Application of DNA Hybridization Biosensor as a Screening Method for the Detection of Genetically Modified Food Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and solutions
2.2. Apparatus and electrodes
2.3. Procedures
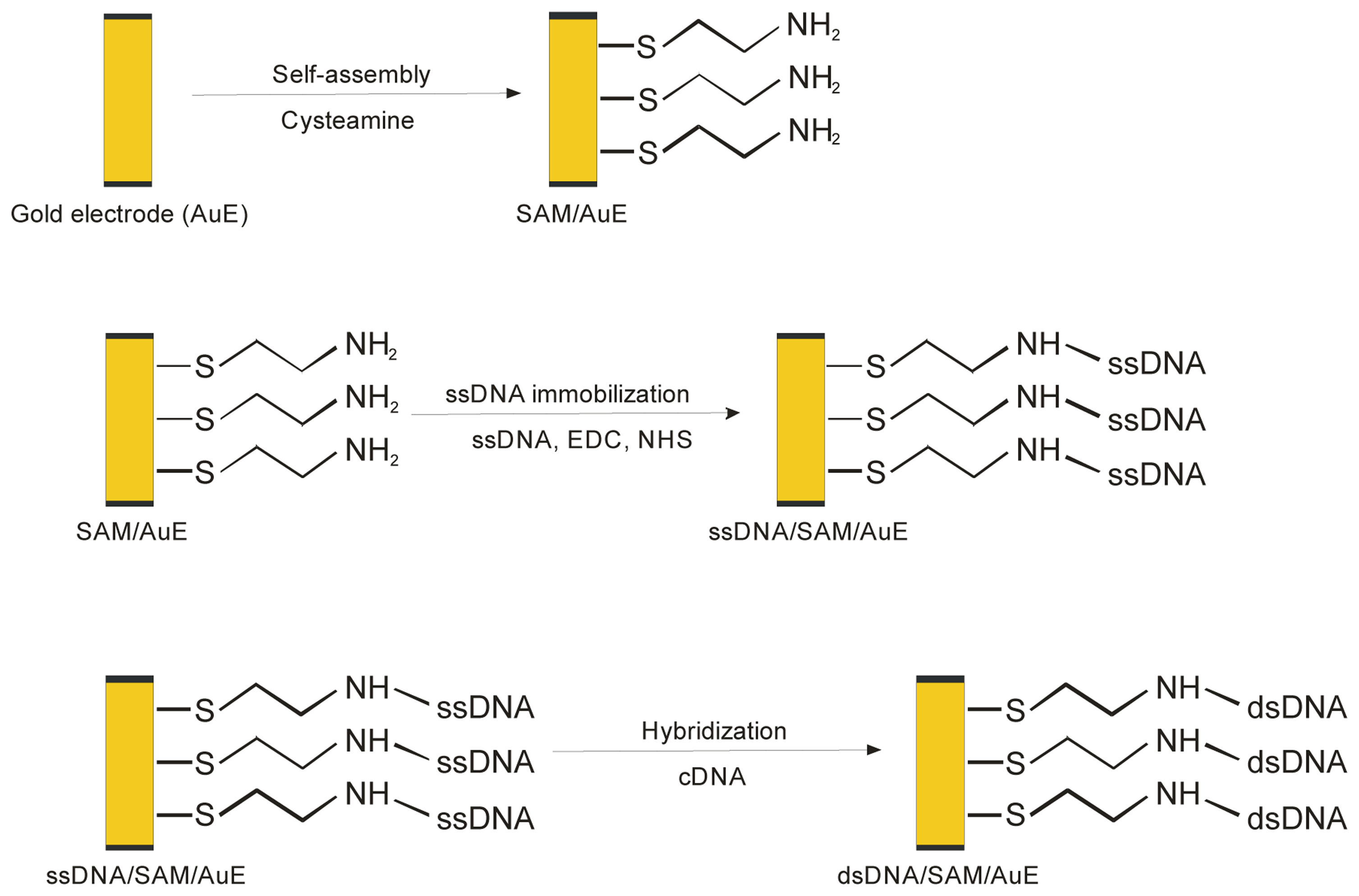
2.3.1. Immobilization of ssDNA probe on the modified gold electrode
2.3.2. DNA hybridization
2.3.3. MB accumulation and electrochemical measurement
3. Results and Discussion
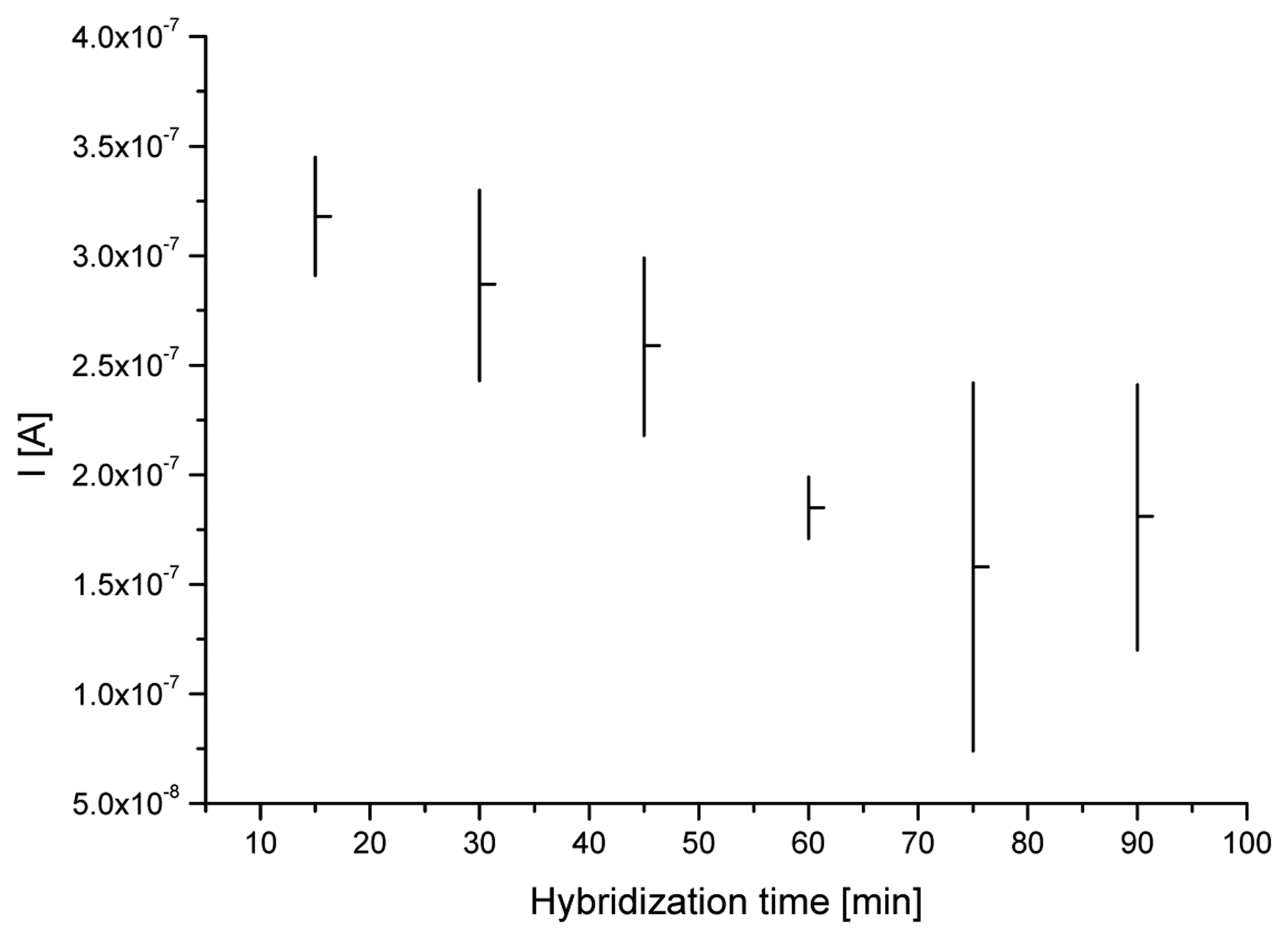
3.1. Optimization of a probe immobilization and hybridization procedures
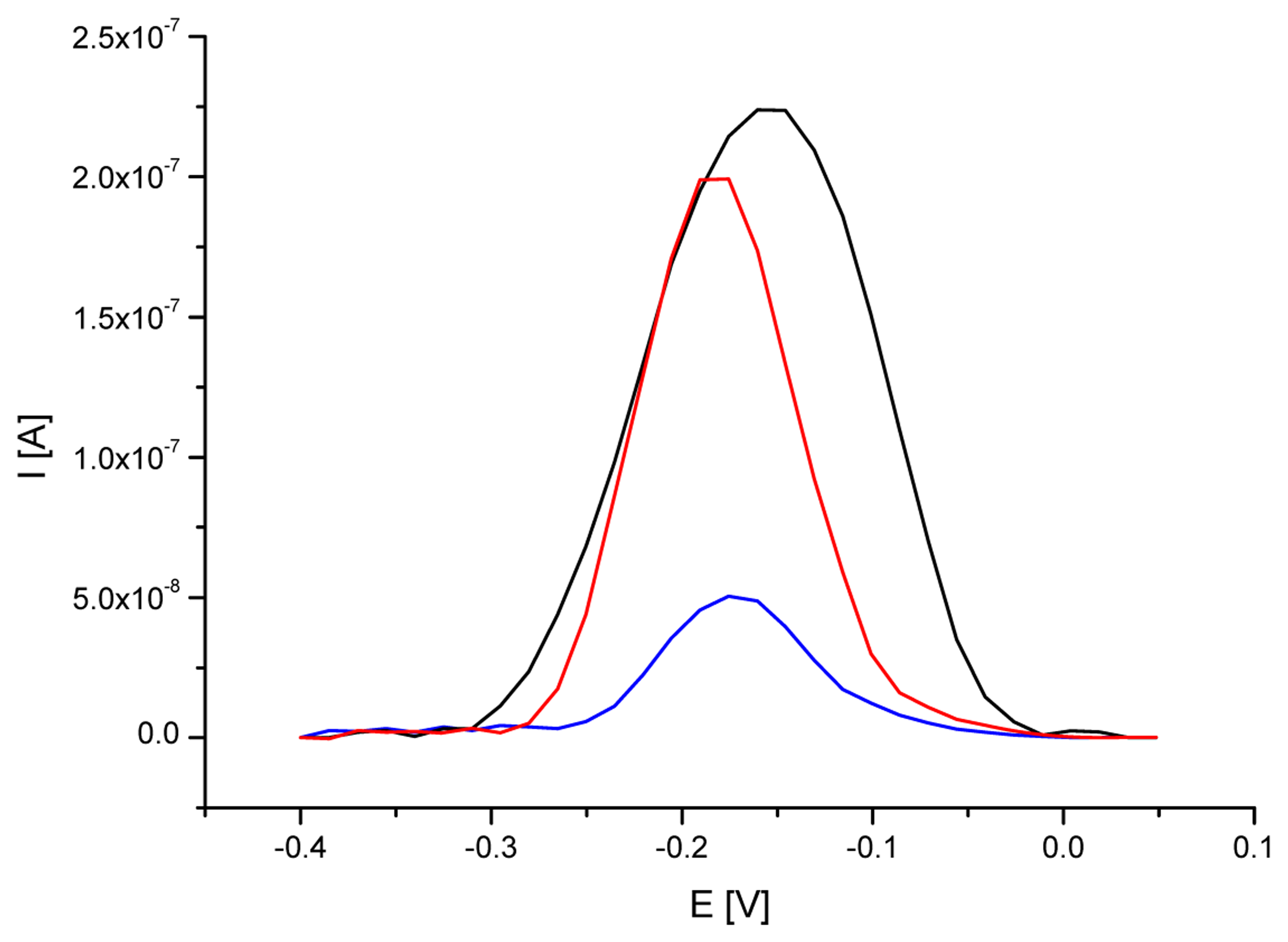
3.2. Analytical approach based on electrochemical detection of 35S promoter
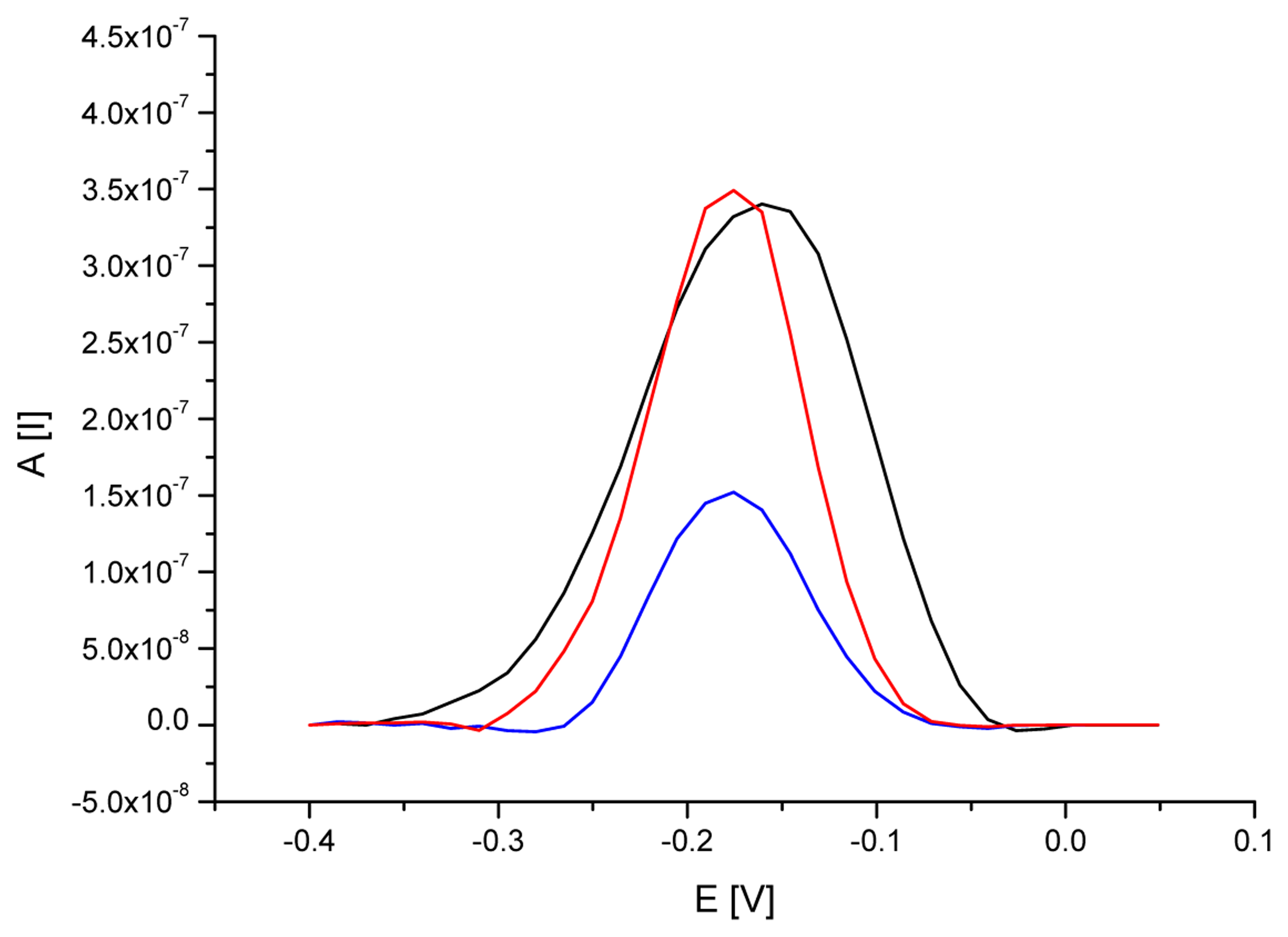
3.3. Analytical approach based on electrochemical detection of nos terminator
Conclusions
References
- Wang, J.; Rivas, G.; Cai, X.; Palecek, E.; Nielsen, P.; Shiraishi, H.; Dontha, N.; Luo, D.; Parrado, C.; Chicharro, M.; Farias, P.A.M.; Valera, F.S.; Grant, D.H.; Ozsoz, M.; Flair, M.N. DNA electrochemical biosensors for environmental monitoring. A review. Anal. Chim. Acta 1997, 347, 1–8. [Google Scholar]
- Marrazza, G.; Chianella, I.; Mascini, M. Disposable DNA electrochemical biosensors for environmental monitoring. Anal. Chim. Acta 1999, 387, 297–307. [Google Scholar]
- Mascini, M.; Palchetti, I.; Marrazza, G. DNA electrochemical biosensors. Fresenius J. Anal. Chem. 2001, 361, 15–22. [Google Scholar]
- Hall, R.H. Biosensor technologies for detecting microbiological foodborne hazards. Microbes Infect. 2002, 4, 425–432. [Google Scholar]
- Palecek, E.; Jelen, F. Electrochemistry of Nucleic Acids and Development of DNA Sensors. Critical Rev. Anal. Chem. 2002, 32, 261–270. [Google Scholar]
