-prop-TPPZn it is overlapped by an intense band at 1,055 cm−1 (Figure 3) originating from O-(CH2)3 vibrations. This intense IR absorption in dendrimers is further increased and shifted to 1,080 cm−1 (Figure 4). The line at 1,052 cm−1 of ZnP is shifted approximately to 1,041 cm−1 in the dendrimers. Since this Cβ-H bending also involves some Cm-Ph bending character, its frequency is sensitive to substituents in the phenyl rings of TPPZn and is reduced in the more flexible bis-MPA dendrimers. The other eu vibrations of low intensity in the IR spectrum of ZnP are not so informative as the intense lines mentioned above and we omit their discussion.
In free-base porphyrin and its derivatives the corresponding eu vibrations of ZnP are split in the D2h point group of the H2P molecule into b2u and b3u modes [,]. The ZnP mode at 993 cm−1 (ν54,55, Table 4) is split into 951 cm−1 b2u and 971 cm−1 b3u modes in H2P (ν53 and ν55, in Table 3). The nature of these modes is the same as in ZnP (asymmetric breathing of the opposite-lying pyrrole rings), but the absence of the Zn-ion and Zn-N stretches releases the force constants and leads to low-frequency shifts. The b2u mode shifts more since it corresponds to unprotonated pyrrole rings. These two lines are not so intense in H2P, like the double-degenerate line at 993 cm−1 of the ZnP molecule (Tables 3 and 4). Instead of the intense ZnP peak in this region, there is a gap (weaker absorption) in the IR spectrum of the H2P molecule and this is the main difference between the two spectra. This trend is also well observed for IR spectra of the HO-TPPZn and HO-TPPH2 molecules (Figure 3), but not in the dendrimers (Figure 4), since the line at 993 cm−1 is not the most intense in acetonide-Zn-prop-TPPZn derivatives, as discussed above. The line corresponding to 993 cm−1 of HO-TPPZn splits in the HO-TPPH2 molecule into 983 and 966 cm−1 lines (analogous the the case of b3u and b2u modes, respectively; Figure 3). As far as the ZnP infrared line 1,052 cm−1 is concerned, the behavior of free-base porphyrin variants is very peculiar; it splits into the 1,043 cm−1 and 1,054 cm−1 bands. They correspond to our ν61 (b3u) and ν62 (b2u) modes, respectively (Table 3). In the H2P molecule they become Cβ- Cβ -H bending vibrations of the out-of-phase type with respect to the opposite pyrrole rings. The ν61 (b3u) vibration involves the protonated rings, and the ν62 (b2u) mode involves the unprotonated rings (Table 3). Only the ν61 (b3u) vibration is mixed with the Cm-Ph bendings and only this mode is seen in IR absorption of dendrimers. The striking difference of ZnP and H2P vibrations of the Cβ-H type has not been stressed before, as will be discussed more below, it is important for our further analysis of the dendrimers.
The separated strong line at 1,731–1,733 cm−1 of all dendrimer samples is definitely connected with the carbonyl groups stretching. A very strong and narrow IR band at 1,080 cm−1 in the region of intense porphyrin absorption was also present in all dendrimer samples. This originated from acetonide vibrations mixed with porphyrin modes. Even though the band is narrow, it consists of few close lying intense lines of similar nature. They include wagging vibrations of CH2 and CH3 groups, deformation of the Ph-O-CH chain and ν61 - ν63 modes of the porphyrin ring (Table 3). The band 1,172 cm−1 of HO-TPPX and HO-prop-TPPX molecules corresponds to the single bond C-O stretching of the terminal COH groups. It disappears in dendrimers because there are no such groups in the acetonide moiety. The C-O-H bending vibrations are assigned to the strong line at about 1220-1240 cm−1 in the IR spectra of the HO-TTPX and HO-prop-TPPX molecules (X= Zn, H2P). The less intense, closely lying bands at about 1,260–1,280 cm−1 correspond to C-C-H bending vibrations of the phenyl rings; in the dendrimers they are shifted to lower frequency and overlapped by absorption of acetonide groups. The line at 1,349 cm−1 of HO-TTPH2 corresponds to the Cm-Phenyl stretching vibrations; it is shifted to 1,369 cm−1 in dendrimers because of mixing with acetonide vibrations. The bands near 1,600 cm−1 at the edge of the HO-TPPX infrared spectra belong to the phenyl C=C vibrations; these are sensitive to substituents and are strongly reduced in the dendrimers because of admixture of the ether-group stretching. In HO-TPPX in the region 1,500-1,420 cm−1, there are a few intense IR bands of C-C stretching and C-C-H bending vibrations of the phenyl rings; some of them are mixed with Cm-Cα asymmetric modes of the porphyrin core. Introduction of the acetylide moieties leads to significant distortion of these bands. The broad strong IR absorption bands at 3,244 cm−1 (HO-TTPZn) and 3,219 cm−1 (HO-TTPH2), data not shown, are attributed to O-H stretching vibrations in agreement with the calculated results. They further split into four modes of ag and bu symmetry; the two latter bu modes correspond to paired combinations of O-H stretches from the opposite sides of the porphyrin ring. In the HO-prop-TPPX molecules these O-H stretching vibrational modes are shifted to higher frequency (about 3,320 cm−1) because of the inductive effect of the alkyl groups.
2.3. Raman Active Modes
The gerade modes which are active in Raman spectra are important for analysis of vibronic bands in dendrimer fluorescence, since they induce mixing between the Q and Soret states. Thus they provide the Hertzberg-Teller (HT) contribution to fluorescence intensity from the second term of Equation 1 (Methodology section). The totally symmetric vibrations are mostly important for the Franck-Condon (FC) terms. Because of the symmetry reduction in dendrimers some acetonide modes are simultaneously active in IR and Raman spectra, and in order to understand and interpret their occurrence in the fluorescence vibronic sub-structure it is necessary to analyse first the Raman activity of the porphyrin core.
In-plane vibrations active in Raman spectra
The resonance Raman (RR) spectra of tetraphenyl complexes with metals (TPPM) were previously assigned using a normal coordinate analysis of biphenyl with regard to assignment of phenyl modes [,]. In our normal coordinate analysis of tetraphenyl complexes the full Hessian obtained from DFT calculations was used. Taking the TPPZn molecule as an example one gets 225 real frequencies. In the low-frequency region the most intense line of the Raman spectrum of the H2P molecule is the mode ν13 =309 cm−1 of ag symmetry (Table 5; thiscorresponds to ν′8 in the numeration of []). This corresponds to Cα-Cm-Cα in-phase bending vibrations and hindered translation of all pyrrole rings and can also be described as a uniform breathing of the whole tetrapyrrole ring [,]. In ZnP it is shifted to 363 cm−1 (ν18 in Table 6). In TPPZn it is detected at 387 cm−1 [,]. This vibration was also seen in the fluorescence spectrum taken using a low-temperature solid matrix [].
It is well known that the RR spectra of the TPP derivatives are usually dominated by the porphyrin skeletal modes due to a resonant effect, although some phenyl modes have also been observed indicating evidence for π-delocalization to phenyl rings []. Resonance enhancement of the Raman scattering occurs only if the vibrational mode involves atoms which are part of the electronically excited chromophore. The dihedral angle between the porphyrin ring and meso-phenyl substituent planes is close to 70° from our DFT optimization, being in general agreement with experimental data (80°) []; thus the π-systems of the porphyrin and phenyl rings should not interact. At the same time the RR spectrum of TPPZn has a strong band at 1,236 cm−1 that has been assigned as the Cm-Ph mode []. From our DFT analysis it is mixed with the internal phenyl C-C stretches. The Raman intensity of this and other phenyl modes can be explained in terms of hyperconjugation. In fact the LUMO eg orbital of TPPZn has large π-expansion coefficients at the Cm atoms; at the same time it has appreciable admixtures of 2s-orbitals at the ortho-carbon atoms of the phenyl rings. Thus the π-σ hyperconjugation occurs upon the π-π* excitation, explaining the Raman activity of the phenyl modes. This could also be observed in the fluorescence spectrum of low-temperature solid TPPZn as the onset of the blue wing of the 0–1 band at 650 nm [].
