Micro Machining of Injection Mold Inserts for Fluidic Channel of Polymeric Biochips
Abstract
:1. Introduction
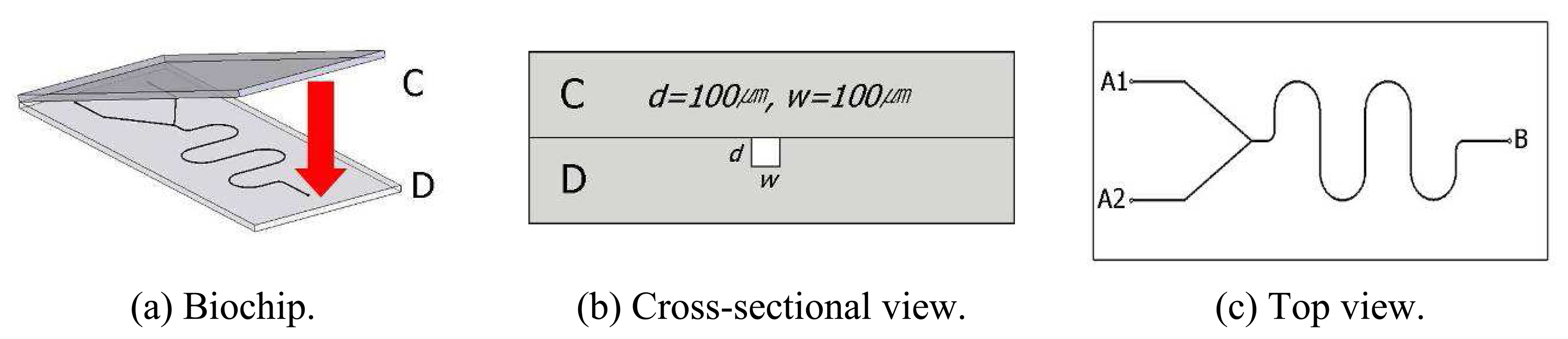
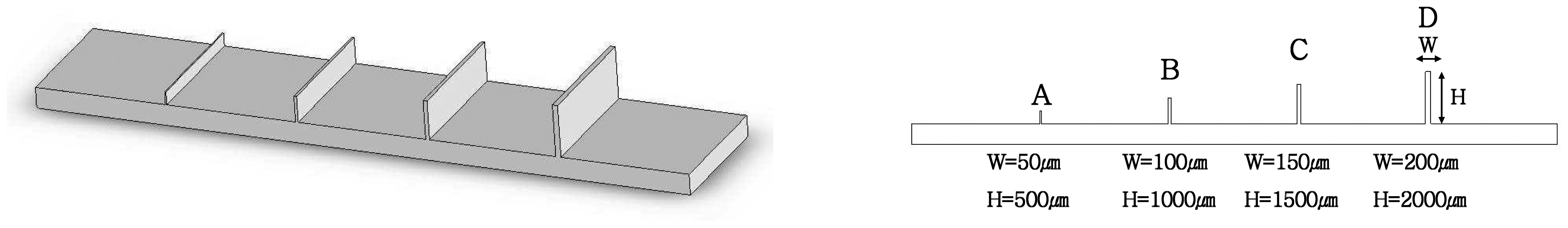
