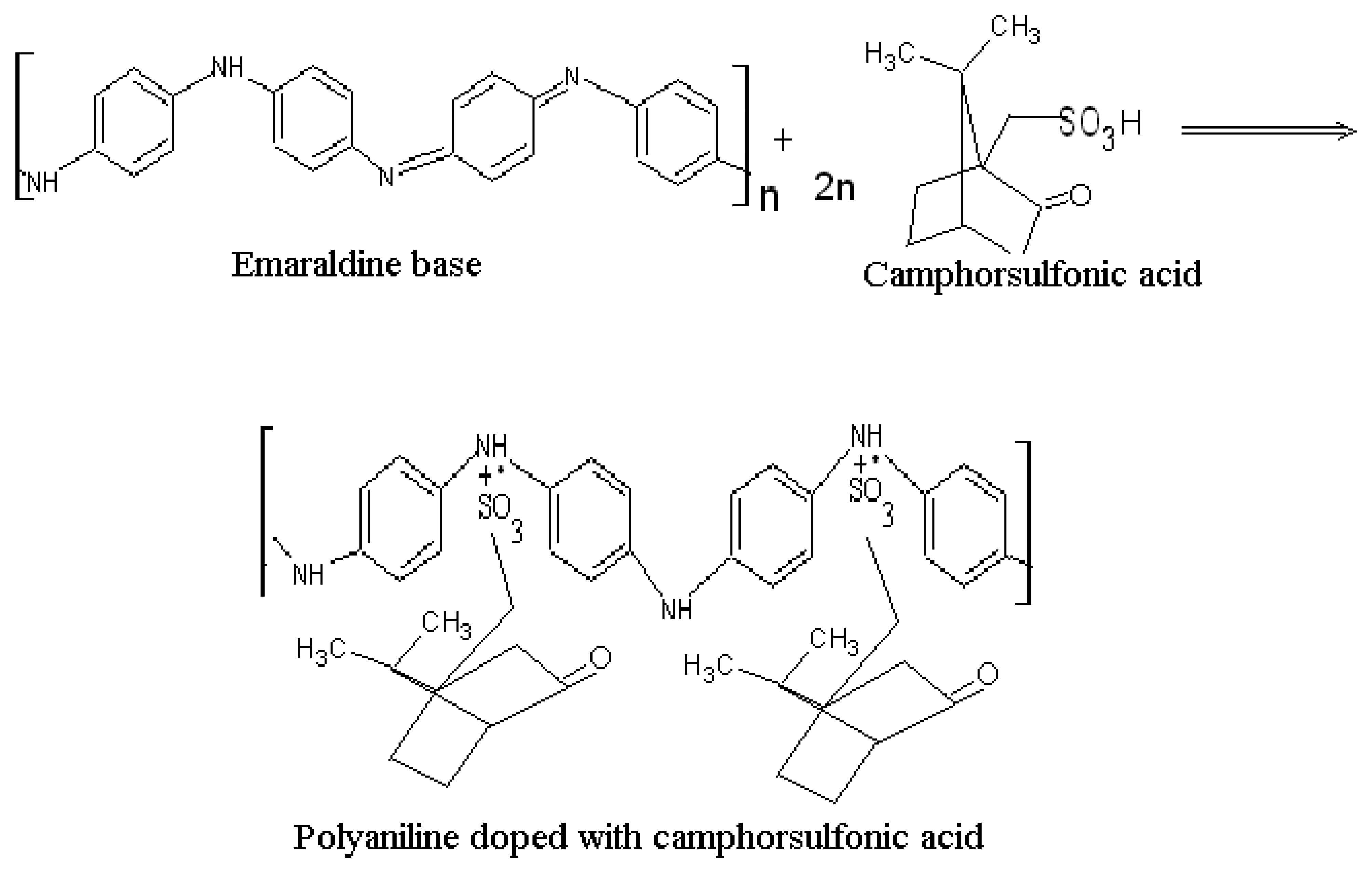
2.1 Characterization of polyaniline
Sergeyeva et al. (1996) reported that the conductivity of non-self doped Pani (Pani chemically polymerized with hydrochloric acid) linearly increased with increasing molecular weight from 10 KDa up to 45 KDa. The conductivity of 100 KDa of the same Pani, however, was reported to be lower than that of the 45 KDa (4). Owing to this report, three commercially-available emeraldine base compounds were self doped with camphorsulfonic acid: two with molecular weights larger than 45 KDa (65 and 50 KDa) and one lower than 45 KDa (20KDa).