The maximum of the 0–1 band in porphyrins is determined by two asymmetric Cα-Cm stretching vibration modes []. In the H2P molecule these modes are very close in frequency (1610 and 1600 cm1; ν94 and ν92 in Table 5), belonging to ag and b1g symmetry, respectively. These are ν′10 and ν′19 modes in notations of Ref. []. The former RR line is very intense, the latter one is weak. In ZnP molecule these two vibrational frequencies are separated by about 50 cm−1 (These are the ν93 and ν91 modes in Table 6). The interaction between adjacent Cα-Cm and Cα-N bonds in ZnP have much larger negative effect on the ν′19 mode than on the ν′10 mode, as also was pointed out in Ref. []. In tetraphenyl porphyrins the ν′19 mode is shifted down by about 25 cm−1, being more in agreement to the results of our DFT calculations. This is because of its mixture with Cα-Cm-Cphenyl vibrations. Our scaled prediction for the TPPZn molecule (ν′19 = 1,545 cm−1) is in a good agreement with the resonance Raman frequency measurement [] (1,548 cm−1). It was natural to propose that a large shift of the ν′19 frequency in ZnP in comparison with the H2P can be responsible for the difference in their fluorescense spectra [].
The next less intense line in H2P fluorescence is ν′20 = 1,388 cm−1 of b1g symmetry [,] (vibrational mode ν79 in Table 5). In ZnP it has slightly lower frequency (1353 cm−1) and belongs to a2g symmetry []. In the D4h point group this mode and the close lying ν′26 vibration are non-active in the Raman spectra. The interpretation of these closely lying vibrations of a2g symmetry (ν′20 and ν′26) in the ZnP molecule is very important for analysis of fluorescence vibronic bands (a2g x Eu = Eu; thus, these modes are active in mixing of the states giving the characteristic Q and Soret bands). In our DFT study (Table 6) they correspond to modes number 75 and 76, respectively. The former includes asymmetric Cm-H stretches accompanied with strong deformations of the pyrrole rings (mostly Cα-N- Cα asymmetric stretching). The ν′26 = 1,322 cm−1 mode [] corresponds to our number 75 in Table 6; including both asymmetric Cβ-H and Cm-H stretches. Thus, it is expected that in tetraphenyl porphyrins the ν′26 mode is shifted down by about 90 cm−1. In TPPZn we calculated it to be 1,233 cm−1. Similar results were obtained for the H2P and the TPPH2 molecules. Accounting the results of vibronic calculations [], it is here suggested that the ν′26 = 1,237 cm−1 (a2g) mode contributes to the formation of the 0–1 band in the fluoresecnce spectrum of the HO-TPPH2 molecule with a wide maximum at about 1200 cm−1. The ν′26 mode includes the Cm-Cphenyl stretching and according to our calculations it is responsible for effective mixing between the Q and Soret states.
In the fluorescence spectra of the TPPH2 molecules the 0-0 band (658 nm) is much more intense than the 0–1 band (714 nm) because the phenyl substituents are not in the porphyrin plane. This deviation from planarity and from the D2h symmetry provides an increase of the electronic 0-0 transition moment of the Qx band. The quantum yield of fluorescence is also increased upon tetra phenyl substitution of the H2P molecule []. Thus the 0-0 line is more intense than all 0–1 lines because of the stronger Franck-Condon mechanism in comparison with the Herzberg-Teller mechanism for borrowing intensity []. The energy gap between the 0-0 and 0–1 bands in the TPPH2 molecule in benzene [] is 1,410 cm−1. This gap depends on the solvent: using a mixture of ethyl-iodide the gap was found to be 1,538 cm−1 []. For H2P the gap is largest []: 1,620 cm−1. This solvent and substituent dependence of the frequency separation between the 0-0 and 0–1 bands in fluorescence of porphyrins has never been explained so far. It is here suggested that it can be interpreted as the result of more involvement of the ν′20 and ν′26 modes of b1g symmetry in H2P upon tetraphenyl substitution. The ν′10 (ag) and ν′26 (b1g) modes at about 1,600 cm−1, which correspond to the Cα-Cm asymmetric stretching vibrations, produce the most intense 0–1 vibronic line in the H2P molecule and are strongly reduced upon tetraphenyl substitution. The massive phenyl groups are naturally admixed into these vibrations and contribute some 60 cm−1 down-shift; more important is a reduction of vibronic mixing and of the corresponding 0–1 lines intensity in fluorescence and Qx band absorption. Keeping in mind these peculiarities of the ν′10, ν′19 and ν′26 modes we now can progress by considering the absorption and fluorescence spectra of porphyrin-cored bis-MPA TPP dendrimers.
2.4. Interpretation of Optical Absorption Spectra
We have to point out that at first glance there are no large differences observed in the absorption and fluorescence spectra of dendrimers of different generations []. This agrees with earlier findings for tetraphenyl porphyrin dendrimers of Frechet type []. The spectra show the typical absorption bands of porphyrins (Soret-band and Q-bands) and the difference in the Q-bands between free base and zinc-containing porphyrins can clearly be seen []. This difference is well-reproduced in TD DFT calculations of the singlet-singlet absorption spectra of the H2P and ZnP molecules [,,] and in their tetraphenyl derivatives (Table 1). The metal porphyrins are characterised by a blue shift of the Q-band with respect to the free bases. For the ZnP and H2P molecules the calculated shift is 0.18 eV; whereas the experimental shift is 0.2 eV (Table 1). For tetraphenyl porphyrins there are red shifts with respect to simple porphyrins: for TPPZn it is 0.14 eV (0.09 eV), for TPPH2 the red shift is equal to 0.12 eV (0.11 eV); experimental data shown in parentheses. The absorption spectra for both free base and zinc porphyrin dendrimers in THF were depicted in Figure 2 of Vestberg et al. [].
The calculated red shift of the Q-band between TPPH2 and HO-TPP2 molecules is very small (0.0087 eV, or 2 nm in wavelength) which agrees well with spectral measurement in dichloromethane []. Further substitution in para-position of the phenyl rings by OCH3 and OCH2CH2CH3 groups provides no shift. This partially explains why no apparent shifts in the Q-bands maxima between the dendrimer generations are observed. The four weak absorption bands of acetonide-Gn-prop-TPPH2 dendrimers at 649, 592, 552 and 514 nm [] can be interpreted as the Qx(0-0), Qx(1-0), Qy(0-0) and Qy (1-0) bands in free-base tetraphenyl porphyrin moiety, respectively, and the peaks apart from the long wavelength absorption are also shown up in fluorescence excitation spectra when monitoring the emission at 660 nm, as shown in Figure 2a. The spectrum is similar to the absorption spectrum of the TPPH2 molecule in the same THF solvent [,]. In Zn-porphyrins there is only one degenerate (11Eu) excited state in this region (Table 1) which is responsible for the Q(0-0) and Q(1-0) absorption bands at 600 and 558 nm, respectively.
With more detailed analysis the absorption spectra of acetonide-G0/G5-prop-TPPH2 and acetonide-G0/G5-prop-TPPZn dendrimers indicate some differences in both classes. In the case of free-base porphyrins, increased absorption just above 450 nm was observed for the fifth generation. This absorption is also present for the higher-generation zinc-cored porphyrin, where it is much more pronounced. One possible reason for this could be connected with the rise of the red wing of the Soret band (shown in Figure 2 of Ref. []). But this explanation is not supported by the fluorescence excitation spectra of the dendrimers (Figure 2a). From our calculations (Table 1) it follows that there are forbidden transitions to the 1B1g states in this region. For ZnP molecule they correlate with the 1A2g and 1B2g states that correspond to π-π* (4eg – 5eg) transitions with large “metal-to-ligand” charge-transfer character. The MO 4eg has about 30 % of the metal 3dπ-orbital contribution; MO 5eg is a double degenerate LUMO of the porphyrin ring. Transition to the 1A2g state is rather peculiar since it is the only one that has a large magnetic dipole transition moment (μz = 1.9 μB, where μB is the Bohr magneton). In the TPPZn molecule this transition is red-shifted by 8.3 nm with respect to ZnP following our DFT/3-21G calculation; besides its magnetic-dipole character it acquires electric dipole transition moment (Dz = 0.01 ea0, where a0 is the Bohr radius).
