Mesoporous Silicate Materials in Sensing
Abstract
:1. Introduction
2. Sensors for Relative Humidity
3. pH Sensors
4. Metal Cation Sensors
4.1. Optical Sensing
4.2. Electrochemical Sensing
5. Small Molecules and Ions
6. TICs, Pesticides, and Other Targets
7. Hard Templates for Sensing Materials
8. Conclusions
Acknowledgments
References and Notes
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, 1990. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; Higgins, J.B.; Schlenker, J.L. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar]
- Inagaki, S.; Fukushima, Y.; Kuroda, K. Synthesis of Highly Ordered Mesoporous Materials from a Layered Polysilicate. J. Chem. Soc. Chem. Commun. 1993, 8, 680–682. [Google Scholar]
- Tanev, P.T.; Pinnavaia, T.J. A Neutral Templating Route to Mesoporous Molecular Sieves. Science 1995, 267, 865–867. [Google Scholar]
- Bagshaw, S.A.; Prouzet, E.; Pinnavaia, T.J. Templating of Mesoporous Molecular Sieves by Nonionic Polyethylene Oxide Surfactants. Science 1995, 269, 1242–1244. [Google Scholar]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar]
- Chen, C.-Y.; Li, H.-X.; Davis, M.E. Studies on mesoporous materials I. Synthesis and characterization of MCM-41. Microporous Mater. 1993, 2, 17–26. [Google Scholar]
- Attard, G.S.; Glyde, J.C.; Goltner, C.G. Liquid-Crystalline Phases as Templates for the Synthesis of Mesoporous Silica. Nature 1995, 378, 366–368. [Google Scholar]
- Attard, G.S.; Edgar, M.; Goltner, C.G. Inorganic Nanostructures from Lyotropic Liquid Crystal Phases. Acta. Mater. 1998, 46, 751–758. [Google Scholar]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid Inorganic-Organic Mesoporous Silicates-Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar]
- Schmidt, H. New Type of Non-Crystalline Solids Between Inorganic and Organic Materials. J. Non-Cryst. Solids 1985, 73, 681–691. [Google Scholar]
- Wilkes, G.L.; Orler, B.; Huang, H. Polym. Prepr.; 1985; Volume 26, pp. 300–301. [Google Scholar]
- Burkett, S.L.; Sims, S.D.; Mann, S. Synthesis of hybrid inorganic-organic mesoporous silica by co-condensation of siloxane and organosiloxane precursors. Chem. Commun. 1996, 1367–1368. [Google Scholar]
- Macquarrie, D.J. Chem. Commun.; 1996; pp. 1961–1962. [Google Scholar]
- Lim, M.H.; Blanford, C.F.; Stein, A. Synthesis and Characterization of a Reactive Vinyl-Functionalized MCM-41: Probing the Internal Pore Structure by a Bromination Reaction. J. Am. Chem. Soc. 1997, 119, 4090–4091. [Google Scholar]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel Mesoporous Materials with a Uniform Distribution of Organic Groups and Inorganic Oxide in Their Frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar]
- Melde, B.J.; Holland, B.T.; Blanford, C.F.; Stein, A. Mesoporous Sieves with Unified Hybrid Inorganic/Organic Frameworks. Chem. Mater. 1999, 11, 3302–3308. [Google Scholar]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar]
- Yohsina-Ishii, C.; Asefa, T.; Coombs, N.; MacLachlan, M.J.; Ozin, G.A. Periodic mesoporous organosilicas, PMOS: fusion of organic and inorganic chemistry “inside” the channel walls of hexagonal mesoporous silica. Chem. Commun. 1999, 2539–2540. [Google Scholar]
- Loy, D.A.; Shea, K.J. Bridged Polysilsesquioxanes. Highly Porous Hybrid Organic-Inorganic Materials. Chem. Rev. 1995, 95, 1431–1442. [Google Scholar]
- Carrington, N.A.; Xue, Z.L. Inorganic Sensing Using Organofunctional Sol-Gel Materials. Acc. Chem. Res. 2007, 40, 343–350. [Google Scholar]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar]
- Trewyn, B.G.; Giri, S.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor system. Chem. Commun. 2007, 3236–3245. [Google Scholar]
- Walcarius, A. Electroanalytical applications of microporous zeolites and mesoporous (Organo)silicas: Recent trends. Electroanalysis 2008, 20, 711–738. [Google Scholar]
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar]
- Ariga, K.; Vinu, A.; Hill, J.P.; Mori, T. Coordination chemistry and supramolecular chemistry in mesoporous nanospace. Coord. Chem. Rev. 2007, 251, 2562–2591. [Google Scholar]
- Domansky, K.; Liu, J.; Wang, L.-Q.; Engelhard, M. H.; Baskaran, S. Chemical sensors based on dielectric response of functionalized mesoporous silica films. J. Mater. Res. 2001, 16, 2810–2816. [Google Scholar]
- Bertolo, J.M.; Bearzotti, A.; Generosi, A.; Palummo, L.; Albertini, V.R. X-rays and electrical characterizations of ordered mesostructured silica thin films used as sensing membranes. Sens. Actuat. B 2005, 111-112, 145–149. [Google Scholar]
- Falcaro, P.; Bertolo, J.M.; Innocenzi, P.; Amenitsch, H.; Bearzotti, A. Ordered Mesostructured Silica Films: Effect of Pore Surface on its Sensing Properties. J. Sol-Gel Sci. Tech. 2004, 32, 107–110. [Google Scholar]
- Innocenzi, P.; Falcaro, P.; Bertolo, J.M.; Bearzotti, A.; Amenitsch, H. Electrical responses of silica mesostructured films to changes in environmental humidity and processing conditions. J. Non-Cryst. Solids 2005, 351, 1980–1986. [Google Scholar]
- Innocenzi, P.; Martucci, A.; Guglielmi, M.; Bearzotti, A.; Traversa, E. Electrical and structural characterisation of mesoporous silic thin films as humidity sensors. Sens. Actuat. B 2001, 76, 299–303. [Google Scholar]
- Geng, W.; Li, X.; Zhang, T.; Wang, W.; Qiu, S. Humidity Sensitivity of Polypyrrole and Polypyrrole/SBA-15 Host-Guest Composite Materials. J. Appl. Polym. Sci. 2006, 102, 3301–3305. [Google Scholar]
- Geng, W.; Wang, R.; Li, X.; Zou, Y.; Zhang, T.; Tu, J.; He, Y.; Li, N. Humidity sensitive property of Li-doped mesoporous silica SBA-15. Sens. Actuat. B 2007, 127, 323–329. [Google Scholar]