2. Design of a Sample Biochip with Micro-channels
3. Machining Experiments Using Micro End-mills
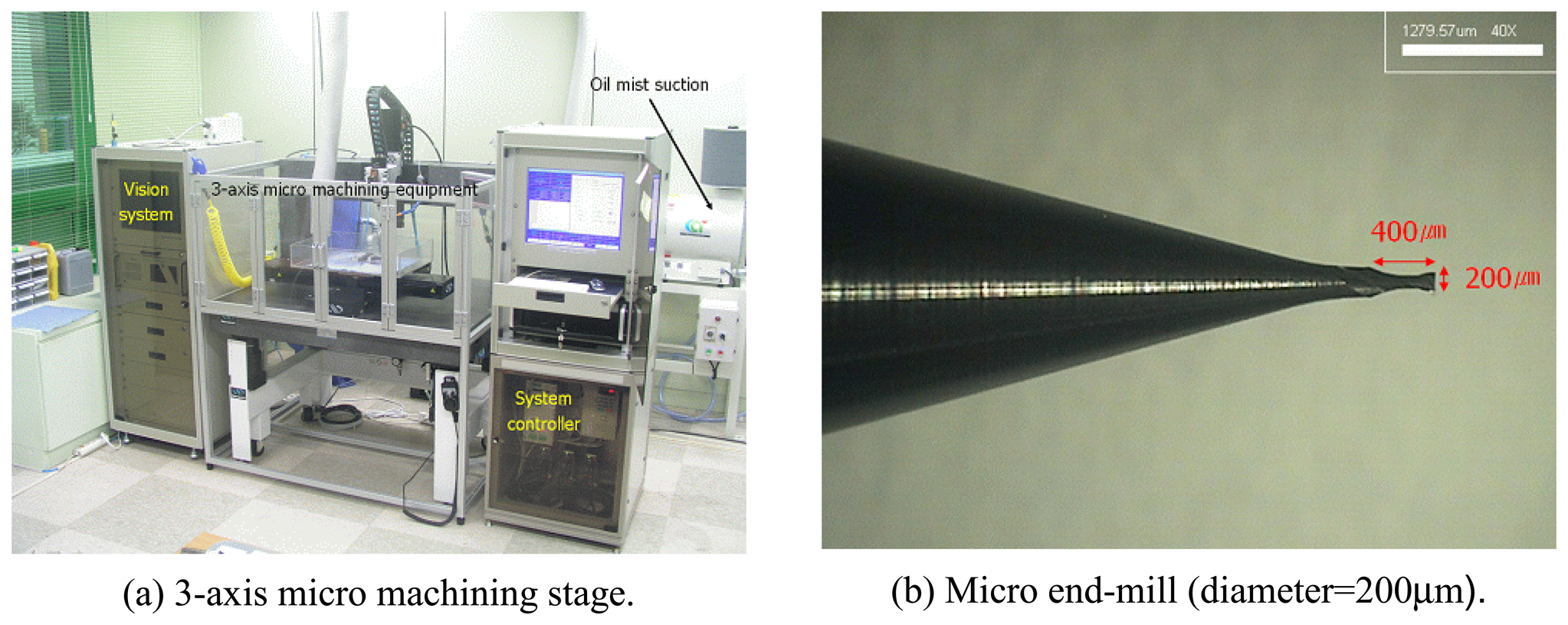
3.1. Experimental Setup
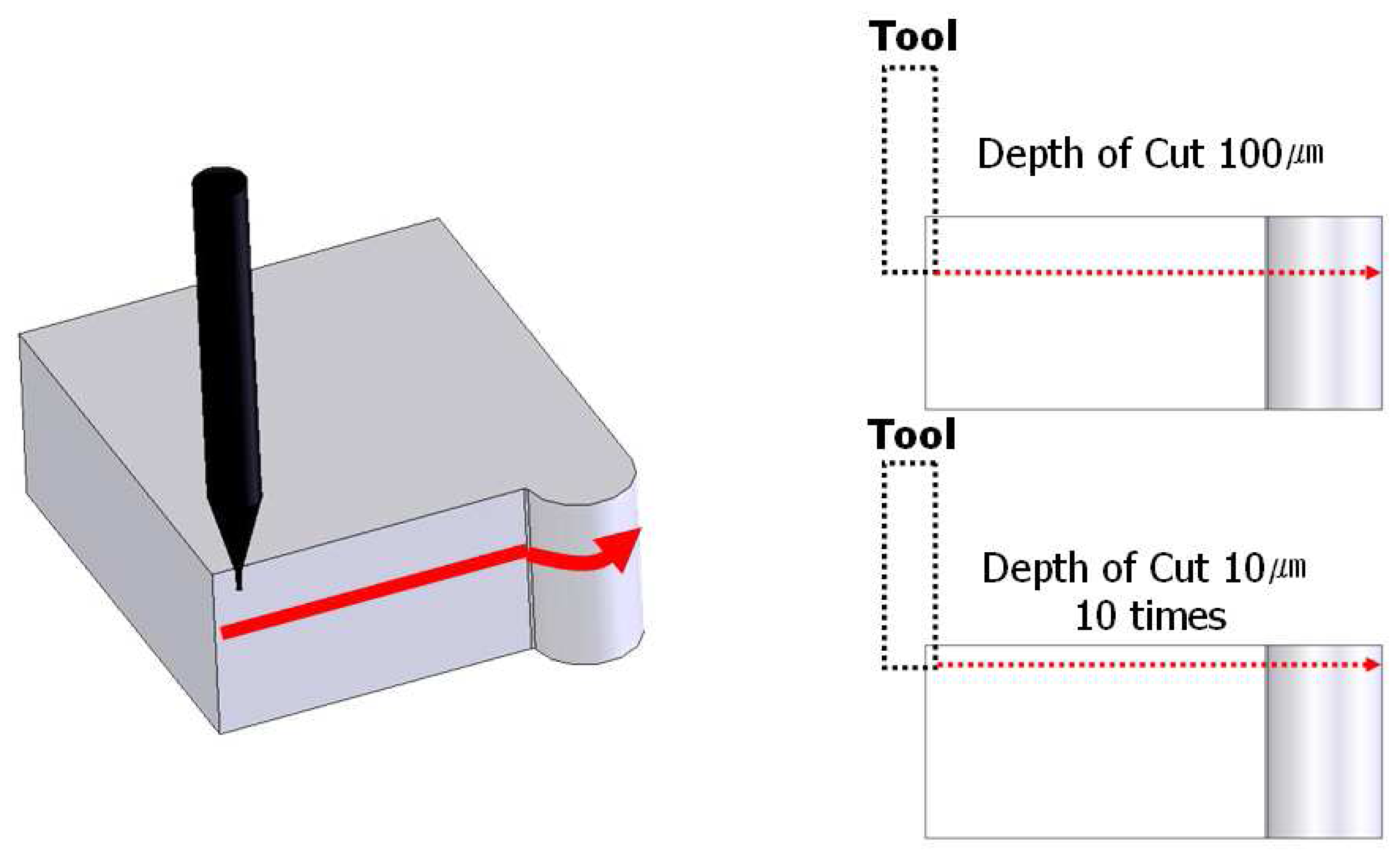
