1. Introduction
There is an increasing demand for cost-effective and long-term stable measuring systems for gas monitoring in the environment [
1,
2]. Beside traditional monitoring tasks (e.g., in research, emission analysis and safety) carbon capture and storage (CCS) develops to an important new application field for subsurface gas monitoring [
3-
6].
The analysis of CO
2 gas comprises a long history going back 180 years with the development of several chemical and physical methods such as: gas chromatography, infrared analysis,
14C isotope measurement, mass spectrometry, FT-IR spectroscopy, gas diffusion-flow injection (GD-FIA) or continious flow systems based on photometric detection with various pH indicator systems, conductimetric sensors, thermistors and acoustic detectors [
7].
Lewicki and Oldenburg [
8] show by numerical investigations that monitoring of CO
2 in the subsurface has greater potential to detect and quantify gas dynamics in heterogeneous ground than above-ground techniques. But up to now the development of a suitable measurement system for
in situ gas monitoring remains to be a challenge, for both scientific and technical reasons. With respect to the heterogeneity of natural systems membrane based monitoring techniques particularly those based on polymers, gain increasing importance for environmental gas measurement.
Typically, membranes are used as a gas-permeable phase boundary. Based on this approach a gas saturometer was introduced already in 1975 to measure the equilibrium gas pressure for a given dissolved gas in a liquid. This technique is still available as
Total Dissolved Gas sensor [
9]. Numerous different applications combining standard analytical techniques and phase separation were developed e.g., [
10-
13]. Due to its low interaction such combinations of standard analytics with phase separating tubes have a significant importance for the
in situ measurements.
The measurement behind a phase separating membrane requires the equilibrium for all permeating substances and therefore, a high permeability of the membrane would be preferable. On the other hand, low gas permeability is required to conserve the equilibrated gas constitution inside the membrane tube during its transport to the analytical device. This problem of optimization restricts the temporal resolution of the readings and the spatial extend of such a measurement system.
To overcome this limitation, we developed a flux-based measurement method [
14] operating near the dynamic equilibrium, which is reached fast in contrast to thermodynamic equilibrium. The gas selectivity of membranes is used as sensory principle and no transport of some gas sample towards an analytical device is required. The robust method is applicable for quantification of the constitution of a multi-component gas [
15] e.g., in soils, aquifers or bodies of water.
One objective of this paper is to demonstrate theoretically the equivalence of a continuous (volume-based) application of the sensor with the discontinuous (pressure based) method. In many practical cases one is interested in the concentration of only one gas component within a given gaseous or liquid phase. Therefore, we present a new concept for a single component analysis, which is a special case of the multi-component theory and which is the main objective of our paper. For this special case the constructive effort can be reduced and the sensor handling becomes relatively simple. We demonstrate the application of the single component analysis for monitoring of O2 and CO2 in a water-unsaturated soil.
3. Lysimeter Experiment
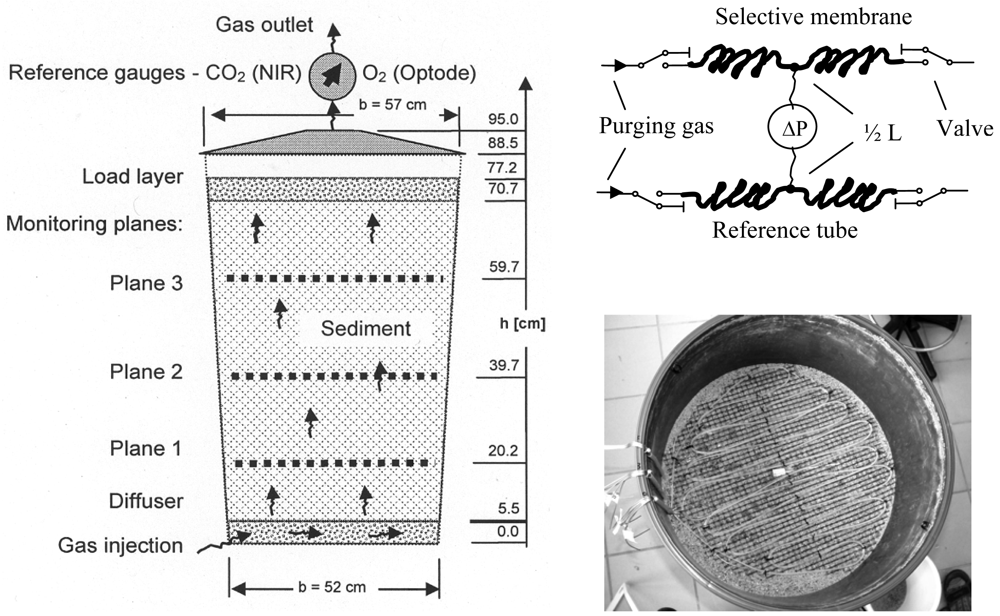
A lysimeter experiment was designed to investigate the accuracy of the sensor under controlled conditions in the lab prior to
in situ measurements in soil. The lysimeter (
Figure 1) was filled by 238 kg dry medium sand (0.1 - 1 mm particle size). During filling three equidistant monitoring planes were installed horizontally. A diffuser (porous PE-sheet over a 21 kg gravel layer) at the bottom of the lysimeter guaranties a homogeneous gas flow. At the top a 28 kg gravel layer was inserted to stabilize the sand pack. Both sediments were divided by gauze (2 mm mashes) to prevent mixing. The lysimeter was closed and sealed.