- Zhang, T.; Wang, R.; Geng, W.; Li, X.; Qi, Q.; He, Y.; Wang, S. Study on humidity sensing properties based on composite materials of Li-doped mesoporous silica A-SBA-15. Sens. Actuat. B 2008, 128, 482–487. [Google Scholar]
- Bearzotti, A.; Bertolo, J.M.; Innocenzi, P.; Falcaro, P.; Traversa, E. Relative humidity and alcohol sensors based on mesoporous silica thin films as synthesized from block copolymers. Sens. Actuat. B 2003, 95, 107–110. [Google Scholar]
- Bearzotti, A.; Bertolo, J.M.; Innocenzi, P.; Falcaro, P.; Traversa, E. Humidity sensors based on mesoporous silica thin films synthesized by block copolymers. J. Eur. Cer. Soc. 2004, 24, 1969–1972. [Google Scholar]
- Wang, C.-T.; Wu, C.-L. Electrical sensing properties of silica aerogel thin films to humidity. Thin Solid Films 2006, 496, 658–664. [Google Scholar]
- Wang, C.-T.; Wu, C.-L.; Chen, I.-C.; Huang, Y.-H. Humidity sensors based on silica nanoparticle aerogel thin films. Sens. Actuat. B 2005, 107, 402–410. [Google Scholar]
- Bearzotti, A. Influence of metal electrodes on the response of humidity sensors coated with mesoporous silica. J. Phys. D: Appl. Phys. 2008, 41, 1–5. [Google Scholar]
- Wirnsberger, G.; Scott, B.J.; Stucky, G.D. pH Sensing with mesoporous thin films. Chem. Commun. 2001, 119–120. [Google Scholar]
- Wirnsberger, G.; Yang, P.; Scott, B.J.; Chmelka, B.F.; Stucky, G.D. Mesostructured Materials for Optical Applications: From Low-k Dielectrics to Sensors and Lasers. Spectrochim. Acta A 2001, 57, 2049–2060. [Google Scholar]
- Li, L.-L.; Sun, H.; Fang, C.-J.; Jin, J.-L.; Yan, C.-H. Optical sensors based on functionalized mesoporous silica SBA-15 for the detection of multianalytes (H+ and Cu+) in water. J. Mater. Chem. 2007, 17, 4492–4498. [Google Scholar]
- Miled, O.B.; Grosso, D.; Sanchez, C.; Livage, J. An optical fibre pH sensor based on dye doped mesostructured silica. J. Phys. Chem. Solids 2004, 65, 1751–1755. [Google Scholar]
- Safavi, A.; Maleki, N.; Bagheri, M. Modification of chemical performance of dopants in xerogel films with entrapped ionic liquid. J. Mater. Chem. 2007, 17, 1674–1681. [Google Scholar]
- Yang, T.-H.; Yee, C.K.; Amweg, M.L.; Singh, S.; Kendall, E.L.; Dattelbaum, A.M.; Shreve, A.P.; Brinker, C.J.; Parikh, A.N. Optical Detection of Ion-Channel Proton Transport in Supported Phospholipid Bilayers. Nano Lett. 2007, 7, 2446–2451. [Google Scholar]
- Nicole, L.; Boissière, C.; Grosso, D.; Hesemann, P.; Moreau, J.; Sanchez, C. Advanced selective optical sensors based on periodically organized mesoporous hybrid silica thin films. Chem. Commun. 2004, 2312–2313. [Google Scholar]
- Balaji, T.; Sasidharan, M.; Matsunaga, H. Optical sensor for the visual detection of mercury using mesoporous silica anchoring porphyrin moiety. Analyst 2005, 130, 1162–1167. [Google Scholar]
- Balaji, T.; Sasidharan, M.; Matsunaga, H. Naked eye detection of cadmium using inorganicorganic hybrid mesoporous material. Anal. Bioanal. Chem. 2006, 384, 488–494. [Google Scholar]
- Métivier, R.; Leray, I.; Lebeau, B.; Valeur, B. A mesoporous silica functionalized by a covalently bound calixarene-based fluoroionophore for selective optical sensing of mercury(II) in water. J. Mater. Chem. 2005, 15, 2965–2973. [Google Scholar]
- Lee, M.H.; Lee, S.J.; Jung, J.H.; Lim, H.; Kim, J.S. Luminophore-immobilized mesoporous silica for selective Hg2+ sensing. Tetrahedron 2007, 63, 12087–12092. [Google Scholar]
- Zhang, H.; Zhang, P.; Ye, K.; Sun, Y.; Jiang, S.; Wang, Y.; Pang, W. Mesoporous material grafted with luminescent molecules for the design of selective metal ion chemosensor. J. Lumin. 2006, 117, 68–74. [Google Scholar]
- Kledzik, K.; Orłowska, M.; Patralska, D.; Gwiazda, M.; Jezierska, J.; Pikus, S.; Ostaszewski, R.; Kłonkowski, A.M. Cu(II) recognition materials: Fluorophores grafted on mesoporous silica supports. Appl. Surf. Sci. 2007, 254, 441–451. [Google Scholar]
- Balaji, T.; El-Safty, S.A.; Matsunaga, H.; Hanaoka, T.; Mizukami, F. Optical Sensors Based on Nanostructured Cage Materials for the Detection of Toxic Metal Ions. Angew. Chem. Int. Ed. 2006, 45, 7202–7208. [Google Scholar]
- El-Safty, S.A.; Prabhakaran, D.; Ismail, A.A.; Matsunaga, H.; Mizukami, F. Nanosensor Design Packages: A Smart and Compact Development for Metal Ions Sensing Responses. Adv. Funct. Mater. 2007, 17, 3731–3745. [Google Scholar]
- El-Safty, S.A.; Prabhakaran, D.; Ismail, A.A.; Matsunaga, H.; Mizukami, F. Three-Dimensional Wormhole and Ordered Mesostructures and Their Applicability as Optically Ion-Sensitive Probe Templates. Chem. Mater. 2008, 20, 2644–2654. [Google Scholar]
- Miled, O.B.; Sanchez, C.; Livage, J. Spectroscopic studies and evanescent optical fibre wave sensing of Cu2+ based on activated mesostructured silica matrix. J. Mater. Chem. 2005, 40, 4523–4530. [Google Scholar]
- Gao, L.; Wang, Y.; Wang, J.; Huang, L.; Shi, L.; Fan, X.; Zou, Z.; Yu, T.; Zhu, M.; Li, Z. A Novel ZnII-Sensitive Fluorescent Chemosensor Assembled within Aminopropyl-Functionalized Mesoporous SBA-15. Inorg. Chem. 2006, 45, 6844–6850. [Google Scholar]
- Gao, L.; Wang, J.Q.; Huang, L.; Fan, X.X.; Zhu, J.H.; Wang, Y.; Zou, Z.G. Novel Inorganic-Organic Hybrid Fluorescence Chemosensor Derived from SBA-15 for Copper Cation. Inorg. Chem. 2007, 46, 10287–10293. [Google Scholar]
- El-Safty, S.A.; Ismail, A.A.; Matsunaga, H.; Mizukami, F. Optical Nanosensor Design with Uniform Pore Geometry and Large Particle Morphology. Chem. Eur. J. 2007, 13, 9245–9255. [Google Scholar]
- Ismail, A.A. A selective optical sensor for antimony based on hexagonal mesoporous structures 2008. 2008, 317, 288–297.