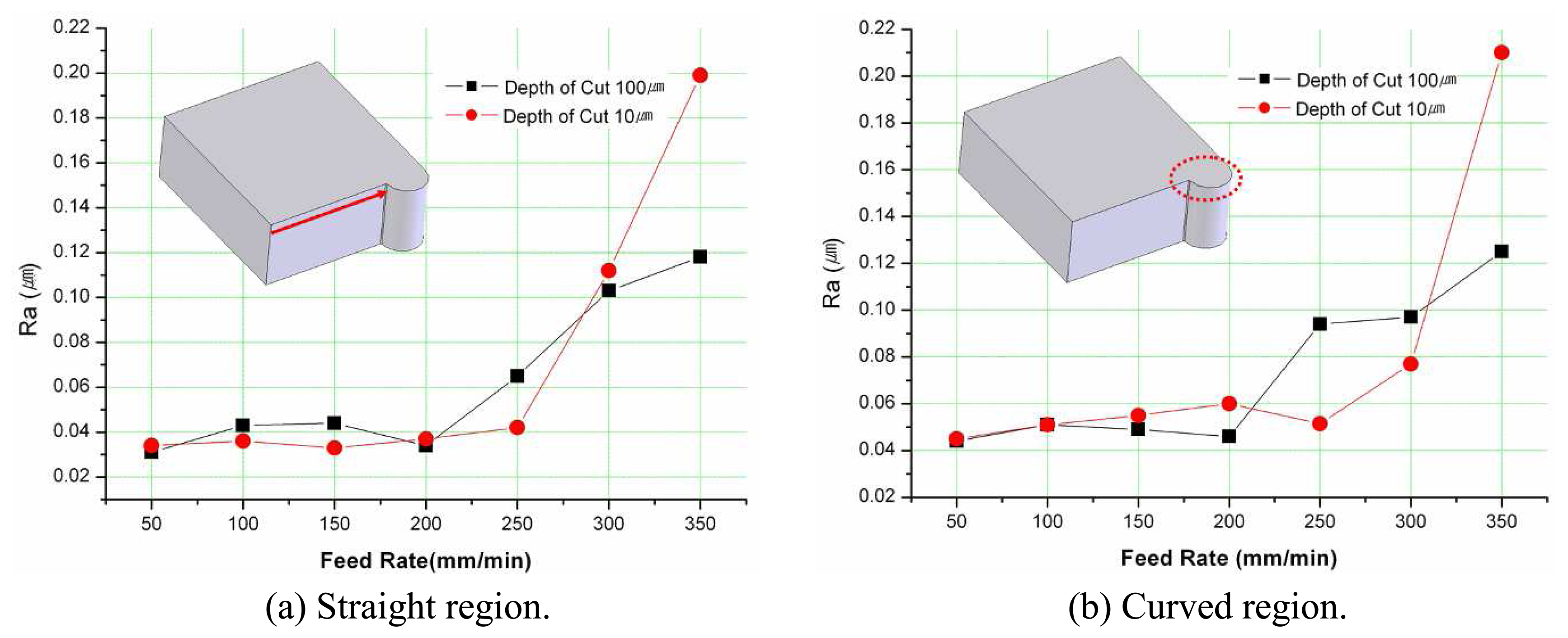
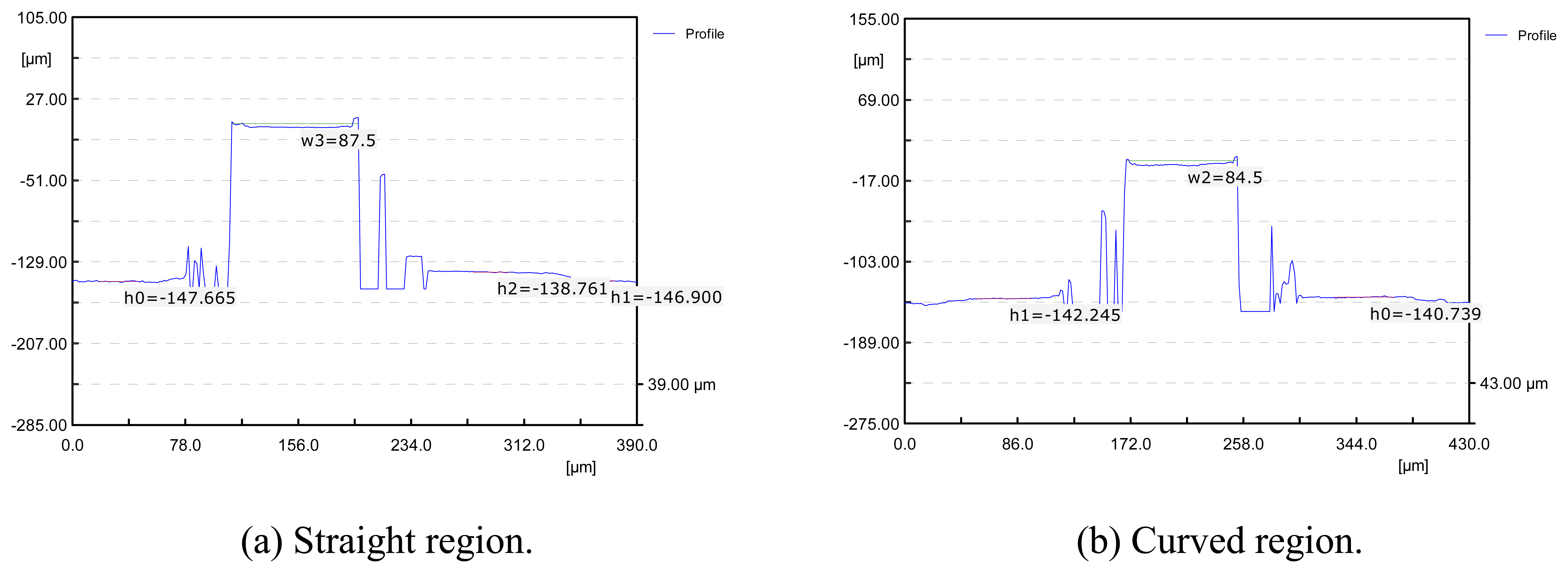
3.2. Experiments for Surface Roughness Improvement
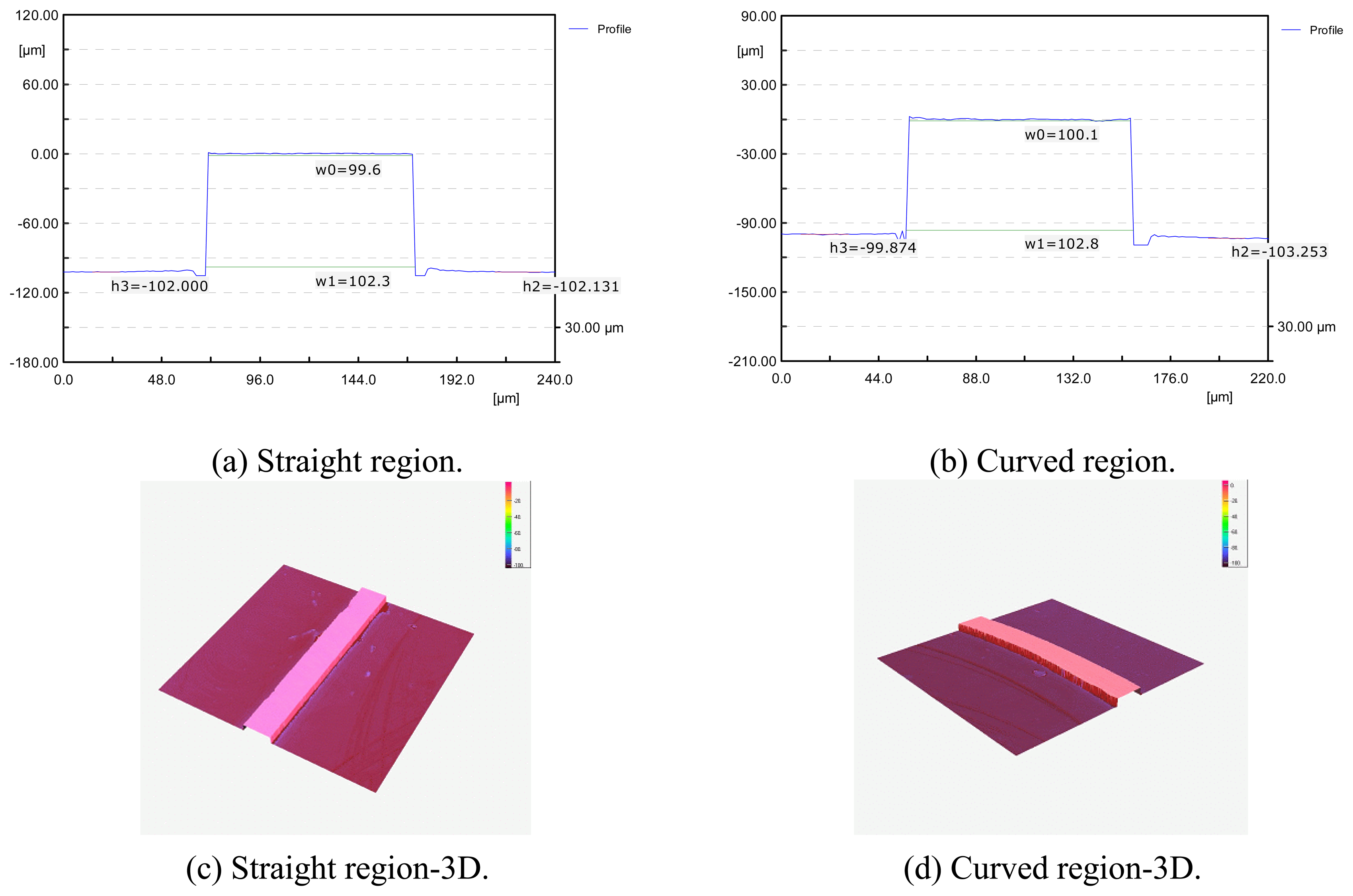
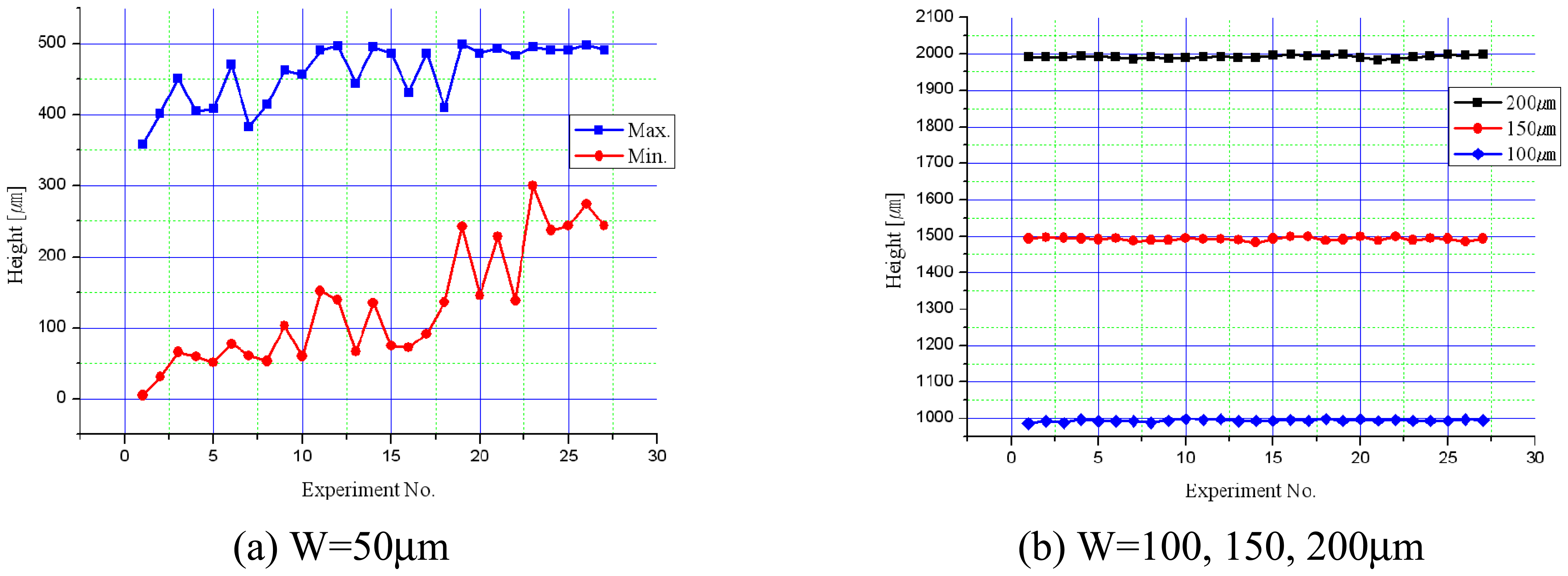
