A Review on Direct Electrochemistry of Catalase for Electrochemical Sensors
Abstract
:1. Introduction
2. Nanomaterial Free Matrices for CAT Immobilization - Versatile Approaches
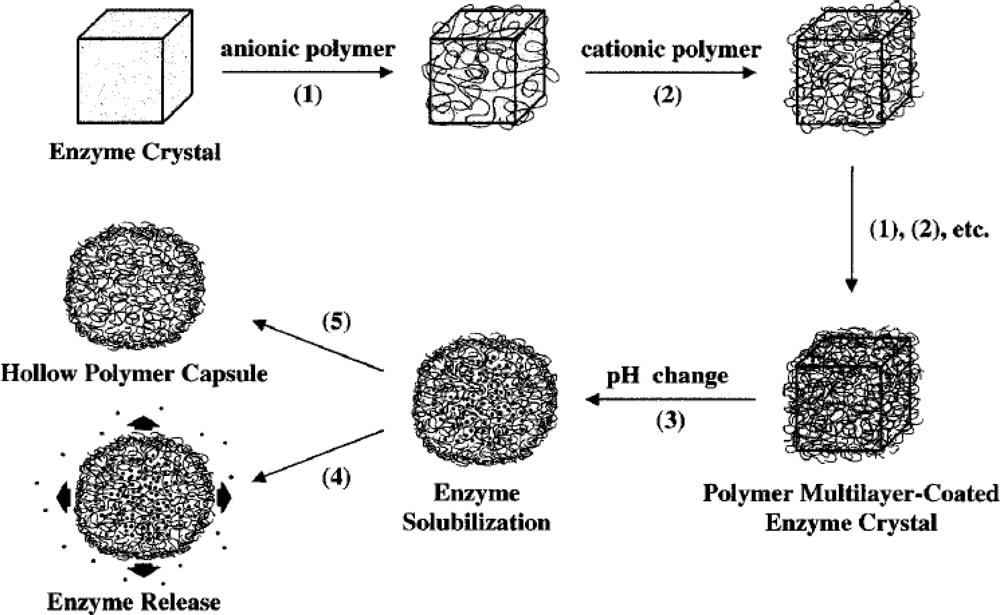
2.1. Polyelectrolyte Encapsulated CAT
2.2. Surfactant Modified Matrices for CAT Immobilization
2.3. Immobilization of CAT at Various Polymers and Gel Matrices
2.4. Dendrimer Matrices for CAT Immobilization
3. Nanomaterial Matrices used for CAT Immobilization
3.1. CAT Immobilized in Single-wall Carbon Nanotubes (SWCNTs) Matrices
3.2. CAT Immobilized in a SWCNTs-CS Matrix
3.3. CAT Immobilized in Nafion-nano Gold-MWCNT Matrices
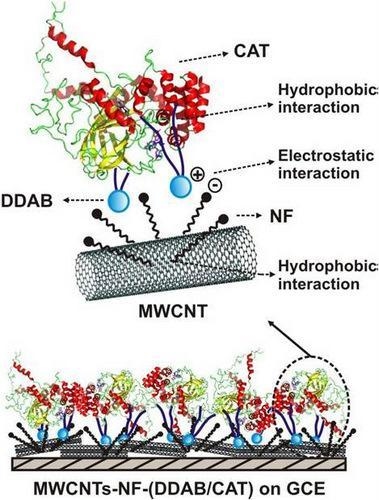
3.4. CAT Immobilized on MWCNTs Incorporated Glassy Carbon Electrodes
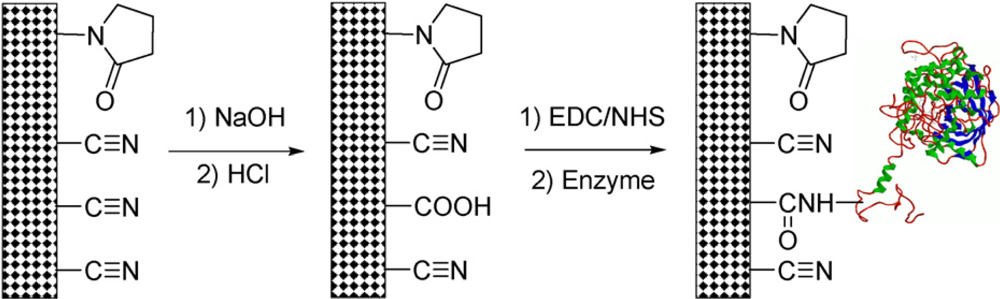
3.5. Covalent Immobilization of CAT on Conductive Composite Nanofiber Meshes
3.6. CAT Immobilized on Nickel Oxide Nanoparticles Modified Glassy Carbon Electrodes
4. Benefits of Nanomaterial Matrices above Nanomaterial Free Matrices for Efficient CAT Immobilization towards the Development of CAT Based Electrochemical Sensors
5. Conclusions
Acknowledgments
References
- Armstrong, F.A.; Wilson, G.S. Recent developments in faradaic bioelectrochemistry. Electrochim. Acta 2000, 45, 2623–2645. [Google Scholar]
- Hill, H.A.O.; Hunt, N.I. Direct and indirect electrochemical investigations of metalloenzymes. Methods Enzymol 1993, 227, 501–522. [Google Scholar]
- Bond, A.M. Chemical and electrochemical approaches to the investigation of redox reactions of simple electron transfer metalloproteins. Inorg. Chim. Acta 1994, 226, 293–340. [Google Scholar]
- Ghindilis, A.L.; Atanasov, P.; Wilkins, E. Enzyme-catalyzed direct electron transfer: Fundamentals and Analytical Applications. Electroanalysis 1997, 9, 661–674. [Google Scholar]
- Ferapontova, E.E.; Ruzgas, T.; Gorton, L. Direct electron transfer of heme and molybdopterin cofactor-containing chicken liver sulfite oxidase on alkanethiol-modified gold electrodes. Anal. Chem 2003, 75, 4841–4850. [Google Scholar]