- El-Safty, S.A.; Ismail, A.A.; Matsunaga, H.; Nanjo, H.; Mizukami, F. Uniformly Mesocaged Cubic Fd3m Monoliths as Modal Carriers for Optical Chemosensors. J. Phys. Chem. C 2008, 112, 4825–4835. [Google Scholar]
- Carrington, N.A.; Thomas, G.H.; Rodman, D.L.; Beach, D.B.; Xue, Z.-L. Optical Determination of Cr(VI) Using Regenerable, Functionalized Sol-Gel Monoliths. Anal. Chim. Acta 2007, 581, 232–240. [Google Scholar]
- Clavier, C.W.; Rodman, D.L.; Sinski, J.F.; Allain, L.R.; Im, H.J.; Yang, Y.; Clark, J.C.; Xue, Z.-L. A Method for the Preparation of Transparent Mesoporous Silica Sol-Gel Monoliths Containing Grafted Organic Functional Groups. J. Mater. Chem. 2005, 15, 2356–2361. [Google Scholar]
- Rodman, D.L.; Pan, H.; Clavier, C.W.; Feng, X.; Xue, Z.-L. Optical Metal Ion Sensor Based on Diffusion Followed by an Immobilizing Reaction. Quantitative Analysis by a Mesoporous Monolith Containing Functional Groups. Anal. Chem. 2005, 77, 3231–3237. [Google Scholar]
- Oh, S.; Moon, J.; Kang, T.; Hong, S.; Yi, J. Enhancement of surface plasmon resonance (SPR) signals using organic functionalized mesoporous silica on a gold film. Sens. Actuat. B 2006, 114, 1096–1099. [Google Scholar]
- Oh, S.; Moon, J.; Kang, T.; Hong, S.; Yi, J. Preparation of a sensor substrate using functionalized mesoporous silica on a gold film for surface plasmon resonance (SPR) spectroscopy. J. Electroceram. 2006, 17, 999–1003. [Google Scholar]
- Bond, A.M.; Miao, W.; Smith, T.D.; Jamis, J. Votammetric reduction of mercury(II), silver(I), lead(II) and copper(II) ions adsorbed onto a new form of mesoporous silica. Anal. Chim. Acta 1999, 396, 203–213. [Google Scholar]
- Walcarius, A.; Despas, C.; Trens, P.; Hudson, M.J.; Bessière, J. Votammetric in situ invesitgation of an MCM-41-modified carbon paste electrode-a new sensor. J. Electroanal. Chem. 1998, 453, 249–252. [Google Scholar]
- Etienne, M.; Cortot, J.; Walcarius, A. Preconcentration Electroanalysis at Surfacactant-Templated Thiol-Functionalized Silica Thin Films. Electroanalysis 2007, 19, 129–138. [Google Scholar]
- Sayen, S.; Etienne, M.; Bessière, J.; Walcarius, A. Tuning the Sensitivity of Electrodes Modified with an Organic-Inorganic Hybrid by Tailoring the Structure of the Nanocomposite Material. Electroanalysis 2002, 14, 1521–1525. [Google Scholar]
- Yantasee, W.; Lin, Y.; Li, X.; Fryxell, G.E.; Zemanian, T.S.; Viswanathan, V.V. Nanoengineered electrochemical sensor based on mesoporous silica thin-film functionalized with thiol-terminated monolayer. Analyst 2003, 128, 899–904. [Google Scholar]
- Yantasee, W.; Lin, Y.; Zemanian, T.S.; Fryxell, G.E. Voltammetric detection of lead(II) and mercury(II) using a carbon paste electrode modified with thiol self-assembled monolayer on mesoporous silica (SAMMS). Analyst 2003, 128, 467–472. [Google Scholar]
- Etienne, M.; Walcarius, A. Evaporation induced self-assembly of templated silica and organosilica thin films on various electrode surfaces. Electrochem. Commun. 2005, 7, 1449–1456. [Google Scholar]
- Yantasee, W.; Lin, Y.; Fryxell, G.E.; Busche, B.J. Simultaneous detection of cadmium, copper, and lead using a carbon paste electrode modified with carbomoylphosphonic acid self-assembled monolayer on mesoporous silica (SAMMS). Anal. Chim. Acta 2004, 502, 207–212. [Google Scholar]
- Yantasee, W.; Timchalk, C.; Fryxell, G.E.; Dockendorff, B.P.; Lin, Y. Automated portable analyzer for lead(II) based on sequential flow injection and nanostructured electrochemical sensors. Talanta 2005, 68, 256–261. [Google Scholar]
- Yantasee, W.; Deibler, L.A.; Fryxell, G.E.; Timchalk, C.; Lin, Y. Screen-printed electrodes modified with functionalized mesoporous silica for voltammetric analysis of toxic metal ions. Electrochem. Commun. 2005, 7, 1170–1176. [Google Scholar]
- Yantasee, W.; Fryxell, G.E.; Lin, Y. Votammetric analysis of europium at screen-printed electrodes modified with salicylamide self-assembled on mesoporous silica. Analyst 2006, 131, 1342–1346. [Google Scholar]
- Tchinda, A.J.; Ngameni, E.; Walcarius, A. Thiol-functionalized porous clay heterostructures (PCHs) deposited as thin films on carbon electrode: Towards mercury(II) sensing. Sens. Actuat. B 2007, 121, 113–123. [Google Scholar]
- Tonle, I.K.; Ngameni, E.; Walcarius, A. Preconcentration and voltammetric analysis of mercury(II) at a carbon paste electrode modified with natural smectite-type clays grafted with organic chelating groups. Sens. Actuat. B 2005, 110, 195–203. [Google Scholar]