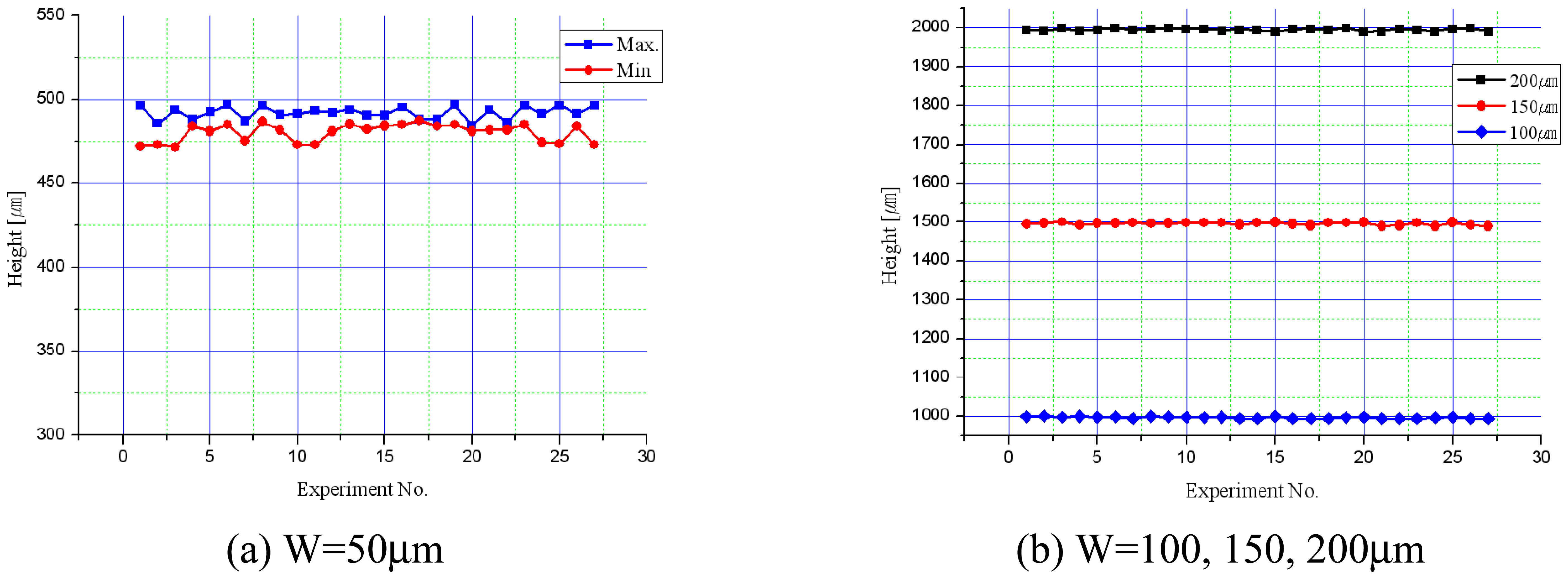
3.3. Experiments for Shape Accuracy Improvement
4. Fabrication of Mold Inserts and Test Injection Experiments
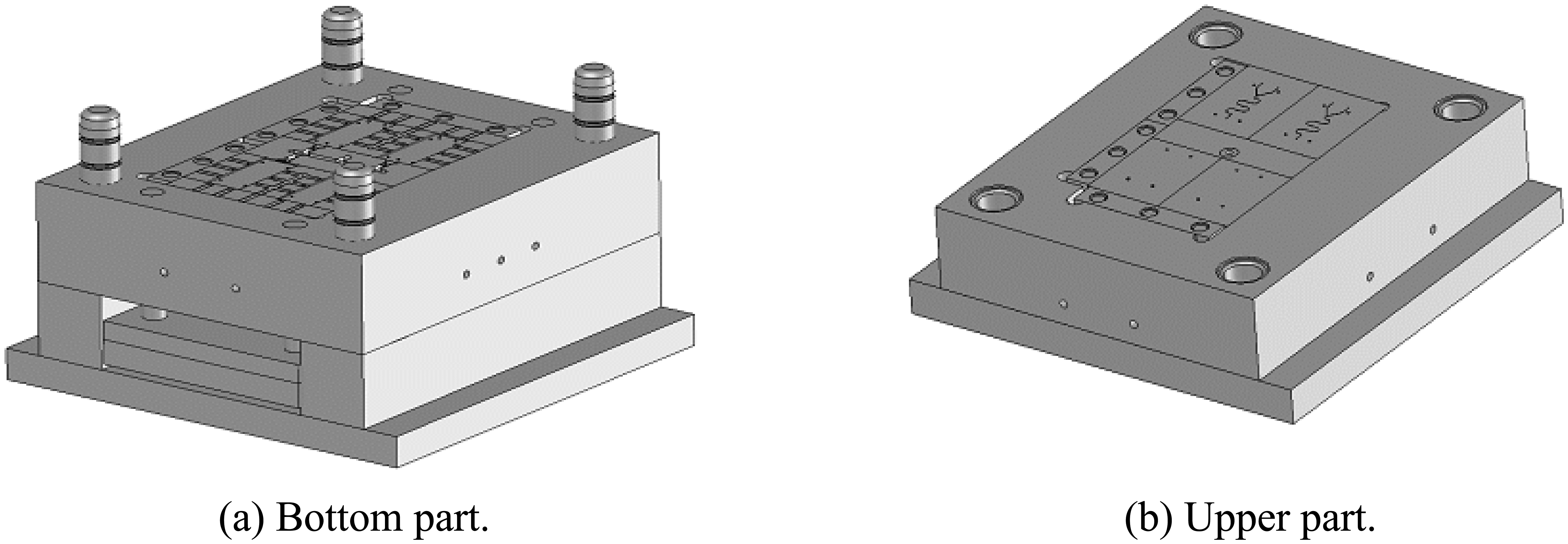