- Gorton, L.; Lindgren, A.; Larsson, T.; Munteanu, F. D.; Ruzgas, T.; Gazaryan, I. Direct electron transfer between heme-containing enzymes and electrodes as basis for third generation biosensors. Anal. Chim. Acta 1999, 400, 91–108. [Google Scholar]
- Yeh, P.; Kuwana, T. Reversible electrode reaction of cytochrome C. Chem. Lett 1977, 6, 1145–1148. [Google Scholar]
- Eddowes, M. J.; Hill, H.A.O. Novel method for the investigation of the electrochemistry of metalloproteins : cytochrome C. J. Chem. Soc., Chem. Commun 1977, 771–772. [Google Scholar]
- Wollenberger, U.; Spricigo, R.; Leimkühler, S.; Schröder, K. Protein electrodes with direct electrochemical communication. Adv. Biochem. Engi. Biotechnol 2008, 109, 19–64. [Google Scholar]
- Nicholls, P.; Schonbaum, G.R.; Boyer, I.; Lardy, P.D.; Myrback, H. The Enzymes; Academic Press: Orlando, 1963; Volume 8, pp. 158–159. [Google Scholar]
- Buleandra, M.; Radu, G.L; Tanase, I. Redox protein electroanalysis: metalloproteins. Roum. Biotechnol. Lett 2000, 5, 423–438. [Google Scholar]
- Lai, M.E.; Bergel, A. Direct electrochemistry of catalase on glassy carbon electrodes. Bioelectrochemistry 2002, 55, 157–160. [Google Scholar]
- Caruso, F.; Trau, D.; Mohwald, H.; Renneberg, R. Enzyme encapsulation in layer-by-layer engineered polymer multilayer capsules. Langmuir 2000, 16, 1485–1488. [Google Scholar]
- Yu, A.; Caruso, F. Thin films of polyelectrolyte-encapsulated catalase microcrystals for biosensing. Anal. Chem 2003, 75, 3031–3037. [Google Scholar]
- Chen, X.; Xie, H.; Kong, J.; Deng, J. Characterization for didodecyldimethylammonium bromide liquid crystal film entrapping catalase with enhanced direct electron transfer rate. Biosens. Bioelectron 2001, 16, 115–120. [Google Scholar]
- Gebicka, L.; Gebicki, J.L. Interaction of sodium bis (2-ethylhexyl) sulfosuccinate (AOT) with catalase and horseradish peroxidase in an aqueous solution and in the reverse micelles of AOT/n-heptane. Biochem. Mol. Biol. Int 1998, 45, 805–811. [Google Scholar]
- Huang, H.; Hu, N.; Zeng, Y.; Zhou, G. Electrochemistry and electrocatalysis with heme proteins in chitosan biopolymer films. Anal. Biochem 2002, 308, 141–151. [Google Scholar]
- Lu, H.Y.; Li, Z.; Hu, N.F. Direct voltammetry and electrocatalytic properties of catalase incorporated in polyacrylamide hydrogel films. Biophys. Chem 2003, 104, 623–632. [Google Scholar]