- Wang, J. Electroanalytical Methods for Biological Materials; Brajter-Toth, A., Chambers, J.Q., Eds.; Marcel Dekker, Inc.: New York, 2002; pp. 27–42. [Google Scholar]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nature Biotech. 2003, 21, 1192–1199. [Google Scholar]
- de-los-Santos-Álvarez, P.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Electrochemistry of nucleic acids at solid electrodes and its applications. Electroanalysis 2004, 16, 1193–1204. [Google Scholar]
- Bakker, E.; Qin, Y. Electrochemical sensors. Anal. Chem. 2006, 78, 3965–3983. [Google Scholar]
- Minunni, M.; Tombelli, S.; Mariotti, E.; Mascini, M.; Mascini, M. Biosensors as new analytical tool for detection of Genetically Modified Organisms (GMOs). Fresenius J. Anal. Chem. 2001, 369, 589–593. [Google Scholar]
- Marrazza, G.; Chianella, I.; Mascini, M. Disposable DNA electrochemical sensor for hybridization detection. Biosens. Bioelectron. 1999, 14, 43–51. [Google Scholar]
- Pividori, M.I.; Merkoçi, A.; Alegret, S. Electrochemical genosensor design: immobilisation of oligonucleotides onto transducer surfaces and detection methods. Biosens. Bioelectron. 2000, 15, 291–303. [Google Scholar]
- Wang, J. From DNA biosensors to gene chips. Survey and summary. Nucleic Acids Res. 2000, 28, 3011–3016. [Google Scholar]
- Xu, Ch.; He, P.; Fang, Y. Electrochemical labeled DNA probe for the detection of sequence-specific DNA. Anal. Chim. Acta 2000, 411, 31–36. [Google Scholar]
- Watterson, J.; Piunno, P.A.E.; Krull, U.J. Practical physical aspects of interfacial nucleic acid oligomer hybridisation for biosensor design. Anal. Chim. Acta 2002, 469, 115–127. [Google Scholar]