The gas inlet at the bottom was connected to a set of calibrated mass flow controllers (MFC 8712, Bürkert Fluid Control Systems), which were used to define the composition of the continuously injected gas phase. The outlet of gas was in the centre of the top cover of the lysimeter. Reference gauzes for O
2 (fiber-optic oxygen meter, Fibox 2,
www.PreSens.de) and CO
2 (near infrared, BCP-CO2,
www.getsens.com) were installed near the outlet.
Each horizontal monitoring plane consists of a 6 - 7 m meander-like membrane tube. We used a commercial polydimethylsiloxane tubing (
Ri = 0.75 mm,
Ra = 1.75 mm, perm-selectivity's:
fO2/N2 = 1.97,
fCO2/N2 = 9.89 [
16]) as sensor membrane. The individual membrane tubes were connected with valves (positioned outside the lysimeter) by stainless steel capillaries (1 mm aperture). The pressure difference between the sensor and the reference tube was measured outside the lysimeter by a pressure sensor (PCLA12X5D1, operating pressure 0 … ±12.5 mbar,
www.sensortechnics.com) which was connected using stainless steel capillaries (1 mm aperture). The valves allowed to close the membrane tubes and to purge it by dry air. To quantify
a1 the pressure development inside the closed tubes was recorded by the pressure sensor over 20 s.
In principle, the membrane sensors could be calibrated for single-gas analysis according
Equation (9) using the extensive data sets available in literature. It should be noted that the actual material properties may differ from those in literature, due to the individual technological process of tube manufacturing and the actual chemical membrane formulation. Therefore, to enable the usage of ordinary, commercial available tubing, the membrane sensors were adjusted for the gas component of interest by simple 2-point calibration of
px =
k1a1 +
k2 prior to the experiments using the MFCs.
Flow rate and composition of the input gas was controlled by a PC, which was also used for the on-line conversion of pressure readings into partial pressures. Different gas mixtures were injected at the bottom of lysimeter with a constant flow rate of 0.5 L/min. They were analyzed within the monitoring planes using the calibrated membrane sensors. The time resolution of the measurement was set to about 6 min.
4. Results and Discussion
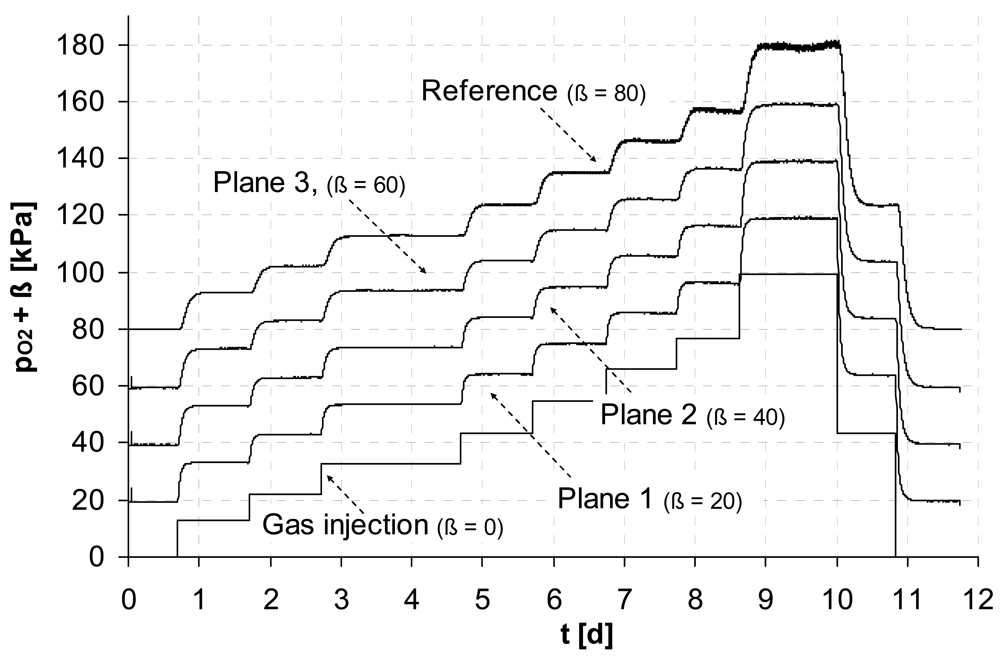
The first two experiments show the detection of O2 and CO2 within the monitoring planes for step-like input functions of the individual gas. To demonstrate the wide area of potential applications the concentration ranges were chosen with respect to typical scales of environmental applications.