- Li, Y.M.; Chen, X.T.; Li, J.; Liu, H.H. Direct voltammetry and catalysis of hemoenzymes in methyl cellulose film. Electrochim. Acta 2004, 49, 3195–3200. [Google Scholar]
- Li, M.; He, P.; Zhang, Y.; Hu, N. An electrochemical investigation of hemoglobin and catalase incorporated in collagen films. Biochim. Biophys. Acta 2005, 1749, 43–51. [Google Scholar]
- Wu, Y.; Shen, Q.; Hu, S. Direct electrochemistry and electrocatalysis of heme-proteins in regenerated silk fibroin film. Anal. Chim. Acta 2006, 558, 179–186. [Google Scholar]
- Sun, Y.X.; Wang, S.F. Direct electrochemistry and electrocatalytic characteristic of heme proteins immobilized in a new sol–gel polymer film. Bioelectrochemistry 2007, 71, 172–179. [Google Scholar]
- Di, J.; Zhang, M.; Yao, K.; Bi, S. Direct voltammetry of catalase immobilized on silica sol–gel and cysteine modified gold electrode and its application. Biosens. Bioelectron 2006, 22, 247–252. [Google Scholar]
- Wang, S.F.; Chen, T.; Zhang, Z.L.; Pang, D.W.; Wong, K.L. Effects of hydrophilic room-temperature ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate on direct electrochemistry and bioelectrocatalysis of heme proteins entrapped in agarose hydrogel films. Electrochem. Commun 2007, 9, 1709–1714. [Google Scholar]
- Shen, L.; Hu, N. Heme protein films with polyamidoamine dendrimer: direct electrochemistry and electrocatalysis. BBA-Bioenergetics 2004, 1608, 23–33. [Google Scholar]
- Salimi, A.; Miranzadeh, L.; Hallaj, R.; Mamkhezri, H. Picomolar detection of hydrogen peroxide at glassy carbon electrode modified with NAD+ and single walled carbon nanotubes. Electroanal 2008, 20, 1760–1768. [Google Scholar]
- Shi, L.; Liu, X.; Niu, W.; Li, H.; Han, S.; Chen, J.; Xu, G. Hydrogen peroxide biosensor based on direct electrochemistry of soybean peroxidase immobilized on single-walled carbon nanohorn modified electrode. Biosens. Bioelectron 2009, 24, 1159–1163. [Google Scholar]
- Cosnier, S.; Ionescu, R.E.; Holzinger, M. Aqueous dispersions of SWCNTs using pyrrolic surfactants for the electro-generation of homogeneous nanotube composites. Application to the design of an amperometric biosensor. J. Mater. Chem 2008, 18, 5129–5133. [Google Scholar]