- Kerman, K.; Kobayashi, M.; Tamiya, E. Recent trends in electrochemical DNA biosensor technology. Meas. Sci. Technol. 2004, 15, R1–R11. [Google Scholar]
- Loaiza, Ó, A.; Campuzano, S.; López-Berlanga, M.; Pedrero, M.; Pingarrón, J.M. Development of a DNA Sensor Based on Alkanethiol Self-Assembled Monolayer-Modified Electrodes. Sensors 2005, 5, 344–363. [Google Scholar]
- Palecek, E.; Fojta, M. Bioelectronics; Willner, I., Katz, E., Eds.; WILEY-VCH Verlag GmbH & Co, KGaA: Weinheim, 2005; pp. 127–192. [Google Scholar]
- Odenthal, K.J.; Gooding, J.J. An introduction to electrochemical DNA biosensors. Analyst 2007, 132, 603–610. [Google Scholar]
- Luong, J.H.T.; Bouvrette, P.; Male, K.B. Developments and applications of biosensors in food analysis. TIBTECH 1997, 15, 369–377. [Google Scholar]
- Wang, J. Towards Genoelectronics: Electrochemical Biosensing of DNA Hybridization. Chem. Eur. J. 1999, 5, 1681–1685. [Google Scholar]
- Schmidt, A.; Bilitewski, U. Instrumentation and sensors in the food industry; Kress-Rogers, E., Brimelov, C.J.B., Eds.; Woodhead Publishing Limited: Cambridge, 2001; pp. 714–739. [Google Scholar]
- Nakamura, H.; Karube, I. Current research activity in biosensors. Anal. Bioanal. Chem. 2003, 377, 446–468. [Google Scholar]
- Elenis, D.S.; Kalogianni, D.P.; Glynou, K.; Ioannou, P.C.; Christopoulos, T.K. Advances in molecular techniques for the detection and quantification of genetically modified organisms. Anal. Bioanal. Chem. 2008. Epublished ahead of print. [Google Scholar]