The LUMO of all porphyrins has large contributions from the Cm atoms. In ZnTPP it is connected by hyperconjugation with the 2s-orbitals at the ortho-carbons of the phenyl rings. Because of the hyperconjugation with phenyl rings the gerade symmetry of the porphine chromophore is removed and the former 4eg – 5eg transition becomes electric-dipole allowed. The analogous 1A2g state of the HO-TPPZn molecule is further red-shifted by 9.4 nm and the X1A1g – 1A2g transition is greatly enhanced. The nature of the transition is changed; here it includes large contribution of charge transfer from π-orbitals of the phenyl rings (oxygen atoms are also included). Substitution in para-position of the phenyl rings by OCH3 groups leads to further increase of the transition moment. One possible explanation could be that the dendrons also interact with the phenyl rings, making these transitions more allowed. The stronger absorption to vibrational levels of the 1A2g and 1B2g states giving the substructure at 450–500 nm could be enhanced by the heavy dendron substitution. This absorption is more enhanced in metal-porphyrin dendrimers, which can be the subject of the Jahn-Teller effect []. This is readily observed in the absorption spectra of Ref. [], as well as in the excitation spectra of the larger dendrimer substitions, when monitoring the emission at 650 nm, Figure 2b. Additional interesting feature of the absorption spectra for the zinc-containing porphyrins is the appearance of a weak shoulder at around 630 nm. This red absorption was clearly observed for the second generation and becomes more pronounced for the larger substituents. We suppose that this is an indication of the Jahn-Teller splitting of the 11Eu state in ZnP upon tetraphenyl substitution (Table 1) and its enhancement in dendrimers.
At this point it is necessary to consider connection with the so-called “hyperporphyrin” spectra [,,]. Hyperporphyrins have been defined as porphyrins that exibits extra absorption bands in the region λ > 320 nm that are not of the π−π* nature of the tetrapyrrole ring []. These extra bands are proposed to be due to charge transfer (CT) interactions between the tertrapyrrole ring and either metal or substituents. Washing the porphyrin-stained laboratory glassware with acid one can see clear manifestation of the hyperporphyrin spectroscopy; acid turns a reddish tetraphenylporphyrin stain into brilliant green []. At the same time porphyrins without tetraphenyl substituents do not undergo such visual change on acidification. The reason is that the protonated TPPH2 molecule is a hyperporphyrins []. In acid solvents the diprotonation occurs and the Qx and Qy bands are transformed into one Q-band red-shifted by 30–140 nm, depending on phenyl-substituent [,]. Such a strong sensitivity to the substituent in the para-phenyl position is important not only for the dication but also (to less extent) for the neutral species. The molecules studied in the present work are not hyperphorphyrins, but the observed small changes in the absorption spectra can be explained by the similar trends found in the tertraphenyl hyperphorphyrins.
To the blue side of the Q(1-0) absorption band (555 nm) of the Zn containing dendrimers there are growing features at about 530 nm []. They also are seen in Frechet-type dendrimers []. In the excitation spectra of the largest dendrimers this increase is even more pronounced as the vibration sublevels collapses into a band at approx. 580 nm. In accord with our B3LYP/3-21G TD FDT calculations this absorption can be interpreted as an enhanced σ(3dx2−y2) – π* transition. In the ZnP molecule this is a forbidden transition (10b1g - 5eg) to the 11Eg state, which is overlapped by the Q-band (Table 1). In H2P there is no metal and no such transition (the σ − π* transition of similar symmetry in the H2P molecule is much higher in energy and is overlapped by the Soret band, Table I). In the TPPZn and HO-TPPZn molecules the σ(3dx2−y2) – π* transition is electric dipole allowed and successively enhanced because of the hyperconjugation of the porphyrin LUMO with tetraphenyl rings and dendrons. The calculated oscillator strength of this transition in TPPZn is very small (3.3 × 10−5) but in the HO-TPPZn molecule the oscillator strength of the σ(3dx2−y2) – π* transition increases by approx. 50%. This effect was examined by changing conformations of a series of acetonide-G1-TPPZn dendrimers by imposing asymmetric distortions to the substituents followed by geometry optimization/relaxation. Although many isomers have essentially the same total energy certain such isomers resulted in that the transition into the 1Eg state is splitted and produces two lines (typically, 517 and 511 nm following from the B3LYP/3-21G TD calculation) with a common oscillator strength increased to 0.0029. For other isomers with more symmetric arrangement of the dendron groups the splitting still exists (515 and 512 nm) but with reduced transition intensity. Thus, only for dendrimers the σ(3dx2−y2) – π* transition became observed. This prediction was obtained only with the 3-21G basis set; the 6-31G** basis set predicts the 11Eg state at higher energy and such interpretation of weak absorption at 530 nm and in the 580 nm regions is not definite.
Calculations of electronic excited states by employing the B3LYP/3-21G TD FDT method indicated that the addition of the OH-group provides a small red-shift of the Q band (73 cm−1, or 2 nm) in HO-TPPZn in comparison with the TPPZn molecule. But for the Soret band of the HO-TPPZn molecule the DFT method predicts a stronger red-shift in comparison with TPPZn in agreement with observations. The reason is connected with the larger involvement of charge transfer from the phenyl rings to those excitations, as determined in the four-orbital scheme. The position of the Soret band in the acetonide-G0/G5-prop-TPPZn dendrimers is slightly shifted to the red side (2–4 nm) upon increase of the dendrimer generation. In free-base analogs, where the Jahn-Teller effect is absent, the shift is negligible. Even the Q(0-0) absorption band of the acetonide-G5-prop-TPPZn dendrimer is blue-shifted as a result of the Jahn-Teller splitting of the 11Eu state. The difference between free-base and zinc-containing dendrimers even is more apparent in fluorescence spectra as will be discussed in the following section.
2.5. Fluorescence of Porphyrin bis-MPA Dendrimers
The emission spectra of bis-MPA coated porphyrins (in THF) in the long wavelength region (600–750 nm) (Figure 5 and 6) indicate quite large differences for higher generations of the HO-prop-TPPZn dendrimers in comparison with the dendron coated free-base variant. The emission of the free-base porphyrin dendrimers shows two peaks, one strong peak at 658 nm and a weaker peak at 714 nm, similar to TPPH2 in different solvents [,]. The former peak corresponds to the 0-0 transition and the latter one to the 0–1 transition. These vibrational frequencies are in the range 1100–1300 cm−1. Moreover, there is no difference in the emission spectra of different generations of the free-base coated dendrimers in this long wavelength region (Figure 5). All free-base porphin-cored bis-MPA dendrimers have the same 0-0 and 0–1 bands in the emission spectra, however, for the zinc-cored porphyrins the spectra show a dramatic different behavior in the region of Q(0-0) and Q(0–1) bands (600–750 nm). The spectrum shows a strong peak at 610 nm, which corresponds to the Q(0-0) band and a smaller one (for Acetonide-G0-prop-TPPZn) around 650 nm, the Q(0–1) band. As can be seen in Figure 6, the emission around 650 nm increases with increasing generation (the difference is largest between the fourth and the fifth generation []; in the latter case a distinct new band at 637 nm appears). The growth of new emission at long wavelengths in TPPZn dendrimers is connected with increased vibronic interaction between porphyrin core and acetonide groups induced by the mixed modes, which touch simultaneousely the ring distortion with the Zn atom in the middle and deformation of phenyl-prop-acetonide moiety.
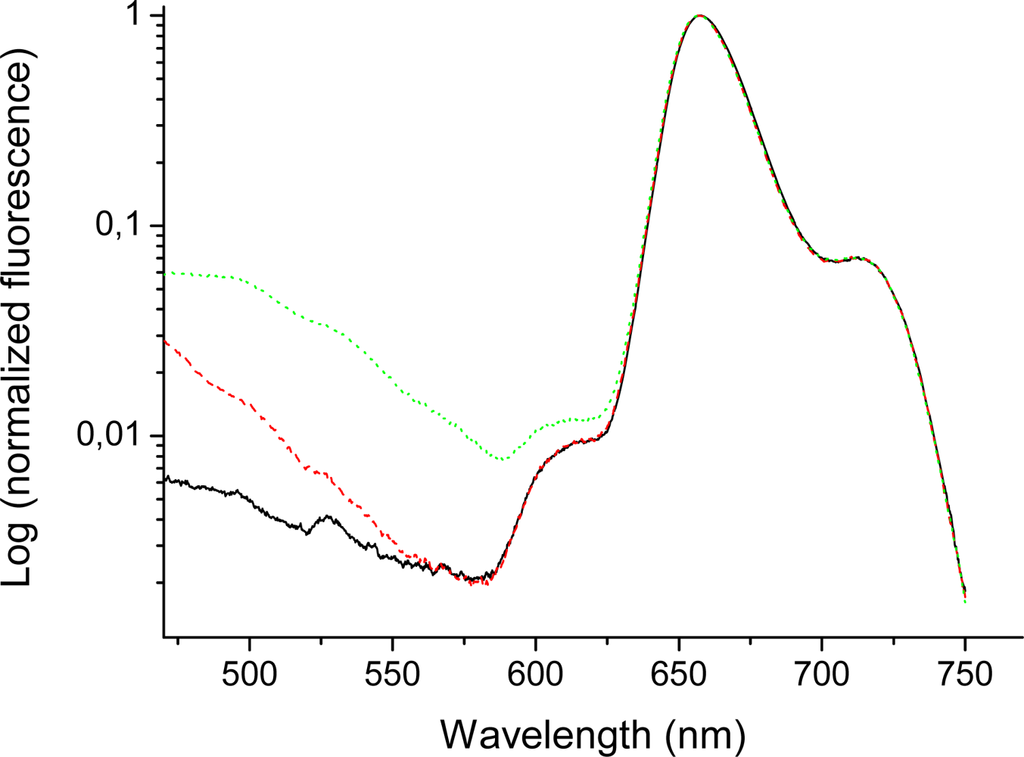
Figure 5.