A wide concentration range of dissolved oxygen is of interest e.g., to control biosparging, a common technology applied for remediation of contaminated groundwater, which uses the injection of air to enhance the activity of microbes. In
Figure 2 (top) a perfect match at the relevant concentration range for O
2 mixed with N
2 (to simulate groundwater-near gas composition) is illustrated between the new measurement technique and the reference optode within all monitoring planes.
An excellent agreement was also found for monitoring pCO
2 (
Figure 2, bottom). In this example we simulate a typical concentration range of CO
2 mixed with air, which can be expected from aerobe microbial activity in soils.
Using the steady states (plateaus) where the gas concentration is the same throughout the entire experimental system, we estimate a mean statistical error for that first in situ sensor test of less then 2% with respect to the reading. The standard error (exemplarily estimated for the data from plane 1) of the regression against the individual reference sensors was for the O2-measurement less than 0.7 kPa (range 0 - 100 kPa) and for the CO2-measurement less than 0.08 kPa (range: 0 - 10 kPa). This smaller error of CO2-measurement can be mainly attributed to the higher selectivity of CO2 (fCO2/N2 = 9.89) with respect to the ones of O2 (fO2/N2 = 1.97) of the used membrane material.
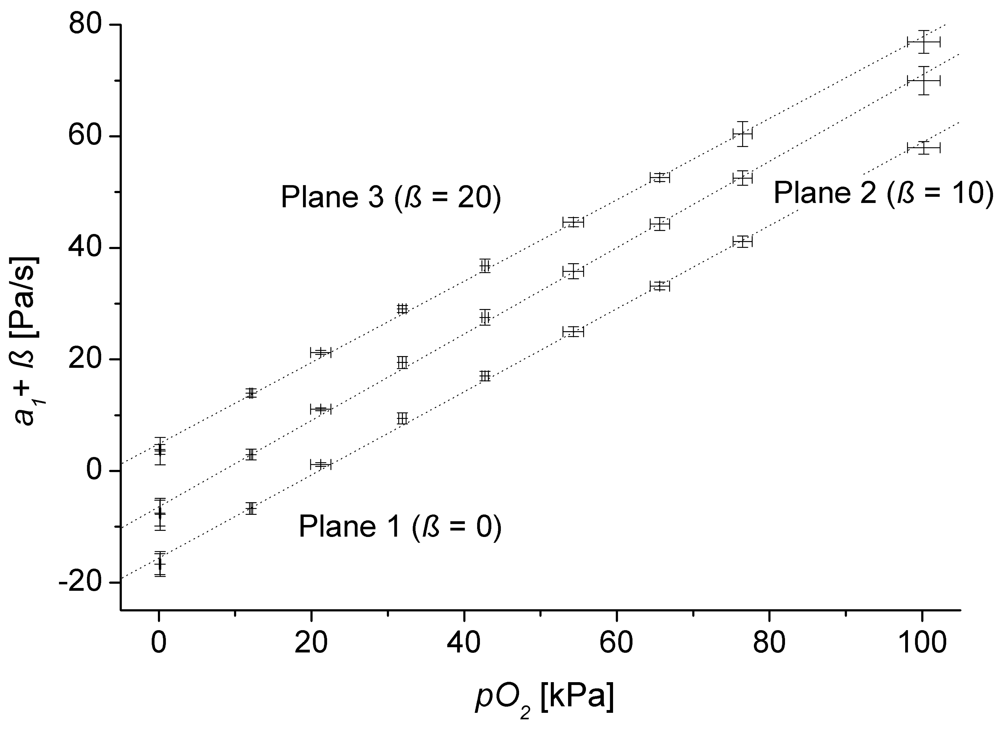
As a prerequisite of the applied theory the gas permeation through the membrane need to be independent of the concentrations within the observed measurement range so, that linearity between concentration and pressure change can be assumed. However it is widely known from literature that this independence is only given for small concentrations. Hence, the corresponding limits need to be known. To investigate this critical aspect the measured coefficients
a1 where correlated with the partial pressure measured by the reference optode (
pO2) at the upper outlet of the lysimeter (
Figure 1). We used the test series for oxygen because of its large measurement range. For the different concentration plateaus (see
Figure 2) the mean values of
a1 and the partial pressure are plotted (
Figure 3) including the 3-fold standard deviation for both
a1 and
pO2.