4.1. Mold design
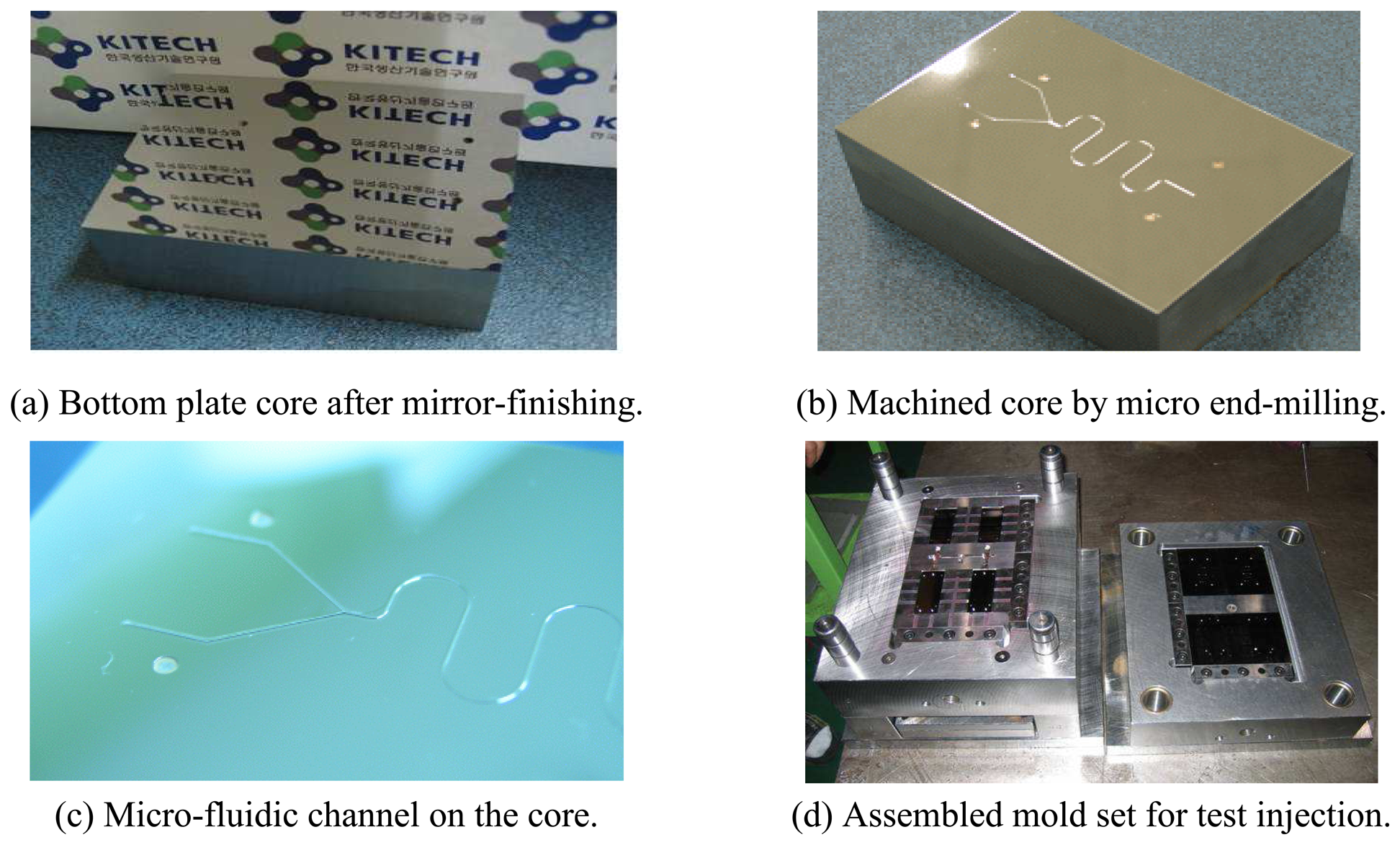
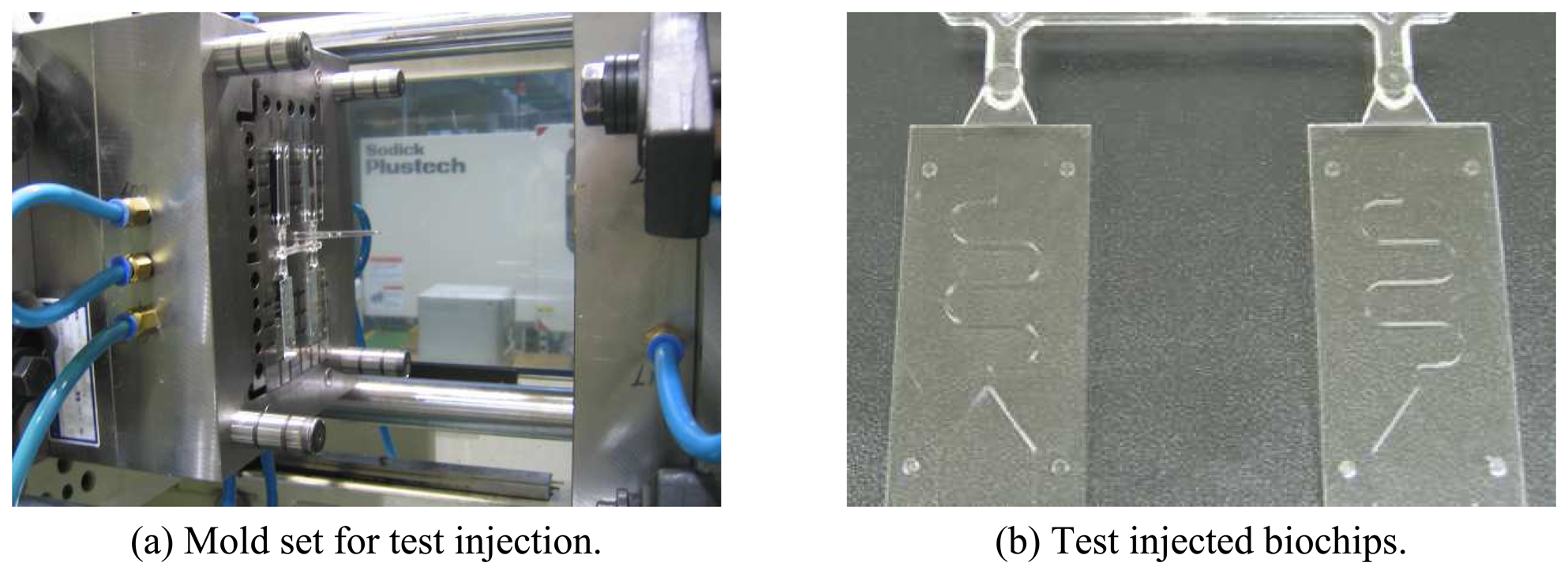
4.2. Mold Fabrication for Biochips
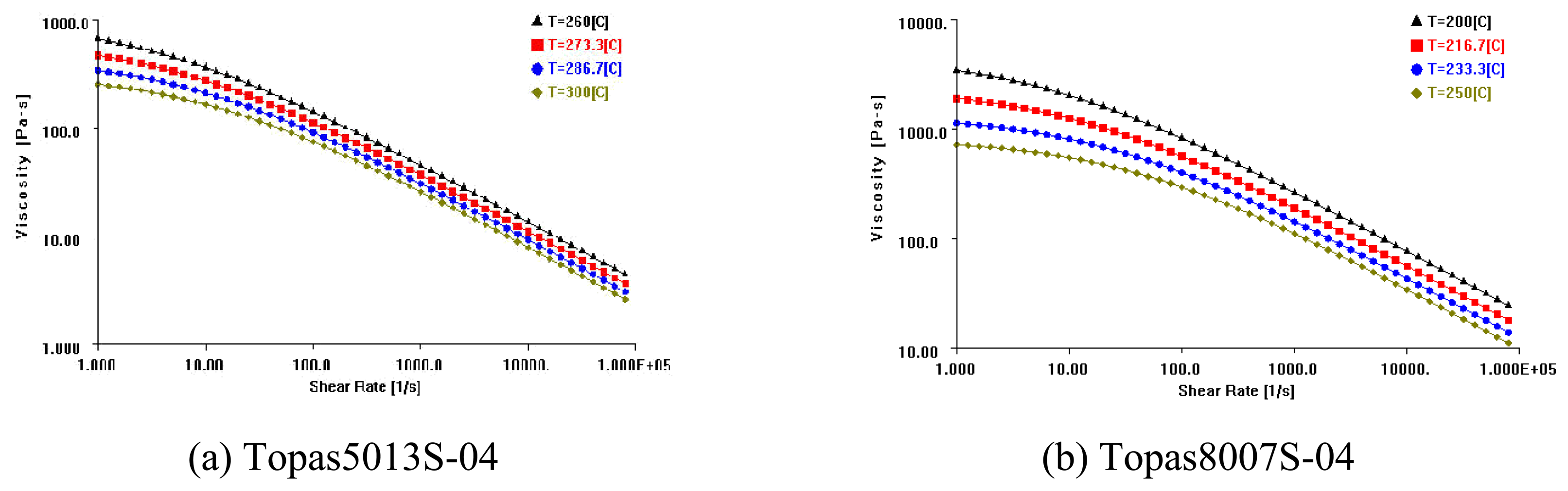
4.3. Injection Molding Characteristics of COC