Emission spectra (logarithmic scale) of Acetonide-G0-prop-TPPH2 (solid/black), Acetonide-G3-prop-TPPH (dashed/red), and Acetonide-G5-prop-TPPH (dotted/green). Concentration 0.010 mM (THF); excitation wavelenth 403 nm. Slits 5 and 10 nm for excitation and emission, respectively.
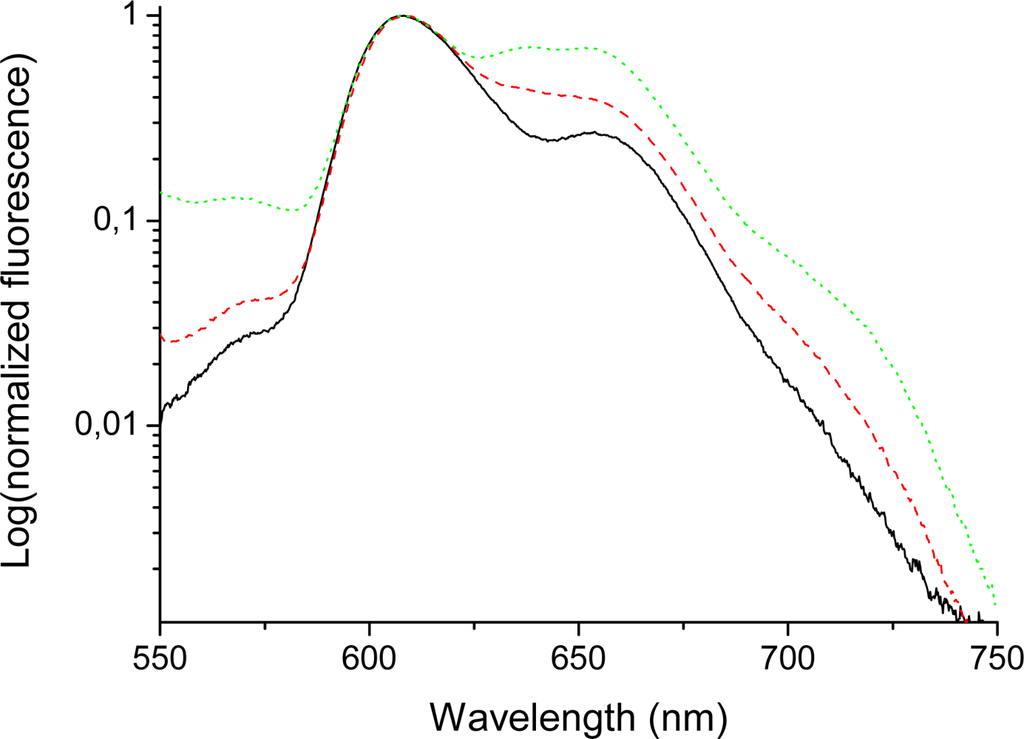
Figure 6.
Emission spectra (logarithmic scale) of Acetonide-G0-prop-TPPZn (solid/black), Acetonide-G4-prop-TPPZn (dashed/red), and Acetonide-G5-prop-TPPZn (dotted/green). Concentration 0.010 mM (THF); excitation wavelenth 403 nm. Slits 5 and 10 nm for excitation and emission, respectively.
This is supported by the time-resolved emission data presented in Figures 7 and 8. The time-decays shown in Figures 7 and 8 are for Acetonide-G2-prop-TPPZn, and the G5 dendrimer, respectively. The molecular systems, here dissolved in THF, were excited at 403 nm and the emission at 650 nm is coded with red dots, whereas the emission at 610 nm is coded with blue triangles. The emission at 650 nm corresponds to the broad vibronic shoulder in the emission spectrum (Figure 6). With no dendrimer, or the smallest dendrimer, the decay-trace can be analysed using a single time-constant of 1.3 - 1.5 ns. This holds true for all dendrimers when detecting the emission at 610 nm. For the larger dendrimers there is an additional contribution from a considerably slower component for the emission at 650. This is readily observed as a “kink” and a decay of slower time-constant in Figure 8. The decay time-constant of this slower decay was between 6.5 and 9 ns (generally, decay times are longer for both the fast and slow components with increasing dendrimer size). Notably, the relative contribution of slow decay in respect to the fast component is larger the larger the dendrimer substituent, so it changes from approximately 5/100 for the G1 dendrimer, up to 35/100 for the G5 dendrimer. There was no change in decay times by varying the dye concentration between 5 and 100 micromolar. Hence, we can attribute this slow component to a true intrinsic “molecular” feature and not to an aggregation effect. The emission decay was also recorded for the series of dendrimer capped TPPH2 variants however, here there was no essential difference between the various dendrimers (data not shown). For the whole series the decay trace could in general be analysed with one dominating (single) decay constant being approximately 9 - 10 ns. Hence, the substituent (dendron) size-effect in the long wavelength region (600–750 nm) of fluorescence emission was exclusive for only the case of dendrimer coated TPPZn.
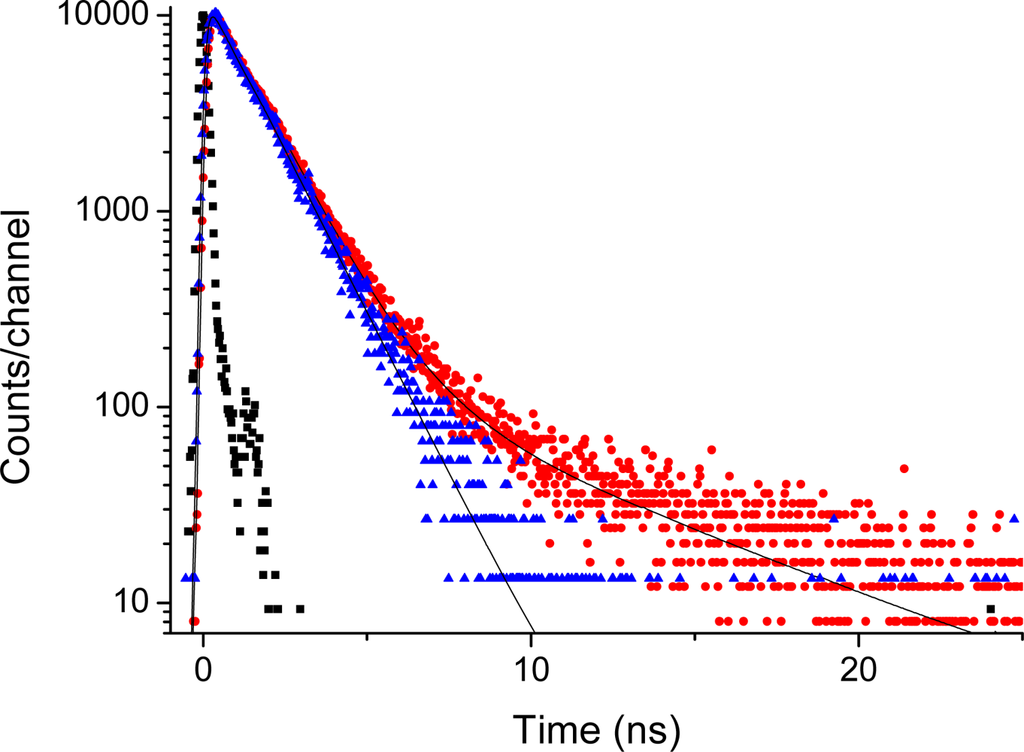
Figure 7.