Table 1 presents the fit results of
a1 = (
c1 ±
δc1)
pO2 + (
c2 ±
δc2) for all monitoring planes (see
Figure 2) where c
iare fit parameters with standard errors
δci,
R² is correlation coefficient.
Both
Figure 3 and
Table 1 confirm our linearity assumption over the whole measurement range. Furthermore, the coefficients
c2 are negative. Due to purging the tube sensors by air the regression crosses zero if the oxygen concentration inside the lysimeter exceeds the one in the purging gas.
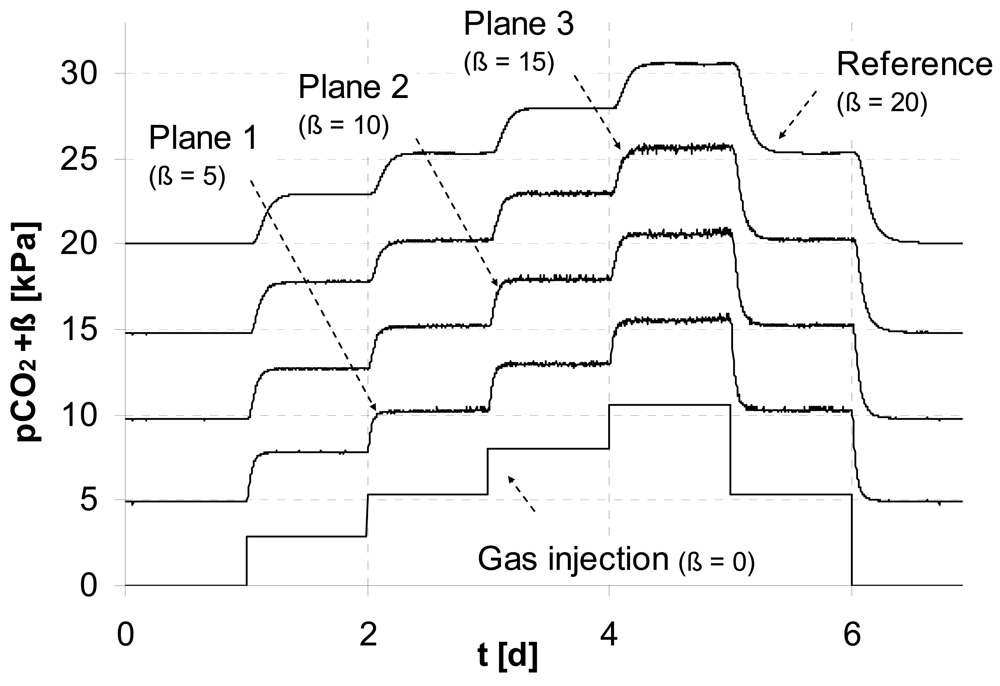
To demonstrate the ability of the new technique to capture fluctuating concentrations in a multi-component gas as typical for natural systems we mix CO
2 and dry air dynamically to obtain an oscillating partial pressure:
PCO2(
t)|
x=0 =
A +
B sin(2
π t/τ) (
A = 6 kPa,
B = 4 kPa,
τ [h] – oscillation period). The flow rate of the gas mixture was adjusted to 0.5 L/min.