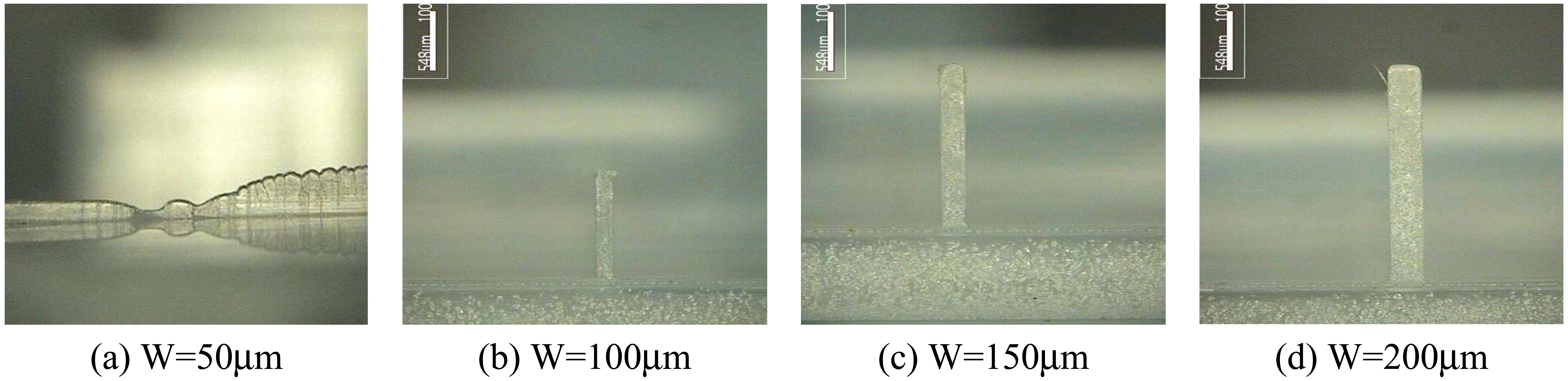
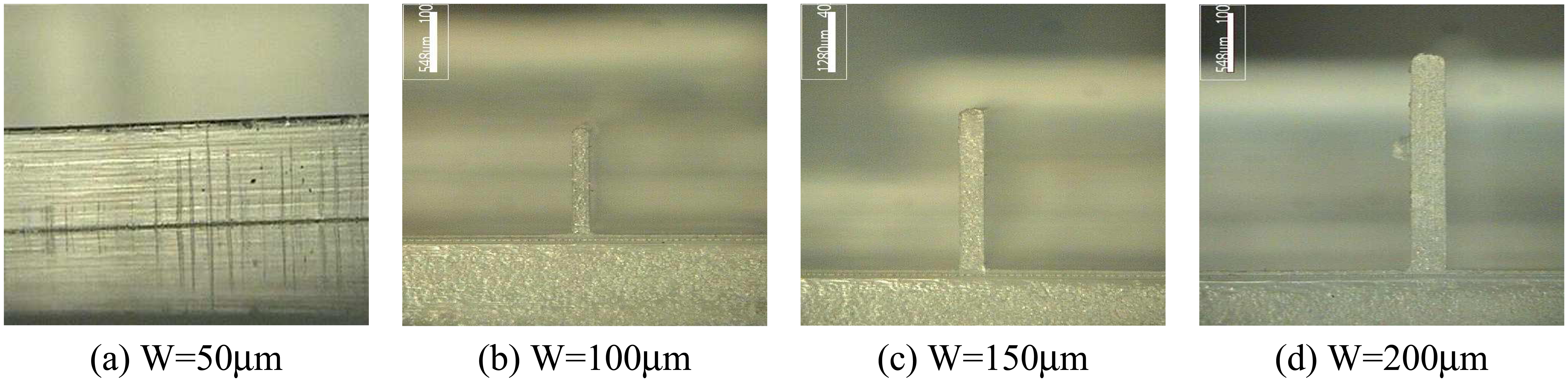
4.4. Test Injection of Sample Bipchips
5. Conclusions
- (1)
- In micro end-milling process, the best surface roughness could be obtained at depth-of-cut was 100μm and feed-rate was 50m/min. Under the condition, surface roughness of Ra=31nm was obtained for the straight regions, and Ra=44nm for curved regions.