Emission decay of Acetonide-G2-prop-TPPZn (0.050 mM). Red dots: Excited at 403 nm with emission monitored at 650 nm. Blue triangles: Excited at 403 nm with emission monitored at 600 nm. Black squares is system response used for de-convolution fit; slit 32 nm.
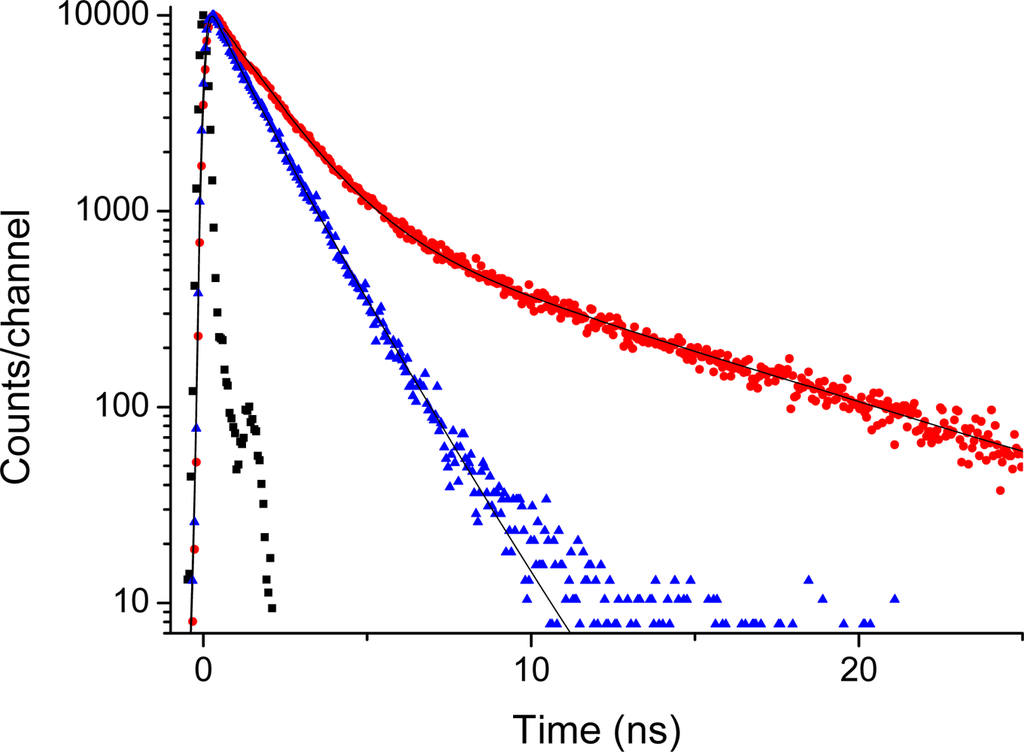
Figure 8.
Emission decay of Acetonide-G5-prop-TPPZn (0.050 mM). Red dots: Excited at 403 nm with emission monitored at 650 nm. Blue triangles: Excited at 403 nm with emission monitored at 600 nm. Black squares is system response used for de-convolution fit; slit 32 nm.
The decay time of all dendron coated free-base variants was in the same order of magnitude as the “slow” component growing in with increased substituents in the TPPZn case. Thus, a plausible reason for appearance of this new emission could be contamination of free base porphyrin in the zinc porphyrin samples since the growing peak occurs in the region of the free base porphyrin fluoresecnce (655 nm). However, no free-base porphyrin was detected in the NMR and UV spectra obtained for the zinc porphyrins []. Another explanation could be that the larger, and notably the fifth-generation, dendrimer was not perfect leading to unsymmetrical substitution of the porphyrin. However, this explanation seems not likely since no traces of such asymmetric units could be observed for the free base variant that was produced using the same procedure. This was also emphasized by the size-exclusion chromatography and the results of other measurements previously reported []. Notably, the different dendrimer substituted TPPH2-cases showed very similar decay traces but a pronounced difference was observed and measured for the time-resolved anisotropy decay, allowing an analysis of their hydrodynamic volume []. Taken together, both photo-physical measurements along with other chemical purification and characterization leads to exclusion of “contamination” effects as a cause of the dendron size effect observed for the substituted TPPZn variants.
We hereby return to the discussions of these results in more detail, in particular the heavy substitution effect observed for the TPPHZn dendrimers. The explanation for this could be interactions between the porphyrin and the dendrons changing the porphyrin conformation and vibrational substructure. Similar changes upon dendron substitution were observed in the absorption spectra as discussed above. The peak at 610 nm in absorption of Zn-containing dendrimers, which is the 0-0 transition to the lowest singlet excited state, corresponds to the 11Eu state of ZnP (Table 1). The excited degenerate state is a subject of the Jahn-Teller distortion and it splits into 11B3u and 11B2u states upon tetraphenyl substitution even at the ground state optimized geometry (Table 1), which in fact is lower than D2h. The nonplanar tetraphenyl substituents do not perturb the degeneracy very much, thus one can speak about the pseudo-Jahn-Teller effect. At this point it is important to mention that the lowest vibrational 0-level in the distorted state is the same in all dendrimer generations. The pseudo-Jahn-Teller effect is calculated by geometry optimization in the excited state of the HO-TPPZn molecule and it is found stronger than in zinc-porphyrin.
The occurence of strong and new peaks to the red-side of the 0,0 band in emission of the Acetonide-G5-prop-TPPZn dendrimer we can explain by manifestation of the pseudo-Jahn-Teller effect in the lowest excited singlet state. Though the D4h symmetry is reduced in TPPZn, in HO-TPPZn and in dendrimers, the quasi-degeneracy of the 11Eu states is still present. The pseudo-Jahn-Teller effect is induced by distortion along the b1g active mode as follows from our geometry optimization in the excited singlet state by employing the TD B3LYP method. The ground state D4h symmetry is reduced to the D2h symmetry in the excited state of the ZnP molecule. The b1g active mode corresponds to the low-frequency vibration (ν7 in Ref []) in the ground state of ZnP molecule. This is a Zn-N stretching and the corresponding translation of all pyrrole rings. A similar analysis was applied for the TPPZn molecule, though it belongs to the C2 point group, since the quasidegeneracy still exists (Table 1). In this case the Zn-N stretching is mixed with the out-of-plane deformations of the C-H bonds in the phenyl rings. In the model of Acetonide-G1-TPPZn dendrimer (Figure 1), calculated with 3-21G basis set, this mode is mixed with the twist and rock vibrations of the methyl groups. The Jahn-Teller effect cannot occur in the molecules of free-base porphyrin type and in their dendrimers; this can explain the large differences in fluorescence spectra of the dendron coated TPPH2- and TPPZn-species (Figures 5 – 8, Ref []).
The vibrational modes connected with phenyl vibrations (frequencies in the range 750–830 cm−1) are becoming more active with increasing generation of TPPZn dendrimers along with the increase of the Jahn-Teller effect. We optimized the HO-TPPHZn molecular geometry in the excited singlet state by the TD B3LYP/3-21G method. The C-OH bond length in the hydroxy groups decreases upon excitation in agreement with the known fact that phenol is a stronger acid in the excited state than in the ground state []. The decrease of the C-OH bond length leads to stronger energy shift of the Q-state (the stronger Jahn-Teller effect) and to an increase of the S0 - S1 transition moment. At the same time the transition moment becomes more sensitive to phenyl vibrations in the range 750–830 cm−1. All these findings can explain the increase of vibronic bands in fluorescence of Acetonide-Gx-prop-TPPZn dendrimers at 650 nm and shorter wavelength for higher generations (Figures 6 and 8).
There are many conformers of dendrimers. For a model shown in Figure 1 we have found more than 20 species by molecular mechanics, but the possible number is much larger. There are six conformers of TPPH2 which are very close in energy (in the range of 1.4 kcal/mol) [] and the number increases to 144 for HO-TPPH2. Being close in the ground state energy they have different frequencies for some vibrational modes of the terminal groups. We can estimate that the number of close-energy conformers of Acetonide-G5-prop-TPPZn dendrimer is larger than a thousand. All of them are present in solvent at room temperature and provide slightly different wavelengths of vibronic transitions. This explains some broadening of vibronic bands in absorption, excitation and emission spectra (Ref. [], Figure 6 and 8).