- Hahn, S.; Mergenthaler, S.; Zimmermann, B.; Holzgreve, W. Nucleic acid based biosensors: The desires of the user. Bioelectrochemistry 2005, 67, 151–154. [Google Scholar]
- Wang, J. Electrochemical nucleic acid biosensors. Anal. Chim. Acta 2002, 469, 63–71. [Google Scholar]
- de-los-Santos-Álvarez, P.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Current strategies for electrochemical detection of DNA with solid electrodes. Anal. Bioanal. Chem. 2004, 378, 104–118. [Google Scholar]
- Chiorcea Paquim, A.-M.; Diculescu, V.C.; Oretskaya, T.S.; Oliveira Brett, A.M. AFM and electrochemical studies of synthetic oligonucleotide hybridization. Biosens. Bioelectron. 2004, 20, 933–944. [Google Scholar]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical Quantitation of DNA Immobilized on Gold. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar]
- Herne, T.M.; Tarlov, M.J. Characterization of DNA Probes Immobilized on Gold Surfaces. J. Am. Chem. Soc. 1997, 119, 8916–8920. [Google Scholar]
- Peterson, A.W.; Heaton, R.J.; Georgiadis, R.M. The effect of surface probe density on DNA hybridization. Nucleic Acids Res. 2001, 29, 5163–5168. [Google Scholar]
- Sun, X.; He, P.; Liu, Sh.; Ye, J.; Fang, Y. Immobilization of single-stranded deoxyribonucleic acid on gold electrode with self-assembled aminoethanethiol monolayer for DNA electrochemical sensor applications. Talanta 1998, 47, 487–495. [Google Scholar]
- Lucarrelli, F.; Marrazza, G.; Turner, A.P.F.; Mascini, M. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens. Bioelectron. 2004, 19, 515–530. [Google Scholar]
- Yang, M.; McGovern, M.E.; Thompson, M. Genosensor technology and the detection of interfacial nucleic acid chemistry. Anal. Chim. Acta 1997, 346, 259–275. [Google Scholar]