- Han, B.-H.; Manners, I.; Winnik, M.A. Oxygen Sensors Based on Mesoporous Silica Particles on Layer-by-Layer Self-assembled Films. Chem. Mater. 2005, 17, 3160–3171. [Google Scholar]
- Zhang, H.; Sun, Y.; Zhang, P.; Wang, Y. Oxygen sensing materials based on mesoporous silica MCM-41 and Pt(II)-porphyrin complexes. J. Mater. Chem. 2005, 15, 3181–3186. [Google Scholar]
- Huo, C.; Zhang, H.; Zhang, H.; Zhang, H.; Yang, B.; Zhang, P.; Wang, Y. Synthesis and Assembly with Mesoporous Silica MCM-48 of Platinum(II) Porphyrin Complexes Bearing Carbazyl Groups: Spectroscopic and Oxygen Sensing Properties. Inorg. Chem. 2006, 45, 4735–4742. [Google Scholar]
- Cardoso, W.S.; Francisco, M.S.P.; Landers, R.; Gushikem, Y. Co(II) porphyrin adsorbed on SiO2/SnO2/phosphate prepared by the sol-gel method. Application in electroreduction of dissolved dioxygen. Electrochim. Acta 2005, 50, 4378–4384. [Google Scholar]
- Cardoso, W.S.; Gushikem, Y. Electrocatalytic oxidation of nitrite on a carbon paste electrode modified with Co(II) porphyrin adsorbed on SiO2/SnO2/Phosphate prepared by the sol-gel method. J. Electroanal. Chem. 2005, 583, 300–306. [Google Scholar]
- Tao, S.; Li, G. Porphyrin-doped mesoporous silica films for rapid TNT detection. Colloid Polym. Sci. 2007, 285, 721–728. [Google Scholar]
- Tao, S.; Li, G.; Zhu, H. Metalloporphyrins as sensing elements for the rapid detection of trace TNT vapor. J. Mater. Chem. 2006, 16, 4521–4528. [Google Scholar]
- Tao, S.; Shi, Z.; Li, G.; Li, P. Hierarchically Structured Nanocomposite Films as Highly Sensitive Chemosensory Materials for TNT Detection. ChemPhysChem 2006, 7, 1902–1905. [Google Scholar]
- Johnson-White, B.; Zeinali, M.; Shaffer, K.M.; Patterson, C.H.; Charles, P.T.; Markowitz, M.A. Detection of organics using porphyrin embedded nanoporous organosilicas. Biosens. Bioelect. 2007, 22, 1154–1162. [Google Scholar]
- Leventis, N.; Elder, I.A.; Rolison, D.R.; Anderson, M.L.; Merzbacher, C.I. Durable Modification of Silica Aerogel Monoliths with Fluorescent 2.7-Diazapyrenium Moieties. Sensing Oxygen near the Speed of Open-Air Diffusion. Chem. Mater. 1999, 11, 2837–2845. [Google Scholar]
- Leventis, N.; Rawashdeh, A.-M.M.; Elder, I.A.; Yang, J.; Dass, A.C. Sotiriou-Leventis, Synthesis and Characterization of Ru(II) Incorporating the 4-Benzoyl-N-methylpyridinium Cation or N-Benzyl-N′-methyl Viologen. Improving the Dynamic Range, Sensitivity, and Response Time of Sol-Gel Based Optical Oxygen Sensors. Chem. Mater. 2004, 16, 1493–1506. [Google Scholar]
- Lei, B.; Li, B.; Zhang, H.; Lu, S.; Wenlian, Z.; Wang, Y. Mesostructured Silica Chemically Doped with RuII as a Superior Optical Oxygen Sensor. Adv. Funct. Mater. 2006, 16, 1883–1891. [Google Scholar]
- Wang, B.; Liu, Y.; Li, B.; Yue, S.; Li, W. Optical oxygen sensing materials based on trinuclear starburst ruthenium(II) complexes assembled in mesoporous silica. J. Lumines. 2008, 128, 341–347. [Google Scholar]
- Zhang, P.; Guo, J.; Wang, Y.; Pang, W. Incorporation of luminescent tris(bipyridine)ruthenium(II) complex in mesoporous silica spheres and their spectroscopic and oxygen-sensing properties. Mater. Lett. 2002, 53, 400–405. [Google Scholar]
- Dai, Z.; Liu, S.; Ju, H.; Chen, H. Direct electron transfer and enzymatic activity of hemoglobin in a hexagonal mesoporous silica matrix. Biosens. Bioelect. 2004, 19, 861–867. [Google Scholar]
- Dai, Z.; Xu, X.; Ju, H. Direct electrochemistry and electrocatalysis of myoglobin immobilized on a hexagonal mesoporous silica matrix. Anal. Biochem. 2004, 332, 23–31. [Google Scholar]
- Liu, Y.; Xu, Q.; Feng, X.; Zhu, J.-J.; Hou, W. Immobilization of hemoglobin on SBA-15 applied to the electrocatalytic reduction of H2O2. Anal. Bioanal. Chem. 2007, 387, 1553–1559. [Google Scholar]
- Liu, Y.; Zhang, J.; Hou, W.; Zhu, J.-J. A Pd/SBA-15 composite: synthesis, characterization and protein biosensing. Nanotechnology 2008, 19, 1–8. [Google Scholar]
- Basallote, M.G.; Blanco, E.; Blaázquez, M.; Fernández-Trujillo, M.J.; Litrán, R.; Máñez, M.Á.; Solar, M.R. Exploring the Properties and Optical Sensing Capability of Sol-Gel Materials Containing a Covalently Bonded Binucleating Cryptand. Chem. Mater. 2003, 15, 2025–2032. [Google Scholar]
- Gojon, C.; Dureault, B.; Hovnanian, N.; Guizard, C. A comparison of immobilized sol-gel methods for an optical chemical hydrazine sensor. Sens. Actuat. B 1997, 38-39, 154–162. [Google Scholar]