To the blue side from the 0-0 peak in the fluorescence spectra of all dendrimers there are number of growing emission bands (Figures 5 and 6) which are determined by forbidden transitions from the 11B3g,11B2g excited states (Table 1) strongly influenced by substitution at para-position of phenyl rings. Here we just want to stress that this emission from the highly excited states (550 nm) was not detected before in porphyrins, as far as we know (we do not consider the blue fluorescence from the Soret state, which is induced at 430 nm by strong laser impulse []). The forbidden transitions to the 11B3g, 11B2g states (Table 1) are observed in absorption spectra of dendrimers (Figure 2 from Ref. []) and in fluorescence excitation spectra in Figure 5. These states have been discussed recently with respect to the MgP and ZnP spectra, calculated at different geometries of the porphyrin core []. In free-base porphyrin they have much higher energy than in Zn porphyrins (Table 1; they are of σ − π* nature in PH2 and 3dσ-π* nature in Zn-porphyrin). Their energy strongly depends on the b1g distortion discussed above, indicating the Jahn-Teller effect in Zn-porphyrin. These states are important for calculation of metal-porphyrin phosphorescence since their triplet counterparts are mixed with the ground singlet state by spin-orbit coupling []. As it was mentioned above this 1Eg-state in TPPZn dendrimers is responsible for absorption at 520 nm and is enhanced in higher generations. The other gerade states (11B1g, 21Ag in PH2 and 11B2g, 21A2g, 11B1g in Zn-porphyrin, Table 1) are of the π − π* nature. They have been considered to be responsible for two-photon absorption in TPPH2 []. The energy of those states are close to the Soret band origin; their vibronic transitions probably contribute to the weak emission of dendrimers below 500 nm.
3. Methodology
3.1. Sample Preparation
The synthesis and characterization of dendron-coated porphyrins up to the fifth generation were previously described in []. The porphyrin used was 5,10,15,20-tetrakis(4-hydroxyphenyl)-21H-,23H-porphyrin. Both free-base and zinc cored tetraphenylporphyrin (TPPH2 and TPPZn) were designed with dendrimer coatings. From the porphyrin the dendrons were divergently grown using the anhydride of acetonide protected bis-MPA (acetonide-2,2-bis(methoxy)propanoic anhydride). Synthetic procedure schemes, structures and other properties are discussed by Vestberg et al. [].
3.2. Spectroscopy
The Fourier transform infrared absorption spectra were collected by employing a Perkin-Elmer Spectrum 2000 FT-IR equipped with a MKII Golden Gate, Single Reflection ATR System from Specac Ltd, London. The ATR-crystal was a MKII heated Diamond 45° ATR Top Plate. 16 scans were recorded for each spectrum. Time-resolved fluorescence decays were recorded using an IBH 5000 U fluorescence lifetime spectrometer system wth a TBX-04 picosecond photon detection module. The emission monochromator resolution was 1 nm. IBH NanoLED-10 (443 nm) and NanoLED-07 (405 nm) were used as excitation source for decay measurements of single photon excitation. Melles Griot colored glass filter was used to block scattered light from the excitation source. The fluorescence lifetime decays were measured using time-correlated single photon counting (TC-SPC) along with the IBH Data Station v2.1 software for operation of the spectrometer and deconvolution and analysis of decays. The DAQ-card settings were chosen to give a time resolution below 10 ps. Steady state absorption and fluoresence spectra of bis-MPA dendrimers were presented in Figures 2 and 3 of Vestberg et al. [] For the sake of discussion we present excitation spectra (corresponding absorption spectra for selected emissions) as well as fluorescence spectra of both acetonide-G0/G5-prop-TPPH2 and acetonide-G0/G5-prop-TPPZn types of dendrimers, the latter in logarithmic scale to provide a better comparison of spectral intensities. The steady state emission is complemented with novel time-resolved data recorded at different emission wavelengths in order to aid in the interpretation.
3.3. Theory
Very few large molecules have attracted such attention among theorists as porphyrins. The early theoretical studies were limited to semiempirical methods [] which explained the main features of the electronic absorption optical spectra, but required some adjustable parameters. Later, a number of ab initio methods [,–] and DFT calculations [–,,–] were applied in order to explore their electronic properties. Nonetheless, in spite of a large amount of experimental and theoretical data, there are still many unknowns regarding the structural, electronic, and optical properties for various porphyrins, and many fine details remain to be elucidated [,]. One of these puzzles is the 0–1 vibronic band in tetraphenyl porphyrins spectra. In H2P and ZnP molecules it is more intense than the 0-0 band [,,] while in tetraphenyl derivatives the 0-0 band is more intense, especially in emission spectra.
In the absorption and fluorescence spectra of HO-TPPH2 and HO-TPPZn molecules and their bis-MPA dendrimers presented in Figures 2–3 of Vestberg et al. [] we found conspicuous differences in the vibronic structure of the 0–1 bands of free-base and zinc porphyrins including bis-MPA dendrimers. These became more prominent with higher generations of the Bis-MPA dendrimers attached to the porphyrin ring. In order to interpret these differences one has to take into account both electronic and vibrational states. The general expansion of the total wave function in the adiabatic Born-Oppenheimer approximation Yi,n(q,Q)=Fi(q,Q)c n(Q) can be used, where Fi is the electronic i-state wave function, q and Q denote electronic and nuclear coordinates, respectively []. In the harmonic approximation the nuclear wave function cn(Q) is a product of wave functions of all harmonic vibrational modes Qa. The 0-n vibronic Ψi,0 → Ψf,n transition intensity is determined by the transition dipole moment [,]
where
The first term corresponds to the Franck-Condon (FC) contribution, the second term corresponds to the Herzberg-Teller (HT) contribution of the vibronic spectrum. The FC mechanism arises from the difference in equilibrium geometry between the ground and excited states. The HT contribution results from vibronic coupling between excited electronic states. Similar types of FC and HT mechanisms occur in vibronic theory of Raman spectra [].
We used direct calculation of the total energy first and second derivatives in respect to atomic displacements by the DFT []. Vibrational modes are obtained by diagonalization of the Hessian; IR and Raman intensities are calculated by numerical differentiation of the dipole moment and polarizability, rescpectively. FC factors are estimated through the gradients []. We use the linear vibronic coupling model [,] recently implemented in the time-domain DFT approach for calculation of free-base porphyrin spectra [,].
4. Conclusions
The synthesis and basic properties of dendrimers based on free-base tetraphenylporphyrin and zinc-tetraphenylporphyrin (TPPH2 and TPPZn) were reported some years ago []. These porphyrins were coated with the acetonide-2,2-bis(methoxy)propanoic anhydride dendrons and preliminary results of their photo-physical properties were reported []. The molecules were further studied by IR and fluorescence spectroscopy as presented herein. Theoretical simulations of these spectra employing density functional theory (DFT) calculations indicated some new assignments in vibrational spectra of related porphyrins and in dendrimers. Account of vibronic interactions in simple porphyrins leads us to conclude that the pseudo-Jahn-Teller effect induced by distortion along the b1g active mode in the quasi-degenerate states of the 11Eu type is responsible for the intensity increase of wide vibronic bands in the fluorescence of dendron substituted TPPZn variants. The strong vibronic interaction leads to increased Jahn-Teller distortion of the prophyrin core at higher dendrimer generations. Specifically, this is manifested as an entirely different behaviour of the emission spectra upon substitution of the TPPH2 and TPPZn variants. The 0–1 emission band of the dendron substituted TPPZn experiences a “heavy substitution” effect, giving rise to a 0–1 emission signal associated with a longer decay time (7 - 8 ns) than for the 0-0 emission (1 - 1.5 ns). This contributes with stronger relative emission yield for larger dendron substituents, also in agreement with the appearance of steady state emission spectra showing increased contribution from the 0–1 emission at 650 nm. Since TPPH2 is originally of lower symmetry, the specific distortion upon dendron substitution is not expected, and this was also in agreement with the experimental findings.
Acknowledgments
We thank Robert Vestberg and Andreas Nyström at the Department of Fibre and Polymer Technology, Royal Institute of Technology, Stockholm for providing samples and IR spectra. Collaboration with Yanhua Wang and Yi Luo who provided the FC factor calculation is greatly acknowledged. BM acknowledges the Research Council of Norway for a guest researcher grant within the project “Dendritic nanoporous materials with multifuntionality (contract no. 163529/S10) as well as the Ukrainian-Romanian joint project No = 11.571/2008 and Visby project No = 01403/2007.