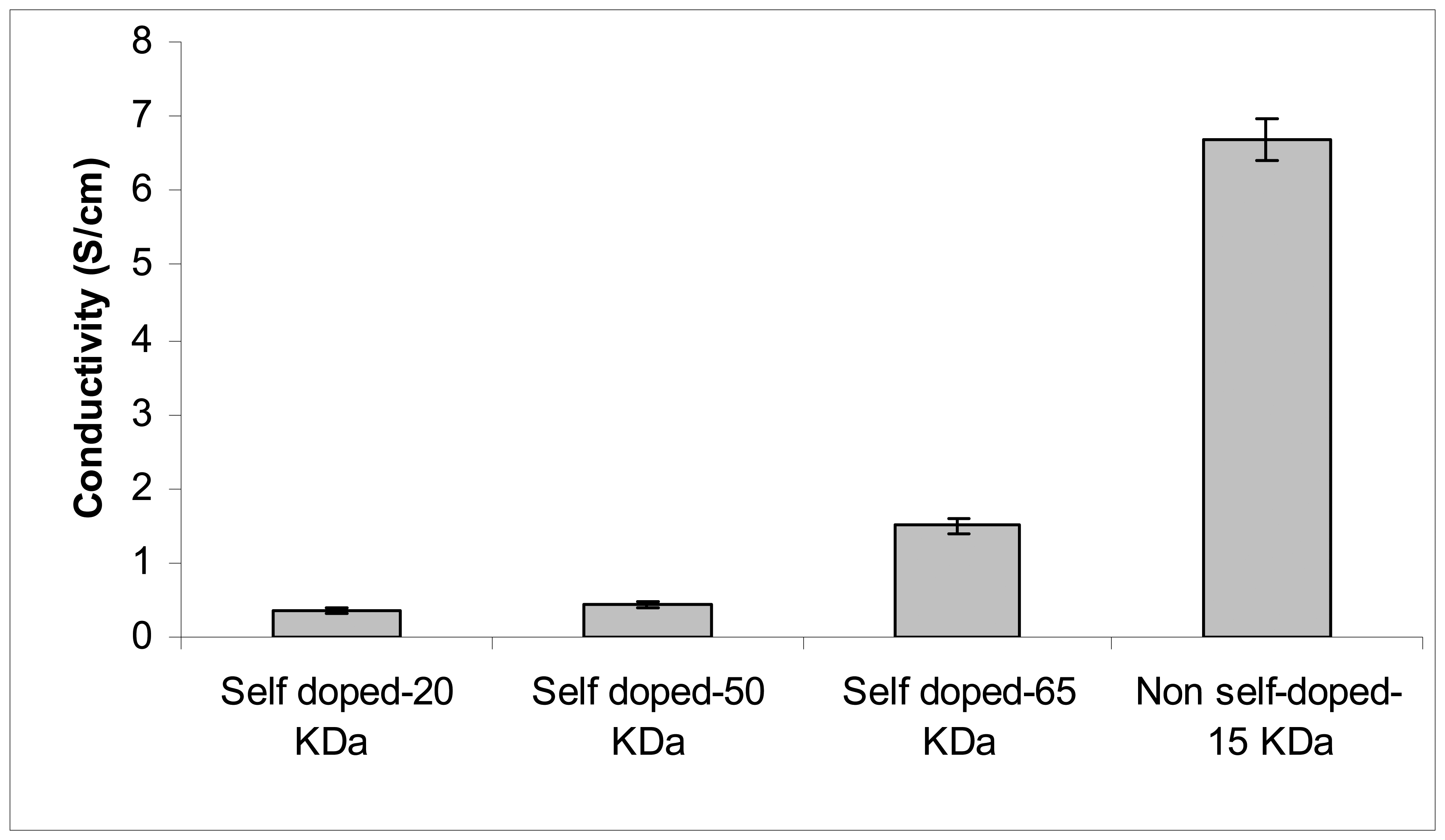
Figure 2 shows the conductivity of self and non-self doped Pani compounds using a four-point probe meter. Among the self doped Pani, the compound with a molecular weight 65-KDa has the highest conductivity at 1.5 Siemens (S)/cm, followed by the 50 (0.44 S/cm) and the 20 (0.36 S/cm) KDa. The conductivities of these self doped Pani are within the range of conductivity levels found in a previous study (11) and are also reported to be increasing with increasing molecular weight. Interestingly, the commercially-available non-self doped Pani has the highest conductivity at 6.7 S/cm even though its molecular weight of ~15 KDa is smaller than the self doped compounds (
Figure 2). This finding may be due to the different doping acids used in the Pani polymerization process (10, 16). As indicated earlier, the self doped Pani was doped with camphorsulfonic acid while the commercially-available non-self doped compound was doped with a proprietary organic acid. This observation was confirmed in the subsequent transmission electron microscope (TEM) analysis.