- Holmstrom, S.D.; Cox, J.A. Solid-State Voltammetric Determination of Gaseous Hydrogen Peroxide Using Nanostructured Silica as the Electrode. Electroanalysis 1998, 10, 597–601. [Google Scholar]
- Holmstrom, S.D.; Sandlin, Z.D.; Steinecker, W.H.; Cox, J.A. Mediated Oxidation and Determination of Gaseous Monomethyl Hydrazine in a Solid-State Voltammetric Cell Employing a Sol-Gel Electrolyte. Electroanalysis 2000, 12, 262–266. [Google Scholar]
- Palaniappan, A.; Li, X.; Tay, F.E.H.; Li, J.; Su, X. Cyclodextrin functionalized mesoporous silica films on quartz crystal microbalance for enhanced gas sensing. Sens. Actuat. B 2006, 119, 220–226. [Google Scholar]
- Yamada, T.; Zhou, H.S.; Uchida, H.; Honma, I.; Katsube, T. Experimental and Theoretical NOx Physisorption Analyses of Mesoporous Film (SBA-15 and SBA-16) Constructed Surface Photo Voltage (SPV) Sensor. J. Phys. Chem. B 2004, 108, 13341–13346. [Google Scholar]
- Yamada, T.; Zhou, H.S.; Uchida, H.; Tomita, M.; Ueno, Y.; Honma, I.; Asai, K.; Katsube, T. Application of a cubic-like mesoporous silica film to a surface photovoltage gas sensing system. Micropor. Mesopor. Mater. 2002, 54, 269–276. [Google Scholar]
- Yuliarto, B.; Honma, I.; Katsumura, Y.; Zhou, H. Preparation of room temperature NO2 gas sensors based on W- and V-modified mesoporous MCM-41 thin films employing surface photovoltage technique. Sens. Actuat. B 2006, 114, 109–119. [Google Scholar]
- Yuliarto, B.; Zhou, H.S.; Yamada, T.; Honma, I.; Asai, K. Synthesis of a Surface Photovoltage Sensor Using Self-Ordered Tin-Modified MCM-41 Films: Enhanced NO2 Gas Sensing. ChemPhysChem 2004, 5, 261–265. [Google Scholar]
- Yuliarto, B.; Zhou, H.S.; Yamada, T.; Honma, I.; Katsumura, Y.; Ichihara, M. Effect of Tin Addition on Mesoporous Silica Thin Film and Its Application for Surface Photovoltage NO2 Gas Sensor. Anal. Chem. 2004, 76, 6719–6726. [Google Scholar]
- Zhou, H.-S.; Yamada, T.; Asai, K.; Honma, I.; Uchida, H.; Katube, T. NO Gas Sensor Based on Surface Photovoltage System Fabricated by Self-Ordered Hexagonal Mesoporous Silicate Film. Jpn. J. Appl. Phys. 2001, 40, 7098–7102. [Google Scholar]
- Palaniappan, A.; Moochhala, S.; Tay, F.E.H.; Su, X.; Phua, N.C.L. Phthalocyanine/silica hybrid films on QCM for enhanced nitric oxide sensing. Sens. Actuat. B 2008, 129, 184–187. [Google Scholar]
- Xie, F.; Li, W.; He, J.; Yu, S.; Yang, H. Directly immobilize polycation bearing Os complexes on mesoporous material MAS-5 and its electrocatalytic activity for nitrite. Mater. Chem. Phys. 2004, 86, 425–429. [Google Scholar]
- Comes, M.; Rodríguez-López, G.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Villaescusa, L.A.; Amorós, P.; Beltrán, D. Host Solids Containing Nanoscale Anion-Binding Pockets and Their Use in Selective Sensing Displacement Assays. Angew. Chem. Int. Ed. 2005, 44, 2918–2922. [Google Scholar]
- Zhang, X.; Wu, W.; Wang, J.; Liu, C.; Qian, S. Molybdenum polyoxometalate impregnated amino-functionalized mesoporous silica thin films as multifunctional materials for photochromic and electrochemical applications. J. Mater. Res. 2008, 23, 18–26. [Google Scholar]
- Feng, Y.; Yao, R.; Zhang, L. Synthesis and sensitivity properties of Pd-doped tin oxide nanoparticles dispersed in mesoporous silica. Mater. Chem. Phys. 2005, 89, 311–314. [Google Scholar]
- Yang, J.; Hidajat, K.; Kawi, S. Synthesis of nano-SnO2/SBA-15 composite as a highly sensitive semiconductor oxide gas sensor. Mater. Lett. 2008, 62, 1441–1443. [Google Scholar]
- Musat, V.; Fortunato, E.; Rego, A.M.B.; Monteiro, R. Sol-gel cobalt oxide-silica nanocomposite thin films for gas sensing applications. Thin Solid Films 2008, 516, 1499–1502. [Google Scholar]
- Fiorilli, S.; Onida, B.; Barolo, C.; Viscardi, G.; Brunel, D.; Garrone, E. Tethering of Modified Reichardt's Dye on SBA-15 Mesoporous Silica: The Effect of the Linker Flexibillity. Langmuir 2007, 23, 2261–2268. [Google Scholar]
- Fiorilli, S.; Onida, B.; Macquarrie, D.; Garrone, E. Mesoporous SBA-15 silica impregnated with Reichardt's dye: a material optically responding to NH3. Sens. Actuat. B 2004, 100, 103–106. [Google Scholar]
- Onida, B.; Fiorilli, S.; Borello, L.; Viscardi, G.; Macquarrie, D.; Garrone, E. Mechanism of the Optical Response of Mesoporous Silica Impregnated with Reichardt's Dye to NH3and Other Gases. J. Phys. Chem. B 2004, 108, 16617–16620. [Google Scholar]