- Zhao, Y.D.; Pang, D.W.; Hu, S.; Wang, Z.L.; Cheng, J.K.; Dai, H.P. DNA-modified electrodes; part 4: optimization of covalent immobilization of DNA on self-assembled monolayers. Talanta 1999, 49, 751–756. [Google Scholar]
- Chaki, N.K.; Vijayamohanan, K. Self-assembled monolayers as a tunable platform for biosensor applications. Biosens. Bioelectron. 2002, 17, 1–12. [Google Scholar]
- Carvalhal, R.F.; Freire, R.S.; Kubota, L.T. Polycrystalline Gold Electrodes: A Comparative Study of Pretreatment Procedures Used for Cleaning and Thiol Self-Assembly Monolayer Formation. Electroanalysis 2005, 17, 1251–1259. [Google Scholar]
- Kerman, K.; Ozkan, D.; Kara, P.; Meric, B.; Gooding, J.J.; Ozsoz, M. Voltammetric determination of DNA hybridization using methylene blue and self-assembled alkanethiol monolayer on gold electrodes. Anal. Chim. Acta 2002, 462, 39–47. [Google Scholar]
- Smith, R.K.; Lewis, P.A.; Weiss, P.S. Patterning self-assembled monolayers. Prog. Surf. Science 2004, 75, 1–68. [Google Scholar]
- Palecek, E.; Fojta, M.; Tomschik, M.; Wang, J. Electrochemical biosensor for DNA hybridization and DNA damage. Biosens. Bioelectron. 1998, 13, 621–628. [Google Scholar]
- Mishima, Y.; Motonaka, J.; Ikeda, S. Utilization of an osmium complex as a sequence recognizing material for DNA-immobilized electrochemical sensor. Anal. Chim. Acta 1997, 345, 45–50. [Google Scholar]
- Erdem, A.; Kerman, K.; Meric, B.; Akarca, U.S.; Ozsoz, M. Novel hybridization indicator methylene blue for the electrochemical detection of short DNA sequences related to the hepatitis B virus. Anal. Chim. Acta 2000, 422, 139–149. [Google Scholar]