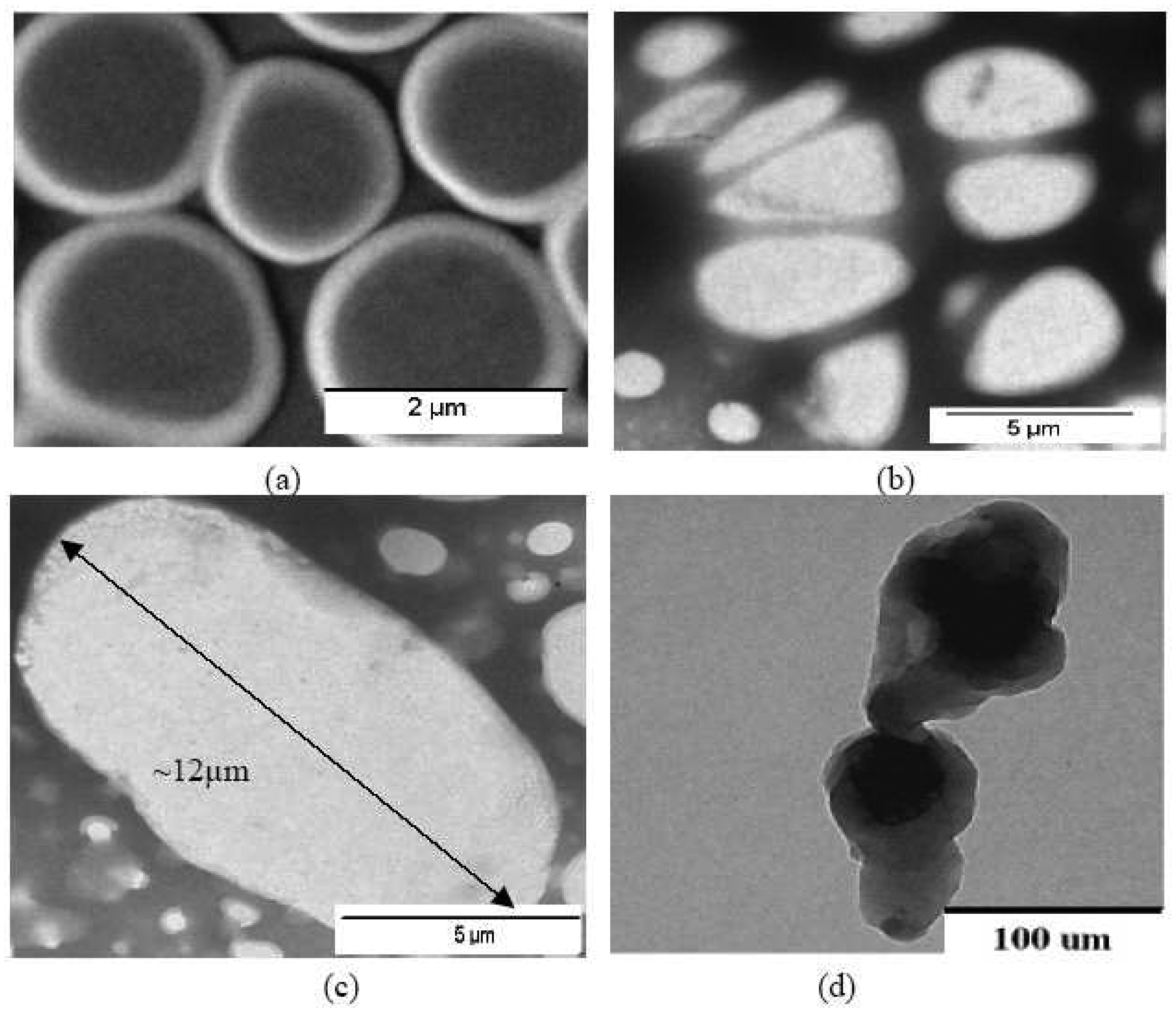
A transmission electron microscope (TEM) was used to study the morphology of Pani compounds (
Figure 3). The TEM images show that the higher the molecular weight of the self doped Pani, the larger is the polymer structure. The 65-KDa self doped Pani is approximately 12μm in diameter (
Figure 3c); the 50 KDa Pani is about 5 μm (
Figure 3b) and the 20 KDa Pani is 2μm (
Figure 3a). Additionally,
Figure 3 also shows that the self doped Pani compounds have more globular shapes and are smaller than the non-self doped Pani. The non-self doped Pani is more of a rod shape with an approximate length of 100μm. Duic et al. (1994) and Stejskal et al. (1998) also observed differences in size and shape of Pani when using different types of doping acids in their polymerization processes.