- Guo, H.; Tao, S. Silver nanoparticles doped silica nanocomposites coated on an optical fiber for ammonia sensing. Sens. Actuat. B 2007, 23, 578–582. [Google Scholar]
- Qi, Z.-M.; Honma, I.; Zhou, H. Ordered-mesoporous-silica-thin-film-based chemical gas sensors with integrated optical polarimetric interferometry. Appl. Phys. Lett. 2006, 88, 053503. [Google Scholar]
- Ueno, Y.; Tate, A.; Niwa, O.; Zhou, H.-S.; Yamada, T.; Honma, I. High benzene selectivity of mesoporous silicate for BTX gas sensing microfluidic devices. Anal. Bioanal. Chem. 2005, 382, 804–809. [Google Scholar]
- Yamada, Y.; Nakamura, T.; Yano, K. Optical Response of Mesoporous Synthetic Opals to the Adsorption of Chemical Species. Langmuir 2008, 24, 2779–2784. [Google Scholar]
- Palaniappan, A.; Su, X.; Tay, F.E.H. Functionalized mesoporous silica films for gas sensing applications. J. Electroceram. 2006, 16, 503–505. [Google Scholar]
- Palaniappan, A.; Su, X.; Tay, F.E.H. Four-Channel QCA Using Mesoporous Silica Films for Gas Sensing Applications. IEEE Sens. J. 2006, 6, 1676–1682. [Google Scholar]
- Jansat, S.; Pelzer, K.; García-Antón, J.; Raucoules, R.; Philippot, K.; Maisonnat, A.; Chaudret, B.; Guari, Y.; Mehdi, A.; Reyé, C.; Corriu, R.J.P. Synthesis of New RuO2@SiO2 Composite Nanomaterials and their Application as Catalytic Filters for Selectvie Gas Detection. Adv. Funct. Mater. 2007, 17, 3339–3347. [Google Scholar]
- Sasahara, T.; Kido, A.; Ishihara, H.; Sunayama, T.; Egashira, M. Highly sensitive detection of volatile organic compounds by an adsorption/combustion-type sensor based on mesoporous silica. Sens. Actuat. B 2005, 108, 478–483. [Google Scholar]
- Xiao, W.; Xiao, D. Aminopyrene functionalized mesoporous silica for the selective determination of resorcinol. Talanta 2007, 72, 1288–1292. [Google Scholar]
- Banet, P.; Legagneux, L.; Hesemann, P.; Moreau, J.J.E.; Nicole, L.; Quach, A.; Sanchez, C.; Tran-Thi, T.-H. Hybrid mesostructured thin films functionalized with DBM as new selective sensors of BF3. Sens. Actuat. B 2008, 130, 1–8. [Google Scholar]
- Innocenzi, P.; Martucci, A.; Guglielmi, M.; Bearzotti, A.; Traversa, E.; Pivin, J.C. Mesoporous silica thin films for alcohol sensors. J. Eur. Cer. Soc. 2001, 21, 1985–1988. [Google Scholar]
- Stevens, N.; Akins, D.L. Dye-doped inorganic/organic composite films as fluorescence sensors for methanol vapor. Sens. Actuat. B 2007, 123, 59–64. [Google Scholar]
- Goettmann, F.; Moores, A.; Boissière, C.; Floch, P.L.; Sanchez, C. A Selective Chemical Sensor Based on the Plasmonic Response of Phosphinine-Stabilized Gold Nanoparticles Hosted on Periodically Organized Mesoporous Silica Thin Layers. Small 2005, 1, 636–639. [Google Scholar]
- Goettmann, F.; Moores, A.; Boissière, C.; Floch, P.L.; Sanchez, C. Periodically organized mesoporous silica thin layers as host for phosphinines-stabilized gold nanoparticles: UV-visible sensing of smal thiols and phosphines. Thin Solid Films 2006, 495, 280–285. [Google Scholar]
- Trammell, S.A.; Zeinali, M.; Melde, B.J.; Charles, P.T.; Velez, F.L.; Dinderman, M.A.; Kusterbeck, A.; Markowitz, M.A. Nanoporous Organosilicas as Preconcentration Materials for the Electrochemical Detection of Trinitrotolune. Anal. Chem. 2008, 80, 4627–4633. [Google Scholar]
- Balkus, K.J., Jr; Pisklak, T.J.; Hundt, G.; Sibert, J.; Zhang, Y. Photoluminescent and redox active periodic mesoporous organosilicas based on 2,7-diazapyrene. Micropor. Mesopor. Mat. 2008, 112, 1–13. [Google Scholar]
- Comes, M.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Villaescusa, L.A.; Amorós, P.; Beltrán, D. Chromogenic Discrimination of Primary Aliphatic Amines in Water with Functionalized Mesoporous Silica. Adv. Mater. 2004, 16, 1783–1786. [Google Scholar]
- Basurto, S.; Torroba, T.; Comes, M.; Martínez-Máñez, R.; Sancenón, F.; Villaescusa, L.; Amorós, P. New Chromogenic Probes into Nanoscopic Pockets in Enhanced Sensing Protocols for Amines in Aqueous Environments. Org. Lett. 2005, 7, 5469–5472. [Google Scholar]
- Comes, M.; Marcos, M.D.; Martínez-Máñez, R.; Millán, M.C.; Ros-Lis, J.V.; Sancenón, F.; Soto, J.; Villaescusa, L.A. Anchoring Dyes into Multidimensional Large-Pore Zeolites: A Prospective Use as Chromogenic Sensing Material. Chem. Eur. J. 2006, 12, 2162–2170. [Google Scholar]