- Ozkan, D.; Kara, P.; Kerman, K.; Meric, B.; Erdem, A.; Jelen, F.; Nielsen, P.E.; Ozsoz, M. DNA and PNA sensing on mercury and carbon electrodes by using methylene blue as an electrochemical label. Bioelectrochemistry 2002, 58, 119–126. [Google Scholar]
- Erdem, A.; Kerman, K.; Meric, B.; Ozsoz, M. Methylene Blue as a Novel Electrochemical Hybridization Indicator. Electroanalysis 2001, 13, 219–223. [Google Scholar]
- Yang, W.; Ozsoz, M.; Hibbert, D.B.; Gooding, J.J. Evidence for the Direct Interaction Between Methylene Blue and Guanine Bases Using DNA-Modified Carbon Paste Electrodes. Electroanalysis 2002, 14, 1299–1302. [Google Scholar]
- Tani, A.; Thomson, A.J.; Butt, J.N. Methylene blue as an electrochemical discriminator of single-or double-stranded oligonucleotides immobilised on gold substrates. Analyst 2001, 126, 1756–1759. [Google Scholar]
- MacCornik, C.A.; Griffin, H.G.; Underwood, H.M.; Gasson, M.J. Common DNA sequences with potential of genetically manipulated organisms in food. J. Appl. Microbiol. 1998, 84, 969–980. [Google Scholar]
- Anklam, E.; Gadani, F.; Heinze, P.; Pijnenburg, H.; Van Den Ende, G. Analytical methods for detection and determination of genetically modified organisms in agricultural crops and plant-derived food products. Eur. Food Res. Technol. 2002, 214, 3–26. [Google Scholar]
- Querci, M.; Maretti, M.; Mazzara, M. Qualitative Detection of MON810 Maize, Bt-176 Maize and Roundup Ready® Soybean by PCR, in. The Analysis of Food Samples for the Presence of Genetically Modified Organisms; Querci, M., Jermini, M., Van den Eede, G., Eds.; European Communities: Luxemburg, 2006; Session 9. [Google Scholar]