The conductive property of Pani was further evaluated by measuring its amperometric response. Both the self and non-self doped Pani compounds were spin-coated on the ITO substrate and tested for their amperometric responses using the electrochemical set-up described in sections 3.1.1 and 3.1.2. These substrates are referred to as ITO-self doped and ITO–non self doped substrates hereafter.
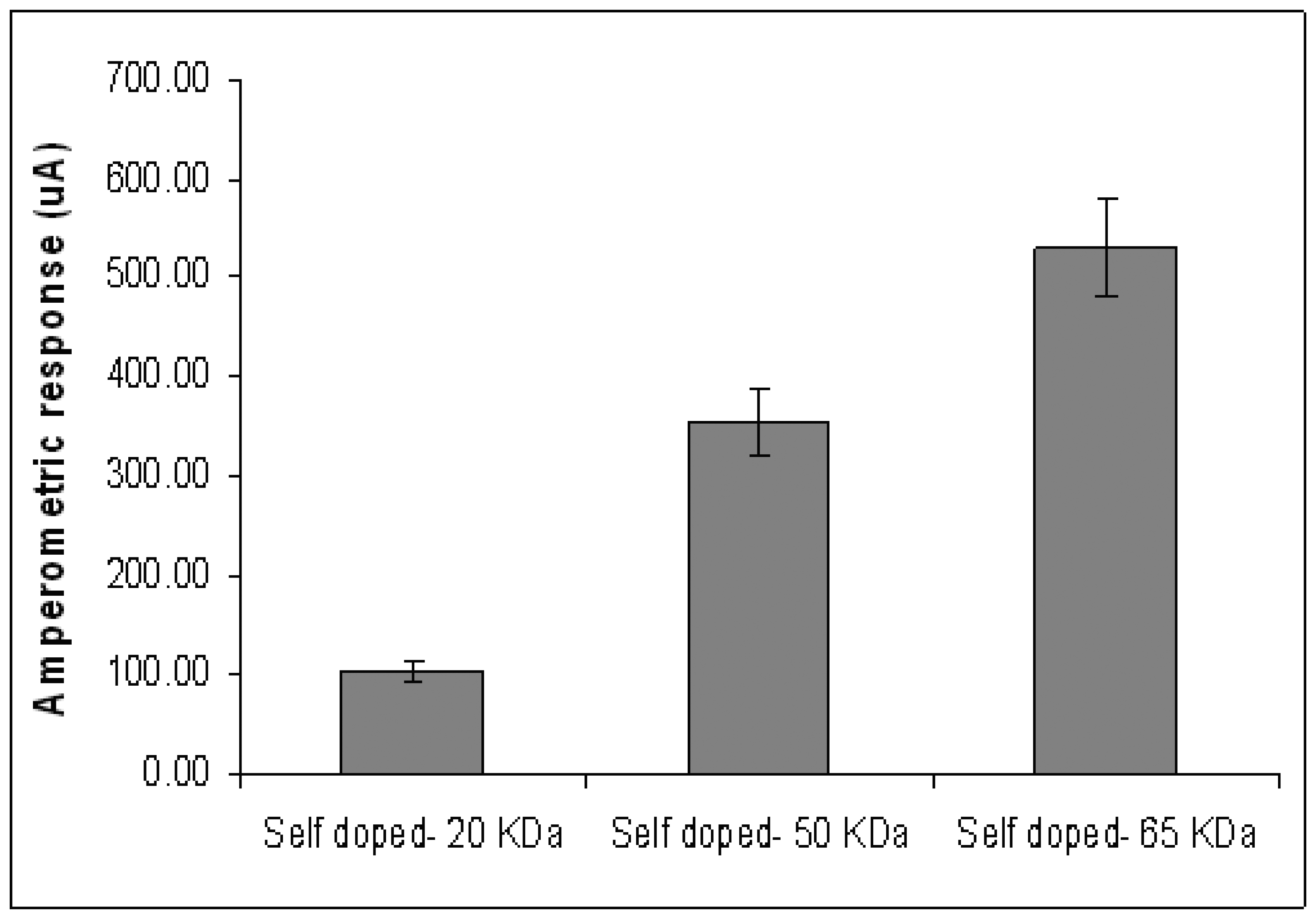
Figure 4 shows the amperometric response of the ITO-self doped substrates in phosphate buffer (PB). The ITO coated with the 65-KDa self doped Pani results in the highest amperometric signal at 530.52 μA. This is followed by the ITO glasses coated with 50KDa (355.78 μA) and 20 KDa (105.13 μA) self doped Pani (
Figure 4). A similar trend was observed in the conductivity experiment (
Figure 2). Therefore, it is appropriate to conclude that the molecular weight of self doped Pani is directly related to its conductivity. This observation