References and Notes
- Hoff, A.J.; Deisenhofer, J. Structure and spectroscopy of reaction centers of purple bacteria. Phys. Rep 1997, 287, 1–21. [Google Scholar]
- Rogers, J.; Nguyen, K.; Hufnagle, D.C.; McLean, D.G.; Su, W.; Gossett, K.M.; Burke, A.R.; Vinogradov, S.A.; Pachter, R.; Fleitz, P.A. Observation and interpretation of annulated porphyrins: studies on the photophysical properties of meso-tetraphenylmetalloporphyrins. J. Phys. Chem. A 2003, 107, 11331–11339. [Google Scholar]
- Krasnovskii, A.A.; Bashtanov, M.E.; Drozdova, N.N.; Yuzhakova, O.A.; Luk’yanets, E.A. Laser induced singlet-oxygen-sensitised delayed fluorescence of dyes in aqueous solutions. Quantum Electron 2002, 32, 83–86. [Google Scholar]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol 1992, 55, 145–157. [Google Scholar]
- Felber, B.; Diederich, F. Synthesis of dendritic metalloporphyrins with distal H-bond donors as model systems for hemoglobin. Helv. Chim. Acta 2005, 88, 120–153. [Google Scholar]
- Oakes, R.E.; Bell, S.E.J. DFT studies of the resonance raman spectra of ground and excited triplet state free base meso-tetraphenylporphyrin (H2TPP). J. Phys. Chem. A 2003, 107, 10953–10959. [Google Scholar]
- Jarzecki, A.A.; Kozlowski, P.M.; Pulay, P.; Ye, B.-H.; Li, X.-Y. Scaled quantum mechanical and experimental vibrational spectra of magnesium and zinc porphyrins. Spectrochim. Acta 1997, 53, 1195–1209. [Google Scholar]
- Nakatsuji, H.; Hasegawa, J.; Hada, M. Excited and ionized states of free base porphin studied by the symmetry adapted cluster-configuration interaction (SAC-CI) method. J. Chem. Phys 1996, 104, 2321–2329. [Google Scholar]
- Ohkawa, K.; Hada, M.; Nakatsuji, H. Excited states of four hemes in a c-type cytochrome subunit of the photosynthetic reaction center of Rhodopseudomonas viridis: SAC-CI calculations. J. Porphyr. Phthalocya 2001, 5, 256–266. [Google Scholar]
- Sundholm, D. Interpretation of the electronic absorption spectrum of free-base porphin using time-dependent density-functional theory. Phys. Chem. Chem. Phys 2000, 2, 2275–2281. [Google Scholar]
- Nguyen, K.A.; Day, P.N.; Pachter, R. Triplet excited states of free-base porphin and its β-octahalogenated derivatives. J. Phys. Chem. A 2000, 104, 4748–4754. [Google Scholar]
- Nguyen, K.A.; Pachter, R. Jahn–Teller triplet excited state structures and spectra of zinc complexes of porphyrin and phthalocyanine: A density functional theory study. J. Chem. Phys 2003, 118, 5802–5810. [Google Scholar]
- Edwards, L.; Dolphin, D.H.; Gouterman, M.; Adler, A.D. Porphyrins XVII. Vapor absorption spectra and redox reactions: tetraphenylporphins and porphin. J. Mol. Spectroscopy 1971, 38, 16–32. [Google Scholar]
- Kharlamov, B.M.; Bykovskaya, L.A.; Personov, R.I. Hole-burning spectra. A new method for obtaining fine structure in absorption spectra of organic molecules. Chem. Phys. Lett 1977, 50, 407–411. [Google Scholar]
- Bykovskaya, L.A.; Gradushko, A.T.; Personov, R.I.; Romanovskii Yu, V.; Solovjov, K.N.; Staruchin, A.S.; Shulga, A.M. Method for determining of the polarization of vibronic transitions of polyatomic molecules in isotropic media upon selective laser excitation. Izv. Akad. Nauk SSSR 1980, 44, 822–826. (in Russian).. [Google Scholar]
- Minaev, B.; Ågren, H. Theoretical DFT study of phosphorescence from porphyrins. Chem. Phys 2005, 315, 215–239. [Google Scholar]
- Minaev, B.; Wang, Y.-H.; Wang, C.-K.; Luo, Y.; Ågren, H. Density functional theory study of vibronic structure of the first absorption Qx band in free-base porphin. Spectrochim. Acta. Part A 2006, 65, 308–323. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; Dapprich, S.; Millam, J.M.; Daniels, A.D.; Kudin, K.N.; Strain, M.C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Cli®ord, S.; Ochterski, J.; Petersson, G.A.; Ayala, P.Y.; Cui, Q.; MoroKUMa, K.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Cioslowski, J.; Ortiz, J.V.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Andres, J.L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E.S.; Pople, J.A. Gaussian 98; 1998; Gaussian Inc: Pittsburgh PA, 1998; ( www.gaussian.com).
- Radziszewski, J.G.; Waluk, J.; Nepras, M.; Michl, J. Fourier transform fluorescence and phosphorescence of porphine in rare gas matrixes. J. Phys. Chem 1991, 95, 1963–1969. [Google Scholar]
- Li, X.Y.; Zgierski, M.Z. Porphine force field: in-plane normal modes of free-base porphine; comparison with metalloporphines and structural implications. J. Phys. Chem 1991, 95, 4268–4287. [Google Scholar]
- Kawa, M.; Fréchet, J.M.J. Self-assembled lanthanide-cored dendrimer complexes: enhancement of the luminescence properties of lanthanide ions through site-isolation and antenna effects. Chem. Mater 1998, 10, 286–296. [Google Scholar]
- Dichtel, W.R.; Hecht, S.; Fréchet, J.M.J. Functionally layered dendrimers: a new building block and its application to the synthesis of multichromophoric light-harvesting systems. Org. Lett 2005, 7, 4451–4454. [Google Scholar]
- Lindgren, M.; Minaev, B.; Glimsdal, E.; Vestberg, R.; Westlund, R.; Malmström, E. Electronic states and phosphorescence of dendron functionalized platinum(II) acetylides. J. Luminesc 2007, 124, 302–310. [Google Scholar]
- Pitois, C.; Hult, A.; Lindgren, M. Lanthanide-cored fluorinated dendrimer complexes: synthesis and luminescence characterization. J. Luminesc 2005, 111, 265–283. [Google Scholar]
- Westlund, R.; Malmstrom, E.; Lopes, C.; Ohgren, J.; Rodgers, T.; Saito, Y.; Kawata, S.; Glimsdal, E.; Lindgren, M. Efficient nonlinear absorbing platinum(II) acetylide chromophores in solid PMMA matrices. Adv. Funct. Mater 2008, 18, 1939–1948. [Google Scholar]
- Vestberg, R.; Westlund, R.; Eriksson, A.; Lopes, C.; Carlsson, M.; Eliasson, B.; Glimsdal, E.; Lindgren, M.; Malmstrøm, E. Dendron decorated platinum(II) acetylides for optical power limiting. Macromolecules 2006, 39, 2238–2246. [Google Scholar]
- Westlund, R.; Glimsdal, E.; Lindgren, M.; Vestberg, R.; Hawker, C.; Lopes, C.; Malmström, E. Click chemistry for photonic applications: triazole-functionalized platinum(II) acetylides for optical power limiting. J. Mater. Chem 2008, 18, 166–175. [Google Scholar]
- Gunnlaugsson, T.; Leonard, J.P. Responsive lanthanide luminescent cyclen complexes: from switching/sensing to supramolecular architectures. Chem. Commun 2005, 25, 3114–3131. [Google Scholar]
- Vestberg, R.; Nystrom, A.; Lindgren, M.; Malmstrom, E.; Hult, A. Porphyrin-cored 2,2-bis(methylol)propionic acid dendrimers. Chem. Mater 2004, 16, 794–2804. [Google Scholar]
- Gillies, E.R.; Fréchet, J.M.J. Designing Macromolecules for Therapeutic Applications: Polyester Dendrimer — Poly(ethylene oxide) “Bow-Tie” Hybrids with Tunable Molecular Weight and Architecture. J. Am. Chem. Soc 2002, 124, 14137–14146. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648–5652. [Google Scholar]