- Meric, B.; Kerman, K.; Marrazza, G.; Palchetti, I.; Mascini, M.; Ozsoz, M. Disposable genosensor, a new tool for the detection of NOS-terminator, a genetic element present in GMOs. Food Control 2004, 15, 621–626. [Google Scholar]
- Mariotti, E.; Minunni, M.; Mascini, M. Surface plasmon resonance biosensor for genetically modified organisms detection. Anal. Chim. Acta 2002, 453, 165–172. [Google Scholar]
- Wang, R.; Tombelli, S.; Minunni, M.; Spiriti, M.M.; Mascini, M. Immobilisation of DNA probes for the development of SPR-based sensing. Biosens. Bioelectron. 2004, 20, 967–974. [Google Scholar]
- Kalogianni, D.P.; Koraki, T.; Christopoulos, T.K.; Ioannou, P.C. Nanoparticicle-based DNA biosensor for visual detection of genetically modified organisms. Biosens. Bioelectron. 2006, 21, 1069–1076. [Google Scholar]
- Passamano, M.; Pighini, M. QCM DNA-sensor for GMOs detection. Sens. Act. B 2006, 118, 177–181. [Google Scholar]
- Stobiecka, M.; Cieśla, J.M.; Janowska, B.; Tudek, B.; Radecka, H. Piezoelectric Sensor for Determination of Genetically Modified Soybean Roundup Ready® in Samples not Amplified by PCR. Sensors 2007, 7, 1462–1479. [Google Scholar]
- Carpini, G.; Lucarelli, F.; Marrazza, G.; Mascini, M. Oligonucleotide-modified screen-printed gold electrodes for enzyme-amplified sensing of nucleic acids. Biosens. Bioelectron. 2004, 20, 167–175. [Google Scholar]
- Lucarelli, F.; Marrazza, G.; Mascini, M. Enzyme-based impedimetric detection of PCR products using oligonucleotide-modified screen-printed gold electrodes. Biosens. Bioelectron. 2005, 20, 2001–2009. [Google Scholar]
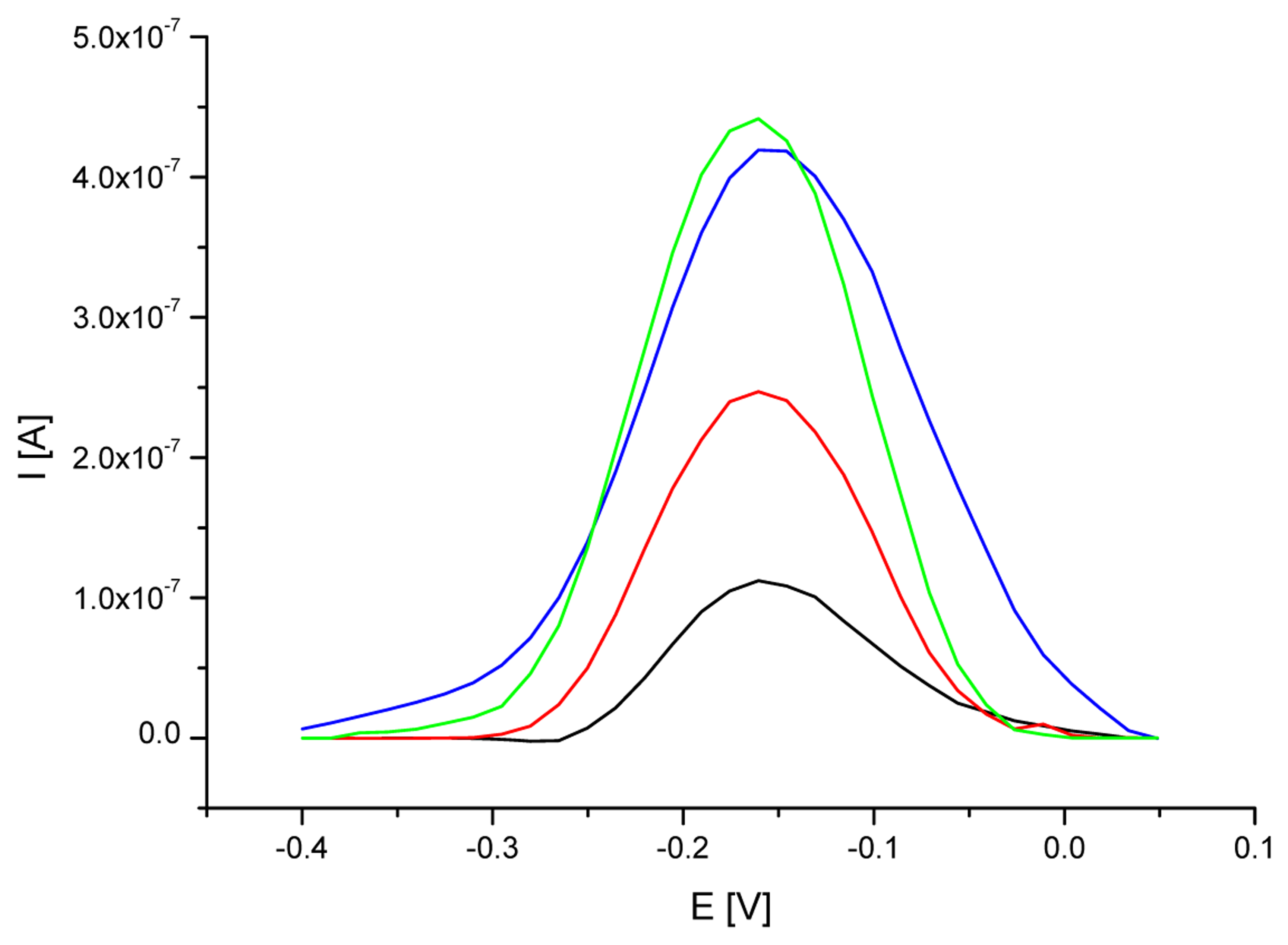