- Wang, L.; Wang, J.; Zhou, F. Direct electrochemistry of catalase at a gold electrode modified with single-wall carbon nanotubes. Electroanal 2004, 16, 627–632. [Google Scholar]
- Miao, Y.; Chia, L.S.; Goh, N.K.; Tan, S.N. Amperometric glucose biosensor based on immobilization of glucose oxidase in chitosan matrix cross-linked with glutaraldehyde. Electroanal 2001, 13, 347–349. [Google Scholar]
- Okuma, H.; Watanabe, E. Flow system for fish freshness determination based on double multi enzyme reactor electrodes. Biosens. Bioelectron 2002, 17, 367–372. [Google Scholar]
- Hikima, S.; Kakizaki, T.; Taga, M.; Hasebe, K. Enzyme sensor for L-lactate with a chitosan mercury film electrode. J. Anal. Chem 1993, 345, 607–609. [Google Scholar]
- Chen, L.; Gorski, W. Bioinorganic composites for enzyme electrodes. Anal. Chem 2001, 73, 2862–2868. [Google Scholar]
- Jiang, H.; Du, C.; Zou, Z.; Li, X.; Akins, D.; Yang, H. A biosensing platform based on horseradish peroxidase immobilized onto chitosan-wrapped single-walled carbon nanotubes. J. Solid State Electrochem 2009, 13, 791–798. [Google Scholar]
- Jiang, H.J.; Yang, H.; Akins, D.L. Direct electrochemistry and electrocatalysis of catalase immobilized on a SWNT-nanocomposite film. J. Electroanal. Chem 2008, 623, 181–186. [Google Scholar]
- Zhou, B.; Wang, J.; Gao, X.; Tian, Y. Attachment of nanoparticles to pyrolytic graphite electrode and its application for the direct electrochemistry and electrocatalytic behavior of catalase. Anal. Lett 2008, 41, 1832–1849. [Google Scholar]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci 1973, 241, 20–22. [Google Scholar]
- Chattopadhyay, D.; Galeska, I.; Papadimitrakopoulos, F. Metal-assisted organization of shortened carbon nanotubes in monolayer and multilayer forest assemblies. J. Am. Chem. Soc 2001, 123, 9451–9452. [Google Scholar]
- Zhang, B.; Zhang, Z.; Wang, B.; Yan, J.; Li, J.; Cai, S.M. Preparation of gold nano-arraied electrode on silicon substrate and its electrochemical properties: Probe into biosensor based on electroluminescence of porous silicon. Acta Chimi. Sin 2001, 59, 1932–1936. [Google Scholar]