- Lei, C.; Valenta, M.M.; Saripalli, K.P.; Ackerman, E.J. Biosensing Paraoxon in Simulated Envrionmental Samples by Immobilized Organphosphorus Hydrolase in Functionalized Mesoporous Silica. J. Environ. Qual. 2007, 36, 233–238. [Google Scholar]
- Dai, Z.H.; Ni, J.; Huang, X.H.; Lu, G.F.; Bao, J.C. Direct electrochemistry of glucose oxidase immobilized on a hexagonal mesoporous silica-MCM-41 matrix. Bioelectrochem 2007, 70, 250–256. [Google Scholar]
- Yao, K.; Wang, P.; Yang, X.; Cheng, P.; Lu, H. ENFET glucose biosensor produced with mesoporous silica microspheres. Mater. Sci. Eng. C 2007, 27, 736–740. [Google Scholar]
- Dai, Z.; Bao, J.; Yang, X.; Ju, H. A bienzyme channeling glucose sensor with a wide concentration range based on co-entrapment of enzymes in SBA-15 mesopores. Biosens. Bioelect. 2008, 23, 1070–1076. [Google Scholar]
- Lin, V.S.-Y.; Lai, C.-Y.; Huang, J.; Song, S.-A.; Xu, S. Molecular Recognition Inside of Multifunctionalized Mesoporous Silicas: Toward Selective Fluorescence Detection of Dopamine and Glucosamine. J. Am. Chem. Soc. 2001, 123, 11510–11511. [Google Scholar]
- Radu, D.R.; Lai, C.-Y.; Wiench, J.W.; Pruski, M.; Lin, V.S.-Y. Gatekeeping Layer Effect: A Poly(lactic acid)-coated Mesoporous Silica Nanosphere-Based Fluorescence Probe for Detection of Amino-Containing Neurotransmitters. J. Am. Chem. Soc. 2004, 126, 1640–1641. [Google Scholar]
- García-Acosta, B.; Comes, M.; Bricks, J.L.; Kudinova, M.A.; Kurdyukov, V.V.; Tolmachev, A.I.; Descalzo, A.B.; Marcos, M.D.; Martínez-Mánez, R.; Moreno, A.; Sancenón, F.; Soto, J.; Villaescusa, L.A.; Rurack, K.; Barat, J.M.; Escriche, I.; Amorós, P. Sensory hybrid host materials for the selective chromo-fluorogenic detection of biogenic amines. Chem. Commun. 2006, 2239–2241. [Google Scholar]
- Descalzo, A.B.; Rurack, K.; Weisshoff, H.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P.; Hoffmann, K.; Soto, J. Rational Design of a Chromo- and Fluorogenic Hybrid Chemosensor Material for the Detection of Long-Chain Carboxylates. J. Am. Chem. Soc. 2005, 127, 184–200. [Google Scholar]
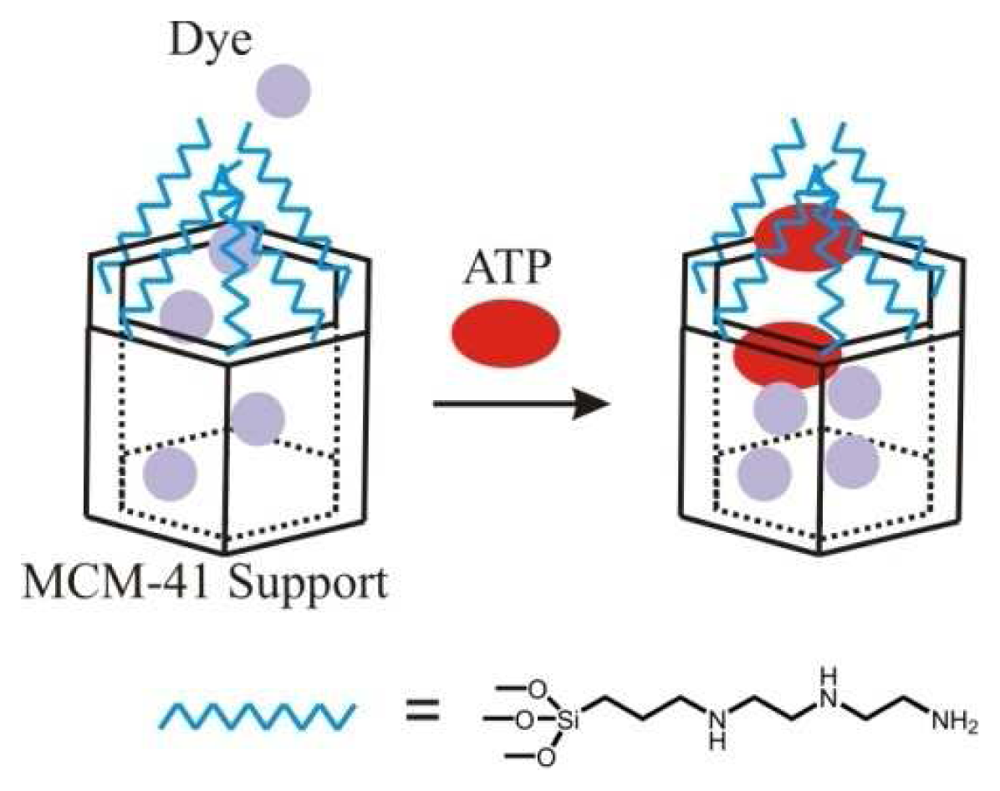
- Descalzo, A.B.; Marcos, M.D.; Martínez-Máñez, R.; Soto, J.; Beltrán, D.; Amorós, P. Anthrylmethylamine functionalised mesoporous silica-based materials as hybrid fluorescent chemosensors for ATP. J. Mater. Chem. 2005, 15, 2721–2731. [Google Scholar]
- Casasús, R.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P. New Methods for Anion Recognition and Signalling Using Nanoscopic Gatelike Scaffoldings. Angew. Chem. Int. Ed. 2006, 45, 6661–6664. [Google Scholar]
- Nozawa, K.; Osono, C.; Sugawara, M. Biotinylated MCM-41 channels as a sensing element in planar bilayer lipid membranes. Sens. Actuat. B 2007, 126, 632–640. [Google Scholar]
- Cho, E.J.; Kang, J.K.; Jung, J.H. A mesoporous silica functionalized by a covalently bound pyridine derivative for selective optical sensing of thymidine. Mater. Lett. 2007, 61, 5157–5160. [Google Scholar]
- Wang, D.; Kou, R.; Gil, M.P.; Jakobson, H.P.; Tang, J.; Yu, D.; Lu, Y. Templated Synthesis, Characterization, and Sensing Application of Macroscopic Platinum Nanowire Network Electrodes. J. Nanosci. Nanotech. 2005, 5, 1904–1909. [Google Scholar]
- Rossinyol, E.; Prim, A.; Pellicer, E.; Arbiol, J.; Hernández-Ramírez, F.; Peiró, F.; Cornet, A.; Morante, J.R.; Solovyov, L.A.; Tian, B.; Bo, T.; Zhao, D. Synthesis and Characterization of Chromium-Doped Mesoporous Tungsten Oxide for Gas-Sensing Applications. Adv. Funct. Mater. 2007, 17, 1801–1806. [Google Scholar]
- Rossinyol, E.; Prim, A.; Pellicer, E.; Rodríguez, J.; Peiró, F.; Cornet, A.; Morante, J.R.; Tian, B.; Bo, T.; Zhao, D. Mesostructured pure and copper-catalyzed tungsten oxide for NO2 detection. Sens. Actuat. B 2007, 126, 18–23. [Google Scholar]
- Prim, A.; Pellicer, E.; Rossinyol, E.; Peiró, F.; Cornet, A.; Morante, J.R. A Novel Mesoporous CaO-Loaded In2O3 Material CO2 Sensing. Adv. Funct. Mater. 2007, 17, 2957–2963. [Google Scholar]
- Wagner, T.; Waitz, T.; Roggenbuck, J.; Fröba, M.; Kohl, C.-D.; Tiemann, M. Ordered mesoporous ZnO for gas sensing. Thin Solid Films 2007, 515, 8360–8363. [Google Scholar]
- Yang, S.M.; Coombs, N.; Ozin, G.A. Micromolding in Inverted Polymer Opals (MIPO): Synthesis of Hexagonal Mesoporous Silica Opals. Adv. Mater. 2000, 12, 1940–1944. [Google Scholar]