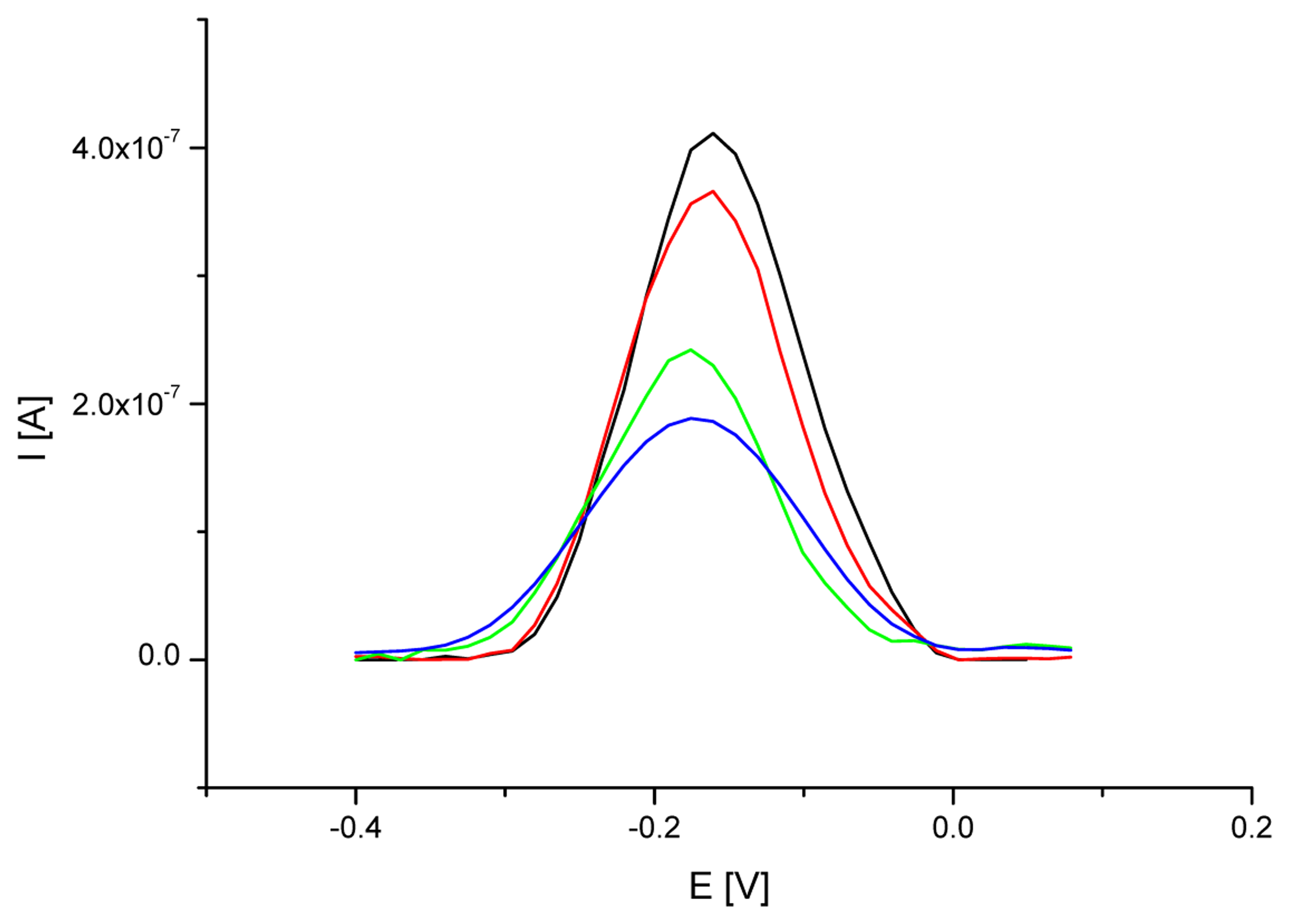
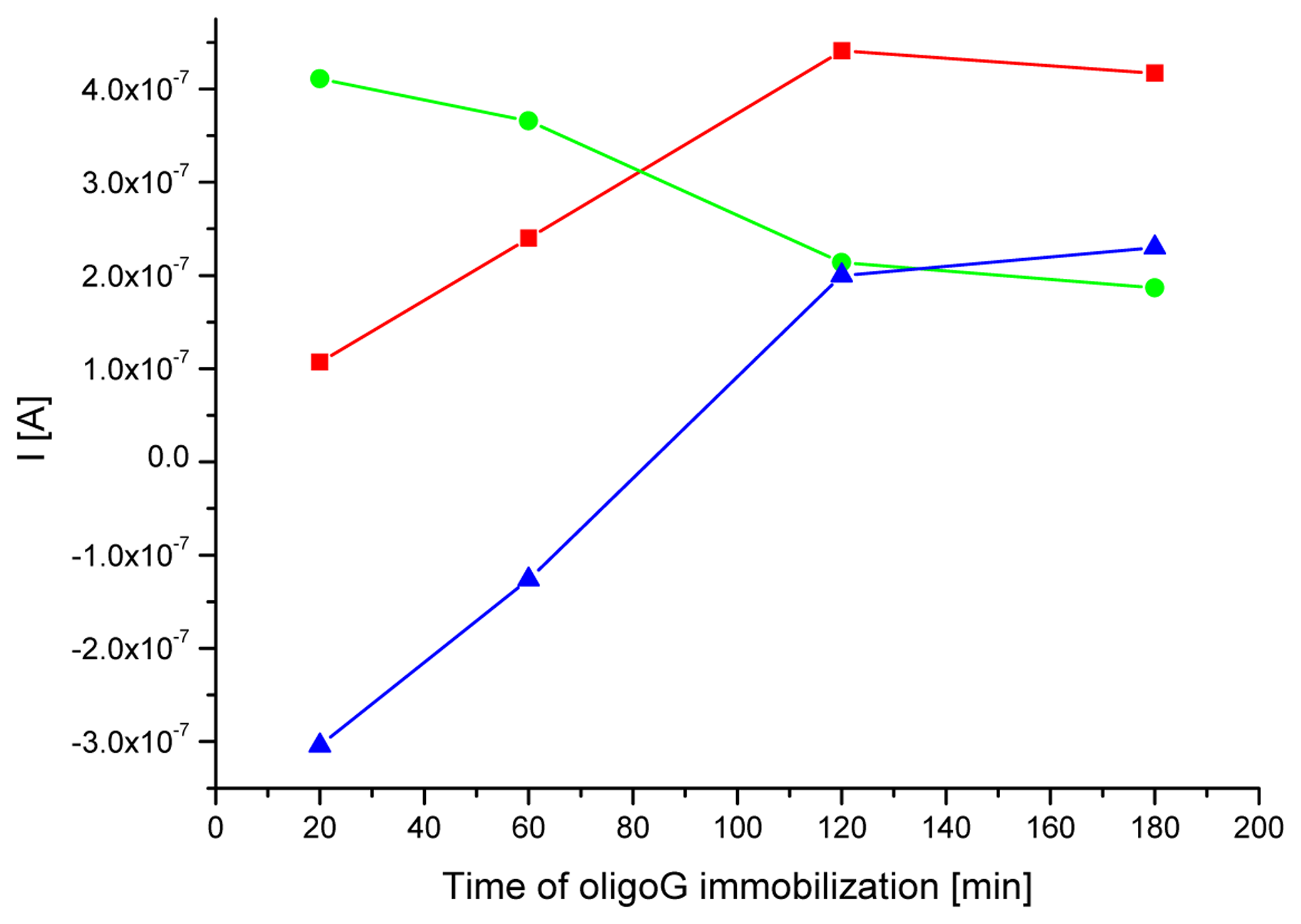
| Electrode | MB peak current [μA] | RSD for MB peak current values [%] | MB peak potential [V] | RSD for MB peak potential [%] |
|---|---|---|---|---|
| ssDNA/SAM/AuE specific for 35S promoter | 0.233 | 16 | -0.160 | 4 |
| ssDNA/SAM/AuE after hybridization with DNA from RR soybean | 0.049 | 14 | -0.176 | 6 |
| ssDNA/SAM/AuE after interaction with DNA from non-GM soybean | 0.191 | 15 | -0.176 | 4 |
| Electrode | MB peak current [μA] | RSD for MB peak current values [%] | MB peak potential [V] | RSD for MB peak potential [%] |
|---|---|---|---|---|
| ssDNA/SAM/AuE specific for nos terminator | 0.339 | 6 | -0.160 | 4 |
| ssDNA/SAM/AuE after hybridization with DNA from RR soybean | 0.151 | 5 | -0.176 | 6 |
| ssDNA/SAM/AuE after interaction with DNA from non-GM soybean | 0.351 | 7 | -0.176 | 5 |
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Tichoniuk, M.; Ligaj, M.; Filipiak, M. Application of DNA Hybridization Biosensor as a Screening Method for the Detection of Genetically Modified Food Components. Sensors 2008, 8, 2118-2135. https://doi.org/10.3390/s8042118
Tichoniuk M, Ligaj M, Filipiak M. Application of DNA Hybridization Biosensor as a Screening Method for the Detection of Genetically Modified Food Components. Sensors. 2008; 8(4):2118-2135. https://doi.org/10.3390/s8042118
Chicago/Turabian StyleTichoniuk, Mariusz, Marta Ligaj, and Marian Filipiak. 2008. "Application of DNA Hybridization Biosensor as a Screening Method for the Detection of Genetically Modified Food Components" Sensors 8, no. 4: 2118-2135. https://doi.org/10.3390/s8042118