- Salimi, A.; Noorbakhsh, A.; Ghadermarz, M. Direct electrochemistry and electrocatalytic activity of catalase incorporated onto multiwall carbon nanotubes-modified glassy carbon electrode. Anal. Biochem 2005, 344, 16–24. [Google Scholar]
- Zhao, G.C.; Zhang, L.; Wei, X.W.; Yang, Z.S. Myoglobin on multi-walled carbon nanotubes modified electrode: direct electrochemistry and electrocatalysis. Electrochem. Commun 2003, 5, 825–829. [Google Scholar]
- Lu, H.; Li, Z.; Hu, N. Direct voltammetry and electrocatalytic properties of catalase incorporated in polyacrylamide hydrogel films. Biophys. Chem 2003, 104, 623–632. [Google Scholar]
- Huang, H.; Hu, N.; Zeng, Y.; Zhou, G. Electrochemistry and electrocatalysis with heme proteins in chitosan biopolymer films. Anal. Biochem 2002, 308, 141–151. [Google Scholar]
- Zhang, Z.; Chouchane, S.; Magiliozzo, R.S.; Rusling, J.F. Direct voltammetry and catalysis with mycobacterium tuberculosis catalase-peroxidase, peroxidases, and catalase in lipid films. Anal. Chem 2002, 74, 163–170. [Google Scholar]
- Bond, A.M. Modern polarographic methods in analytical chemistry; Marcel Dekker: New York, 1980. [Google Scholar]
- Zhou, H.; Lu, T.H.; Shi, H.X.; Dai, Z.H.; Huang, X.H. Direct electrochemistry and electrocatalysis of catalase immobilized on multi-wall carbon nanotubes modified glassy carbon electrode and its application. J. electroanal. Chem 2008, 612, 173–178. [Google Scholar]
- Prakash, P.A.; Yogeswaran, U.; Chen, S.M. Direct electrochemistry of catalase at multiwalled carbon nanotubes - nafion in presence of needle shaped DDAB for H2O2 sensor. Talanta. [CrossRef]
- Wang, Z.G.; Ke, B.B.; Xu, Z.K. Covalent immobilization of redox enzyme on electrospun nonwoven poly(acrylonitrile-co-acrylic acid) nanofiber mesh filled with carbon nanotubes: a comprehensive study. Biotechnol. Bioeng 2007, 97, 708–720. [Google Scholar]
- Wan, L.S.; Ke, B.B.; Xu, Z.K. Electrospun nanofibrous membranes filled with carbon nanotubes for redox enzyme immobilization. Enzyme Microb. Technol 2008, 42, 332–339. [Google Scholar]