- Alben, J.O.; Choi, S.S.; Adler, A.D.; Caughey, WS. Infrared spectroscopy of porphyrins. Ann. N. Y. Acad. Sci 1973, 206, 278–295. [Google Scholar]
- Kincaid, J.R.; Nakamoto, K. Vibrational spectra of transition metal complexes of tetraphenylporphine. J. Inorg. Nucl. Chem 1975, 37, 85–89. [Google Scholar]
- Kozuka, M.; Nakamoto, K. Vibrational studies of (tetraphenylporphyrinato)cobalt(II) and its adducts with carbon monoxide, nitric oxide, and oxygen in gas matrixes. J. Am. Chem. Soc 1981, 103, 2162–2168. [Google Scholar]
- Li, X.Y.; Czernuszewicz, R.S.; Kincaid, J.R.; Su, Y.O; Spiro, T.G. Consistent porphyrin force field. 1. Normal-mode analysis for nickel porphine and nickel tetraphenylporphine from resonance Raman and infrared spectra and isotope shifts. J. Phys. Chem 1990, 94, 31–47. [Google Scholar]
- Guo, H.; Jiang, J.; Shi, Y.; Wang, Y.; Liu, J.; Dong, S. UV-vis spectrophotometric titrations and vibrational spectroscopic characterization of meso-(p-hydroxy-phenyl)porphyrins. J. Phys. Chem. B 2004, 108, 10185–10191. [Google Scholar]
- Andersson, L.A.; Loehr, T.M.; Thompson, R.G.; Strauss, S.H. Influence of symmetry on the vibrational spectra of Zn(TPP), Zn(TPC), and Zn(TPiBC). Inorg. Chem 1990, 29, 2142–2147. [Google Scholar]
- Kozlowski, P.M.; Jarzecki, A.A.; Pulay, P.; Li, X.Y.; Zgierski, M.Z. Vibrational assignment and definite harmonic force field for porphine. 2. Comparison with nonresonance Raman data. J. Phys. Chem 1996, 100, 13985–13992. [Google Scholar]
- Tazi, M.; Lagant, P.; Vergoten, G. Use of the Resonance Raman Intensities To Check the Density Functional Theory Derived Force Field of the Free Base Porphine. J. Phys. Chem. A 2000, 104, 618–625. [Google Scholar]
- Oakes, R.E.; Spence, S.J.; Bell, S.E.J. Resonance Raman and DFT studies of tetra-tert-butyl porphine: assignment of strongly enhanced distortion modes in a ruffled porphyrin. J. Phys. Chem. A 2003, 107, 2964–2973. [Google Scholar]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectroscopy 1961, 6, 138–163. [Google Scholar]
- Almlöf, J. Ab Initio calculation on porphin. Int. J. Quant. Chem 1974, 8, 915–924. [Google Scholar]
- Nakatsuji, H.; Takashima, H.; Hada, M. Spin-orbit effect on the magnetic shielding constant using the ab initio UHF method. Chem. Phys. Lett 1995, 233, 95–101. [Google Scholar]
- Serrano-Andrés, L.; Merchán, M.; Rubio, M.; Roos, B.O. Interpretation of the electronic absorption spectrum of free base porphin by using multiconfigurational second-order perturbation theory. Chem. Phys. Lett 1998, 295, 195–203. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett 1996, 256, 454–464. [Google Scholar]
- Minaev, M. Theoretical study of the external heavy atom effect on phosphorescence of free-base porphin molecule. Spectrochim. Acta. Part A 2004, 60, 3213–3224. [Google Scholar]
- Loboda, O.; Tunnell, I.; Minaev, B.; Ågren, H. Theoretical study of triplet state properties of free-base porphin. Chem. Phys 2005, 312, 299–309. [Google Scholar]
- Liao, M.S.; Scheiner, S. Electronic structure and bonding in metal porphyrins, metal=Fe, Co, Ni, Cu, Zn. J. Chem. Phys 2002, 117, 205–219. [Google Scholar]
- Kruk, M.; Karotki, A.; Drobizhev, M.; Kuzmitsky, V.; Gael, V.; Rebane, A. Two-photon absorption of tetraphenylporphin free base. J. Luminesc 2003, 105, 45–55. [Google Scholar]
- Gouterman, M. Optical spectra and electronic structure of porphyrins and related rings. In The Porphyrins, 2nd Ed; Dolphin, D., Ed.; Academic Press: New York, 1978; Volume 3. [Google Scholar]
- Albrecht, A. Vibronic-spin-orbit perturbations and the assignment of the lowest triplet state of benzene. J. Chem. Phys 1963, 38, 354–365. [Google Scholar]
- Köppel, H.; Domcke, W.; Cederbaum, L.S. Multi-mode molecular dynamics beyond the Born-Oppenheimer approximation. Adv. Chem. Phys 1984, 57, 59–246. [Google Scholar]
- Stanton, J.F.; Sattelmeyer, K.W.; Gauss, J.; Allan, M.; Skalicky, T.; Bally, T. On the photoelectron spectrum of p-benzoquinone. J. Chem. Phys 2001, 115, 1–4. [Google Scholar]
- Gladkov, L.L.; Gradyushko, A.T.; Shulga, A.M.; Solovyov, K.N-; Starukhin, A.S. Experimental and theoretical investigation of infrared spectra of porphin, its deuterated derivatives and their metal complexes. J. Mol. Struct 1978, 47, 463–493. [Google Scholar]
- Kozlowski, P.M.; Jarzecki, A.A.; Pulay, P. Vibrational assignment and definite harmonic force field for porphine. 1. Scaled quantum mechanical results and comparison with empirical force field. J. Phys. Chem 1996, 100, 7007–7013. [Google Scholar]
- Gladkov, L.L.; Solovyov, K.N. The normal coordinate analysis of porphin and its derivatives based on the solution of the inverse spectral problem for porphin and Cu porphin - I. A valence force field for in-plane vibrations of the porphin molecule. Spectrochim. Acta Part A 1985, 41, 1437–1442. [Google Scholar]
- Kitagawa, T.; Ozaki, Y. Infrared and Raman spectra of metalloporphyrins. Struct. Bond 1987, 64, 71–114. [Google Scholar]
- Kim, D.; Terner, J.; Spiro, T.G. Excited triplet state resonance Raman spectra of magnesium, zinc, and palladium tetraphenylporphine. J. Am. Chem. Soc 1986, 108, 2097–2099. [Google Scholar]
- Matos, M.; Hofkens, J.; Verheijen, W.; De Schryver, F.C.; Hecht, S.; Pollak, K.W.; Fréchet, J.M.J.; Forier, B.; Dehaen, W. Effects of generation number and solvent on photophysical and hydrodynamic properties of dendrimers with porphyrin and metalloporphyrin as core. Macromolecules 2000, 33, 2967–2973. [Google Scholar]
- Minaev, B.F.; Minaev, A.B. Calculation of the phosphorescence of porphyrins by the density functional method. Opt. Spectrosc 2005, 98, 214–219. [Google Scholar]
- Weinkauf, J.R.; Cooper, S.W.; Schweiger, A.; Wamser, C.C. Substituent and solvent effects on the hyperporphyrin spectra of diprotonated tetraphenylporphyrins. J. Phys. Chem. A 2003, 107, 3486–3496. [Google Scholar]
- Retsek, J.L.; Medforth, C.J.; Nurco, D.J.; Gentemann, S.; Chirvony, V.S.; Smith, K.M.; Holten, D. Conformational and electronic effects of phenyl-ring fluorination on the photophysical properties of nonplanar dodecaarylporphyrins. J. Phys. Chem. B 2001, 105, 6396–6411. [Google Scholar]
- Wasbotten, I.H.; Conradie, J.; Ghosh, A. Electronic absorption and resonance Raman signatures of hyperporphyrins and nonplanar porphyrins. J. Phys. Chem. B 2003, 107, 3613–3623. [Google Scholar]
- Bour, P.; Záruba, K.; Urbanová, M.; Setnicka, V.; Matejka, P.; Fiedler, Z.; Král, V.; Volka, K. Vibrational circular dichroism of tetraphenylporphyrin in peptide complexes? A computational study. Chirality 2000, 12, 191–198. [Google Scholar]
- Tobita, S.; Kaizu, Y.; Kobayashi, H.; Tanaka, I. Study of higher excited singlet states of zinc(II)-tetraphenylporphin. J. Chem. Phys 1984, 81, 2962–2969. [Google Scholar]
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).