- (2)
- When tool diameter was 400μm, better shape accuracy of the micro-channel could be obtained than the 200μm diameter tool.
- (3)
- The mold inserts for micro-channel forming was machined based on the experimental data.
- (4)
- In micro rib filling experiments, Topas8007S-04 showed better filling characteristics than Topas5013S-04.
- (5)
- Test injection process was performed successfully. Thus, it can be shown that the applied method can be a way for the mass production of biochips.
- (6)
- To form more precise micro channel patterns using injection molding process for biochip production, more extended studied such as tool deflection compensation, micro-fluidics analysis for the given polymers, etc. are needed as the future works.
Acknowledgments
References and Notes
- Song, S.; Lee, K.Y. Polymers for Microfluidic Chips. Macromolecular Research 2006, 14, 121–128. [Google Scholar]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.A.; Manz, A. Micro Total Analysis System. 1. Introduction, Theory and Technology. Analytical Chemistry 2002, 74, 2623–2636. [Google Scholar]
- Ahn, C.H. Appication of Micro Molding Technology to BioMEMS Components and Systems; 2006; University of Cincinnati; Internal technical report to KITECH; Korea. [Google Scholar]
- Blattert, C. Separation of Blood Cells and Plasma in Microchannel Bend Structures. Proceedings of μTAS; 2004; 1, pp. 483–485. [Google Scholar]
- Michael, J.H. DNA Micro Array Technology: Devices, Systems and Applications. Annual Review of Biomedical Engineering 2002, 4, 129–153. [Google Scholar]
- Kurian, K. M.; Watson, C. J.; Wylie, A. H. DNA Chip Technology. Journal of Pathology 1999, 187, 267–271. [Google Scholar]
- Jakeway, S. C.; de Mello, A. J.; Russell, E. L. Miniaturized Total Analysis Systems for Biological Analysis. Analytical Chemistry 2000, 66, 525–539. [Google Scholar]
- Hupert, M.L.; Guy, W.J.; Llopis, S.D.; Shadpour, H.; Rani, S.; Nikitopoulos, D.E.; Soper, S.A. Evaluation of micromilled metal mold masters for the replication of microchip electrophoresis devices. Microfluidics and Nanofluidics 2007, 3, 1–11. [Google Scholar]
- Mecomber, J.S.; Stalcup, A.M.; Hurd, D.; Halsall, H.B.; Heineman, W.R.; Seliskar, C.J.; Wehmeyer, K.R.; Limbach, P.A. Analytical Performance of Polymer-Based Microfluidic Devices Fabricated By Computer Numerical Controlled Machining. Analytical Chemistry 2006, 78, 936–941. [Google Scholar]
- Mecomber, J.S.; Hurd, D.; Limbach, P.A. Enhanced machining of micro-scale features in microchip molding masters by CNC milling. Int. J. of Machine Tools and Manufacture 2005, 45, 1542–1550. [Google Scholar]
- Zhao, D.S.; Roy, B.; McCormick, M.T.; Kuhr, W.G.; Brazill, S.A. Rapid fabrication of a poly(dimethylsiloxane) microfluidic capillary gel electrophoresis system utilizing high precision machining. Lab on a Chip 2003, 3, 93–99. [Google Scholar]

| Feed-rate | 50mm/min | RPM | 17,000 |
| Depth-of-cut | 100μm | Tool | Union 400μm |
| Side depth-of-cut | 10μm | Machine | DMU 100T |
| COC | 5013S-04 | 8007S-04 |
| Melt temperature | 260°C | 230°C |
| Glass transition temperature | 136°C | 80°C |
| Viscosity index (MPI data) | VI(260)0058 | VI(260)0091 |
| No. | Polymer Temp.(°C) | Injection Pressure (kgf/cm2) | Flow Rate (cm3/sec) | No. | Polymer Temp.(°C) | Injection Pressure (kgf/cm2) | Flow Rate (cm3/sec) | ||
|---|---|---|---|---|---|---|---|---|---|
| 5013S-04 | 8007S-04 | 5013S-04 | 8007S-04 | ||||||
| 1 | 270 | 250 | 1263 | 23 | 15 | 280 | 260 | 1768 | 41.4 |
| 2 | 33.2 | 16 | 2273 | 23 | |||||
| 3 | 41.4 | 17 | 33.2 | ||||||
| 4 | 1768 | 23 | 18 | 41.4 | |||||
| 5 | 33.2 | 19 | 290 | 270 | 1263 | 23 | |||
| 6 | 41.4 | 20 | 33.2 | ||||||
| 7 | 2273 | 23 | 21 | 41.4 | |||||
| 8 | 33.2 | 22 | 1768 | 23 | |||||
| 9 | 41.4 | 23 | 33.2 | ||||||
| 10 | 280 | 260 | 1263 | 23 | 24 | 41.4 | |||
| 11 | 33.2 | 25 | 2273 | 23 | |||||
| 12 | 41.4 | 26 | 33.2 | ||||||
| 13 | 1768 | 23 | 27 | 41.4 | |||||
| 14 | 33.2 | ||||||||
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jung, W.-C.; Heo, Y.-M.; Yoon, G.-S.; Shin, K.-H.; Chang, S.-H.; Kim, G.-H.; Cho, M.-W. Micro Machining of Injection Mold Inserts for Fluidic Channel of Polymeric Biochips. Sensors 2007, 7, 1643-1654. https://doi.org/10.3390/s7081643
Jung W-C, Heo Y-M, Yoon G-S, Shin K-H, Chang S-H, Kim G-H, Cho M-W. Micro Machining of Injection Mold Inserts for Fluidic Channel of Polymeric Biochips. Sensors. 2007; 7(8):1643-1654. https://doi.org/10.3390/s7081643
Chicago/Turabian StyleJung, Woo-Chul, Young-Moo Heo, Gil-Sang Yoon, Kwang-Ho Shin, Sung-Ho Chang, Gun-Hee Kim, and Myeong-Woo Cho. 2007. "Micro Machining of Injection Mold Inserts for Fluidic Channel of Polymeric Biochips" Sensors 7, no. 8: 1643-1654. https://doi.org/10.3390/s7081643