- Wan, L.S.; Ke, B.B.; Wu, J.; Xu, Z.K. Catalase immobilization on electrospun nanofibers: effects of porphyrin pendants and carbon nanotubes. J. Phys. Chem. C 2007, 111, 14091–14097. [Google Scholar]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nano-scale islands of nickel oxide. Biophys. Chem 2007, 125, 540–548. [Google Scholar]

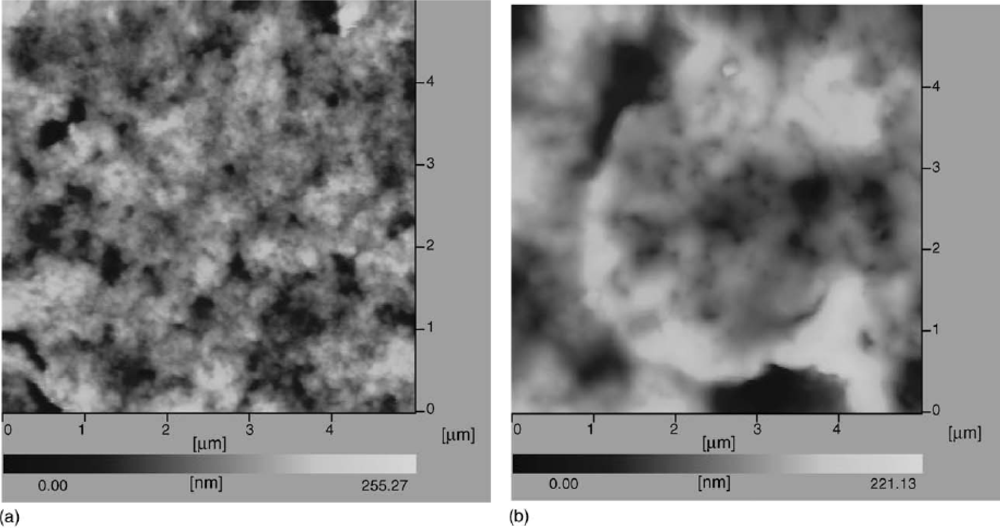


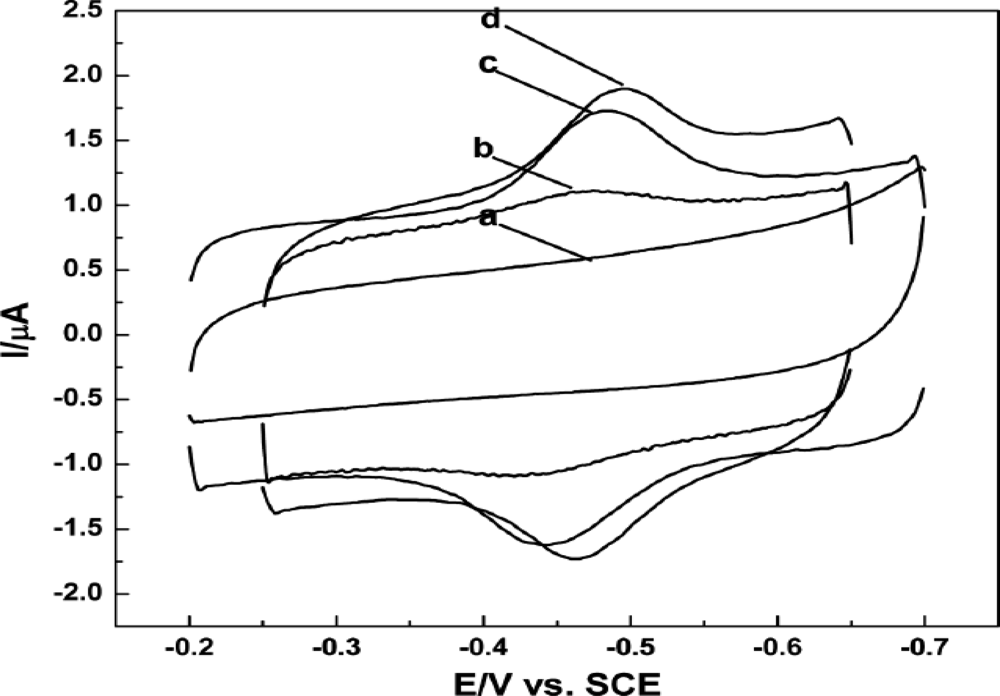

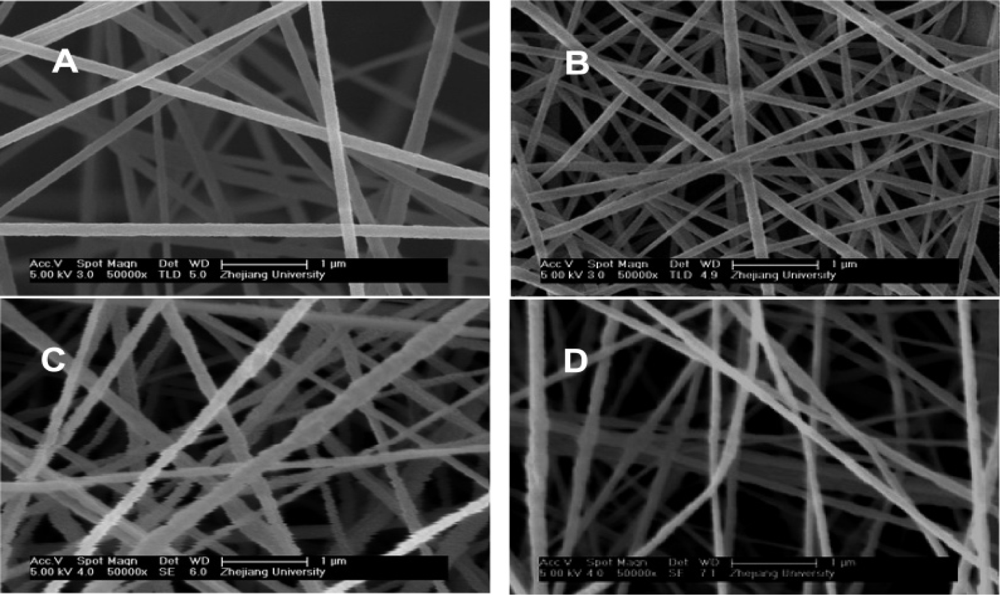
| Modified Electrode | Techniques | Epaa (or) Epcb (V) | Linear range (mM) | Sensitivity (μA mM−1 cm−2) | Detection limit (μM) | Ref |
|---|---|---|---|---|---|---|
| CAT-PAM | CV | −0.5b | 0.4 – 0.8 | - | - | [18] |
| CAT-MC | CV | −0.4b | 0.02–0.12 | - | - | [19] |
| CAT-SF | i-t | −0.2b | 0.003–0.158 | - | - | [21] |
| CAT-PNM | i-t | −0.25b | 0.002–0.035 | - | - | [22] |
| CAT/cysteine/Si sol–gel | i-t | 0.1b | 0.001–0.03 | - | 0.4 | [23] |
| CAT-agarose | CV | −0.24b | 0.001 – 0.818 | - | - | [24] |
| CAT-SWCNTs | CV | −0.4a | 0.7–1.1 | - | 4.0 | [29] |
| CAT-SWCNTs-CS | CV | −0.52b | 5–50 | 6.32 | 2.5 | [35] |
| NF-MWCNTs-CAT–GNP | CV | −0.29a | 1–5 | - | - | [36] |
| CAT-MWCNTs | i-t | −0.3b | 0.01–0.1 | 3.3 | 1.0 | [40] |
| CAT-MWCNTs | CV | −0.56b | 1.0 – 4.8 | - | - | [46] |
| MWCNTs-NF-(DDAB/CAT) | CV | −0.38b | 0.5 – 1.2 | 35.62 | 150 | [47] |
| CAT-NiO | i-t | −0.3b | 0.001–1.0 | 15.9 | 0.60 | [51] |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prakash, P.A.; Yogeswaran, U.; Chen, S.-M. A Review on Direct Electrochemistry of Catalase for Electrochemical Sensors. Sensors 2009, 9, 1821-1844. https://doi.org/10.3390/s90301821
Prakash PA, Yogeswaran U, Chen S-M. A Review on Direct Electrochemistry of Catalase for Electrochemical Sensors. Sensors. 2009; 9(3):1821-1844. https://doi.org/10.3390/s90301821
Chicago/Turabian StylePrakash, Periasamy Arun, Umasankar Yogeswaran, and Shen-Ming Chen. 2009. "A Review on Direct Electrochemistry of Catalase for Electrochemical Sensors" Sensors 9, no. 3: 1821-1844. https://doi.org/10.3390/s90301821
APA StylePrakash, P. A., Yogeswaran, U., & Chen, S.-M. (2009). A Review on Direct Electrochemistry of Catalase for Electrochemical Sensors. Sensors, 9(3), 1821-1844. https://doi.org/10.3390/s90301821