- Holland, B.T.; Blanford, C.F.; Do, T.; Stein, A. Synthesis of Highly Ordered, Three-Dimensional, Macroporous Structures of Amorphous or Crystalline Inorganic Oxides, Phosphates, and Hybrid Composites. Chem. Mater. 1999, 11, 795–805. [Google Scholar]
- Nakanishi, K.; Kanamori, K. Organic-Inorganic Hybrid Poly(silsesquioxane) Monoliths with Controlled Macro- and Mesopores. J. Mater. Chem. 2005, 15, 3776–3786. [Google Scholar]
- Nakanishi, K.; Amatani, T.; Yano, S.; Kodaira, T. Multiscale Templating of Siloxane Gels via Polymerization-Induced Phase Separation. Chem. Mater. 2008, 20, 1108–1115. [Google Scholar]
- Inagaki, S.; Guan, S.; Ohsuna, T.; Terasaki, O. An ordered mesoporous organosilica hybrid material with a crystal-like wall structure. Nature 2002, 416, 304–307. [Google Scholar]
- Kapoor, M.P.; Yang, Q.; Inagaki, S. Self-Assembly of Biphenylene-Bridged Hybrid Mesoporous Solid with Molecular-Scale Periodicity in the Pore Walls. J. Am. Chem. Soc. 2002, 124, 15176–15177. [Google Scholar]
- Vallet-Regí, M. Ordered Mesoporous Materials in the Context of Drug Delivery Systems and Bone Tissue Engineering. Chem. Eur. J. 2006, 12, 5934–5943. [Google Scholar]
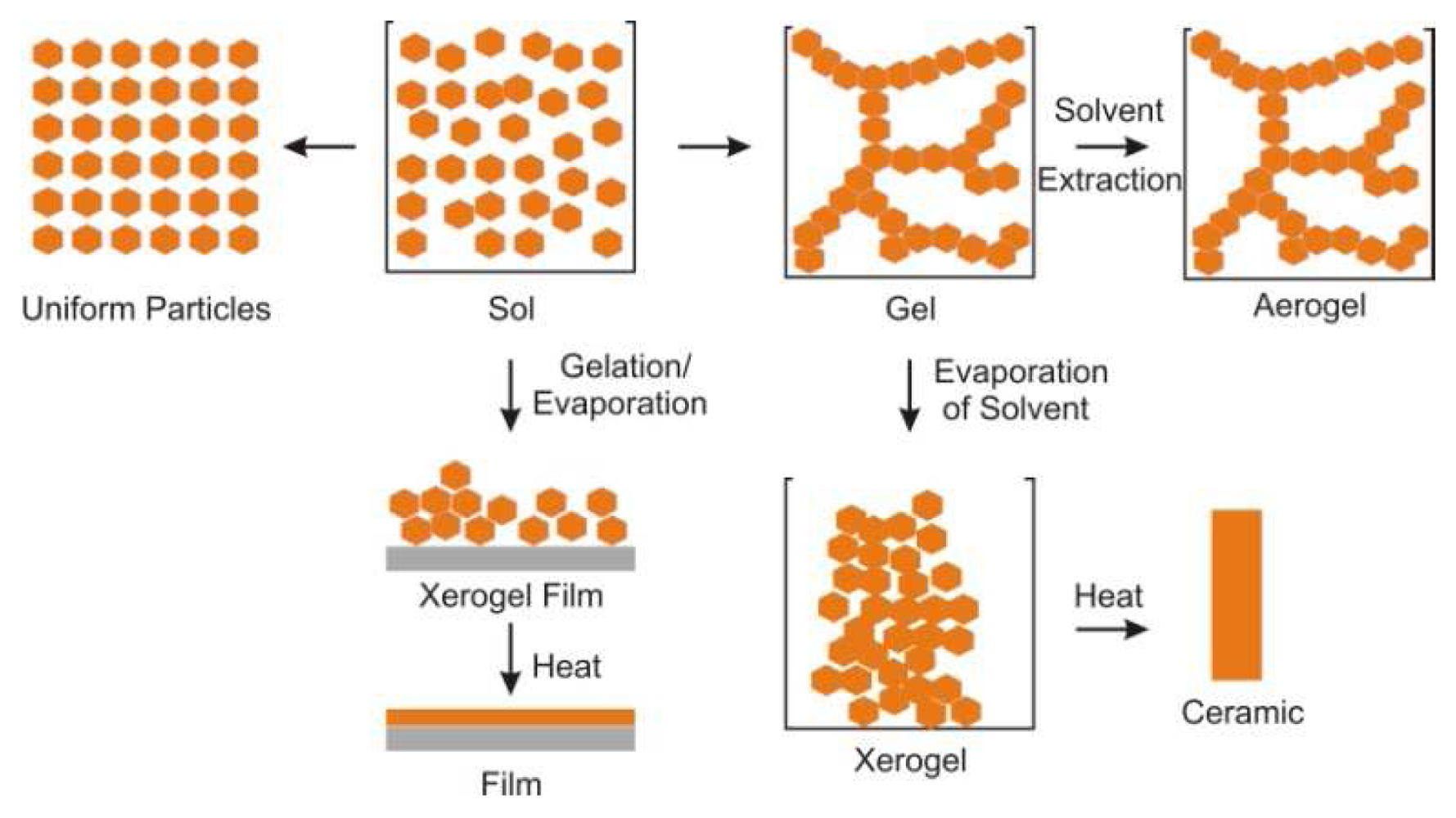
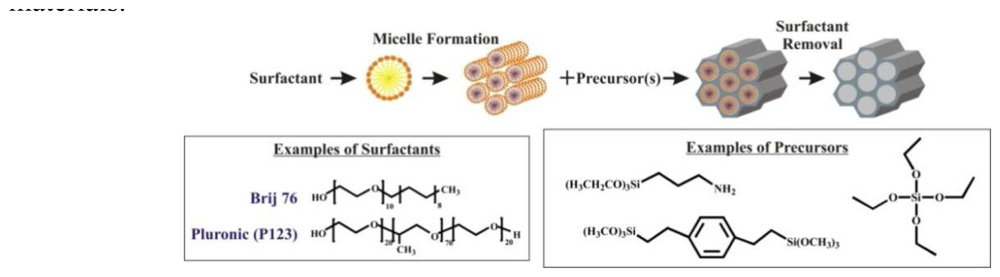
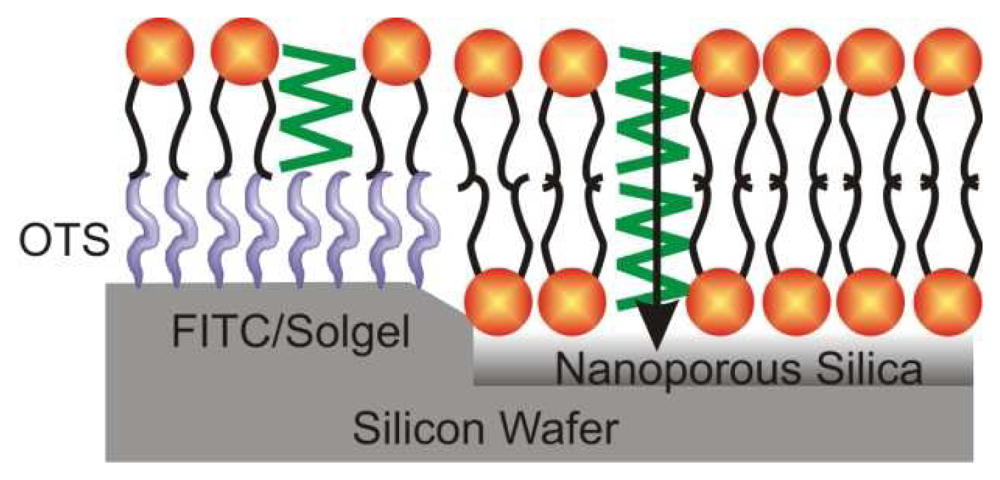
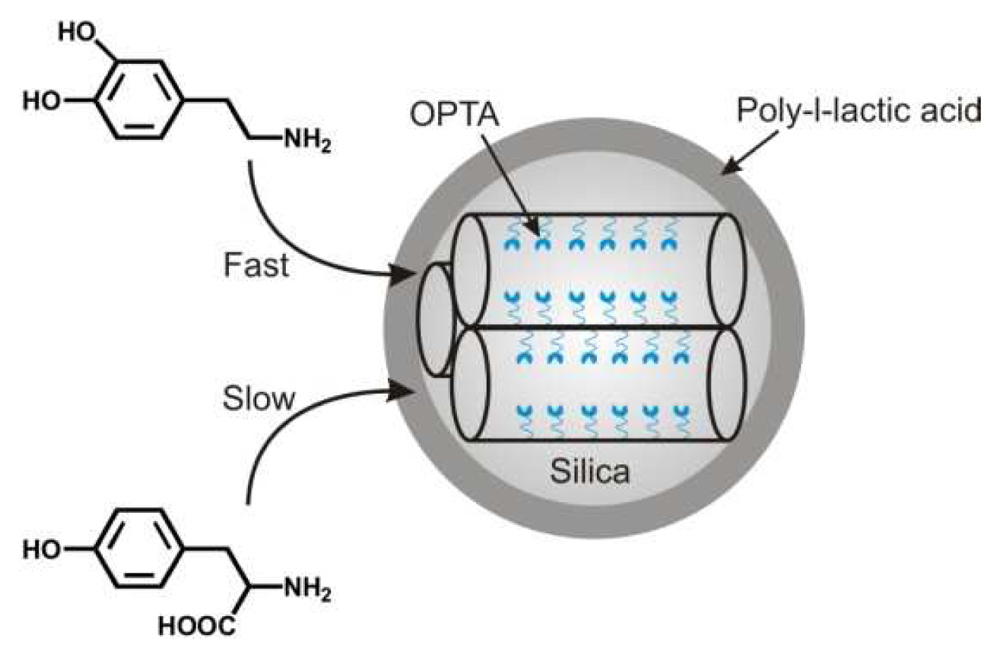
| Dye | Cation | Detection limit | Reference |
|---|---|---|---|
| Dibenzoylmethane | Uranium (VI) | 1 ppm | Nicole et al. [48] |
| Calixarene with two dansyl groups | Mercury (II) | 3.3 × 10-7 M | Métivier et al. [51] |
| Eriochrome cyanine R | Copper(II) | 5 × 10-5 M | Miled et al. [58] |
| Meso-tetra(4-sulfonatophenyl)porphine | Mercury(II) | 1.75 × 10-8 M | Balaji et al. [49] |
| 4-(2-pyridylazo)resorcinol | Cadmium(II) | 1.75 × 10-8 M | Balaji et al. [50] |
| Dithizone α,β,γ,δ-tetrakis(1-methylpyridinium-4-yl)porphine p-toluenesulfonate Pyrogallol red meso-tetra(4-sulfonatophenyl)porphine | Lead(II) Cadmium(II) Antimony(III) Mercury(II) | 2.38 × 10-9 M 1.35 × 10-8 M 3.37 × 10-8 M 6.34 × 10-8 M | Balaji et al. [55] |
| 4-chloroaniline-N-salicylidene | Zinc(II) | 0.2 ng/mL | Gao et al. [59] |
| [4-(2-hydroxyphenyl)methylene]-benzenesulfonamide | Copper(II) | 0.1 ppm | Gao et al. [60] |
| 2-hydroxybenzaldehyde | Copper(II) | N/A | Zhang et al. [53] |
| N-pyrene-1-yl-succinamic acid 4-(pyrene-1-ylcarbamoyl)-butyric acid | Copper(II) | N/A | Kledzik et al. [54] |
| Rhodamine | Mercury(II)+ | ≤ 1.0 × 10-5 M | Lee et al. [52] |
| Ethylpyridine with diphenylcarbazide, | Chromium(VI) | 10 ppb | Carrington et al. [64] |
| 4-n-dodecyl-6-(2-thiazoylazo)resorcinol 4-n-dodecyl-6-(2-pyridylazo)phenol diphenylcarbazide | Cadmium(II) Lead (II) | 0.1 ppb 9 × 10-9 M | El-Safty et al. [56] El-Safty et al. [57] |
| Pyrogallol red | Antimony(III) | 1 × 10-9 M | El-Safty et al. [61]; Ismail et al. [62] |
| Diphenylthiocarbazone | Bismuth(III) | 6.5 × 10-10 M | El-Safty et al. [63] |
| Target | Porphyrin | Reference |
|---|---|---|
| Mercury (II) | meso-tetra(4-sulfonatophenyl)porphine | Balaji et al. [49, 55] |
| Oxygen | Pt (II) 2,3,7,8,12,13,17,18-octaethyl porphine Pd (II) 2,3,7,8,12,13,17,18-octaethyl porphine Pt(II) meso-tetraphenylporphine Pt (II) meso tetra (pentafluorophenyl)porphine | Han et al. [82] |
| Oxygen | Pt(II) meso-tetra(4-N-pyridyl)porphyrin | Zhang et al. [83] |
| Oxygen | Pt(II) meso-tetra(3,5-dihydroxyphenyl)porphyrin Pt(II) meso-tetra(3,5-di[(N-carbazyl)-n-octyloxyphenyl])porphyrin Pt(II) meso-tetra(3,5-di[(N-carbazyl)-n-hexyloxyphenyl])porphyrin Pt(II) meso-tetra(3,5-di[(N-carbazyl)-n-butyloxyphenyl])porphyrin | Huo et al. [84] |
| NO2 | Co(II) meso-tetra(1-methyl-4-pyridyl) porphyrin | Cardoso et al. [85, 86] |
| Nitroenergetic Compounds | meso-tetra(4-siloxyphenyl)porphyrin Cd(II) meso-tetra(4-siloxyphenyl)porphyrin Zn(II) meso-tetra(4-siloxyphenyl)porphyrin | Tao et al. [87-89] |
| 2,4,6-Trinitrotoluene RDX p-Cresol p-Nitrophenol | meso-tetra(4-carboxyphenyl)porphyrin | Johnson-White et al. [90] |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Melde, B.J.; Johnson, B.J.; Charles, P.T. Mesoporous Silicate Materials in Sensing. Sensors 2008, 8, 5202-5228. https://doi.org/10.3390/s8085202
Melde BJ, Johnson BJ, Charles PT. Mesoporous Silicate Materials in Sensing. Sensors. 2008; 8(8):5202-5228. https://doi.org/10.3390/s8085202
Chicago/Turabian StyleMelde, Brian J., Brandy J. Johnson, and Paul T. Charles. 2008. "Mesoporous Silicate Materials in Sensing" Sensors 8, no. 8: 5202-5228. https://doi.org/10.3390/s8085202