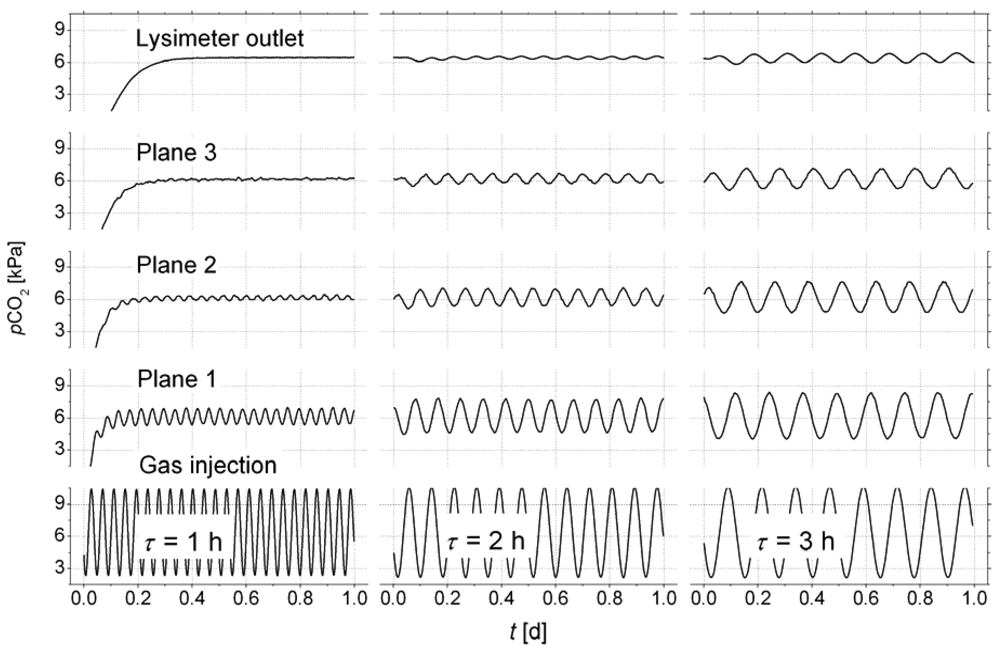
Figure 4 shows the dampening of the signal along the flow path, which demonstrates the accuracy of the measurement.
The first test was performed with an oscillation period of
τ = 1h, which was completely smoozed inside the sediment. Using an oscillation period of two hours, the reference sensor on the top of the lysimeter started to see the oscillation. For
τ = 3h the signal on top was sufficient to analyse cross-correlations of the signals between the monitoring planes. The lag of the first correlation maximum marks the mean travel time of the concentration wave for the distance between two monitoring planes i, j: Δ
t1,2 = 27.3 min, Δ
t2,3 = 28.6 min. Using the distances between the monitoring planes (see
Figure 1) the mean distance velocity can be calculated (
v1,2 = 0.71 cm/min,
v2,3 = 0.70 cm/min).
The porosity of φ = 0.35 was calculated from bulk density of the sediment. Together with the known cross sections of the lysimeter the volumetric gas flow was calculated based on the velocities v1,2, and v2,3. We obtained Q1,2 = 0.578 L/min, and Q2,3 = 0.579 L/min, which differ by less than 1 %.
The comparison with the actually applied flow rate of 0.5 L/min indicates an overestimation of the flow. However, due to the complex geometry of the pore space, it can be assumed that not the entire air-filled cross section contributes to flow. Based on our measurements we could estimate an ‘effective’ porosity of 0.31.
5. Conclusions
This study presents a novel in situ sensor concept. The membrane-based sensors have demonstrated its long term stability in a lab-lysimeter over a couple of years. Using such sensors with tubular geometry it is possible to measure an average gas concentration value over a certain line with negligible impact on the environment by the sensor. As demonstrated by monitoring of O2 and CO2 in a lysimeter, this technique is highly attractive for monitoring the gas dynamics in soil. To expand the number of measurable gases (e.g., CH4, H2S) further experimental work is necessary.
If such sensors are installed in a specific pattern (e.g., regular, hierarchic, site specific), it is possible to calculate a meaningful (representative) average of gas concentrations over a larger area. Therefore, the measuring tube can replace a large number of individual sensors, reducing the cost for representative measurements by previous methods. It will also be possible to gain scale-depended insights into the spatial variability of gas behavior (formation, migration) in saturated and unsaturated porous media. Another advantage of the sensor is the possibility to use ordinary and easily available tube materials after a simple calibration.
Potential technical applications for membrane-based gas sensors are environmental remediation (e.g., measuring of O2- and CO2-distributions in the heterogeneous subsurface for aerobic biodegradation) like biosparging or bioventing of organic contaminants in ground- and seepage water and landfill monitoring.
Due to the fast answer of such line-sensor networks the technology could be also advantageous for safety monitoring of CO2-sequestration, gas pipelines or sewers. The principle can be applied in pure liquids for monitoring of e.g., surface waters and boreholes.