could be explained in terms of polymer backbone density. As the molecular weight increases, the length of the polymer backbone per unit area increases as well (
17), enhancing the flow of electrons and subsequently increasing the conductivity. A similar characteristic was also reported by Ryu et al. (1999).
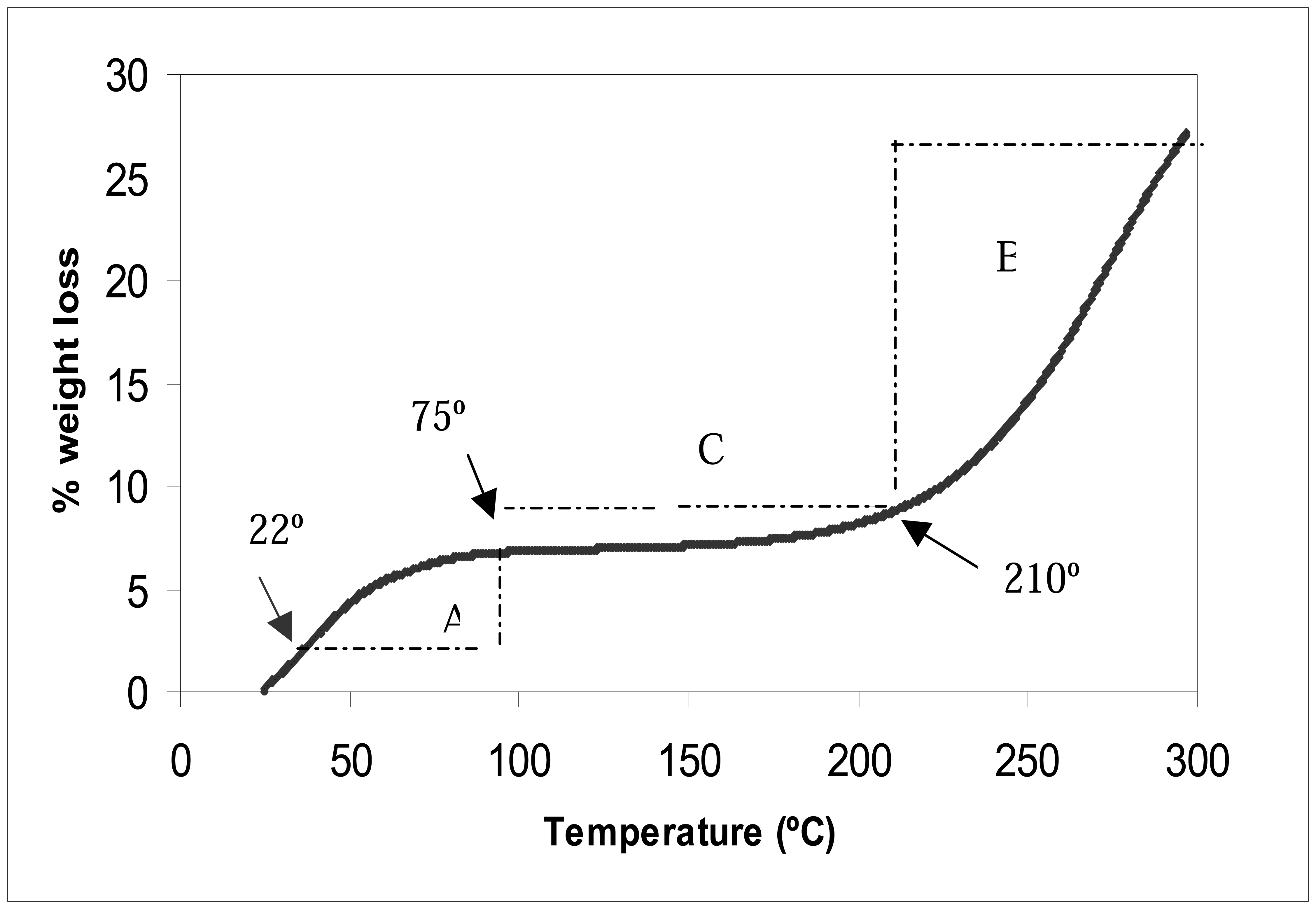
Thermogravimetric (TG) analysis was conducted to study the effect of temperature on the Pani weight.
Figure 5 shows the changes in the weight loss of the self doped Pani (65 KDa) after exposing the compound to temperatures ranging from 22°C to 300°C. A similar trend was also observed when testing the 50 and 20 KDa Pani compounds.
Figure 5 shows a steep rate of weight loss change in regions A (22°C to 75°C) and B (210°C to 300°C), and a smaller rate of change in region C (75°C to 210°C). From this finding, it is optimal to use the Pani between temperatures ranging from 75°C to 210°C (region C) since a temperature fluctuation within this region would only lead to a small change in Pani weight loss (
Figure 5). Since it was shown earlier that the weight of Pani affected the conductive property of the polymer, the use of Pani in region C would ensure minimal changes in the polymer conductive property. Temperature levels at this region, however, are too high for any biological element, such as antibodies, in the biosensor design. An antibody thermal stability study concluded that a heat treatment at 60°C resulted in the cleavage of the antibody heavy and light chains and promoted the denaturation of the protein (
18). For this reason, most biosensor operations are conducted in room temperature (~ 25°C) (
19,
20). Since the Pani weight is sensitive to temperature changes between 22 °C and 75°C (region A), a temperature-controlled mechanism needs to be introduced to the biosensor design to minimize temperature-dependent variations in the polymer properties.
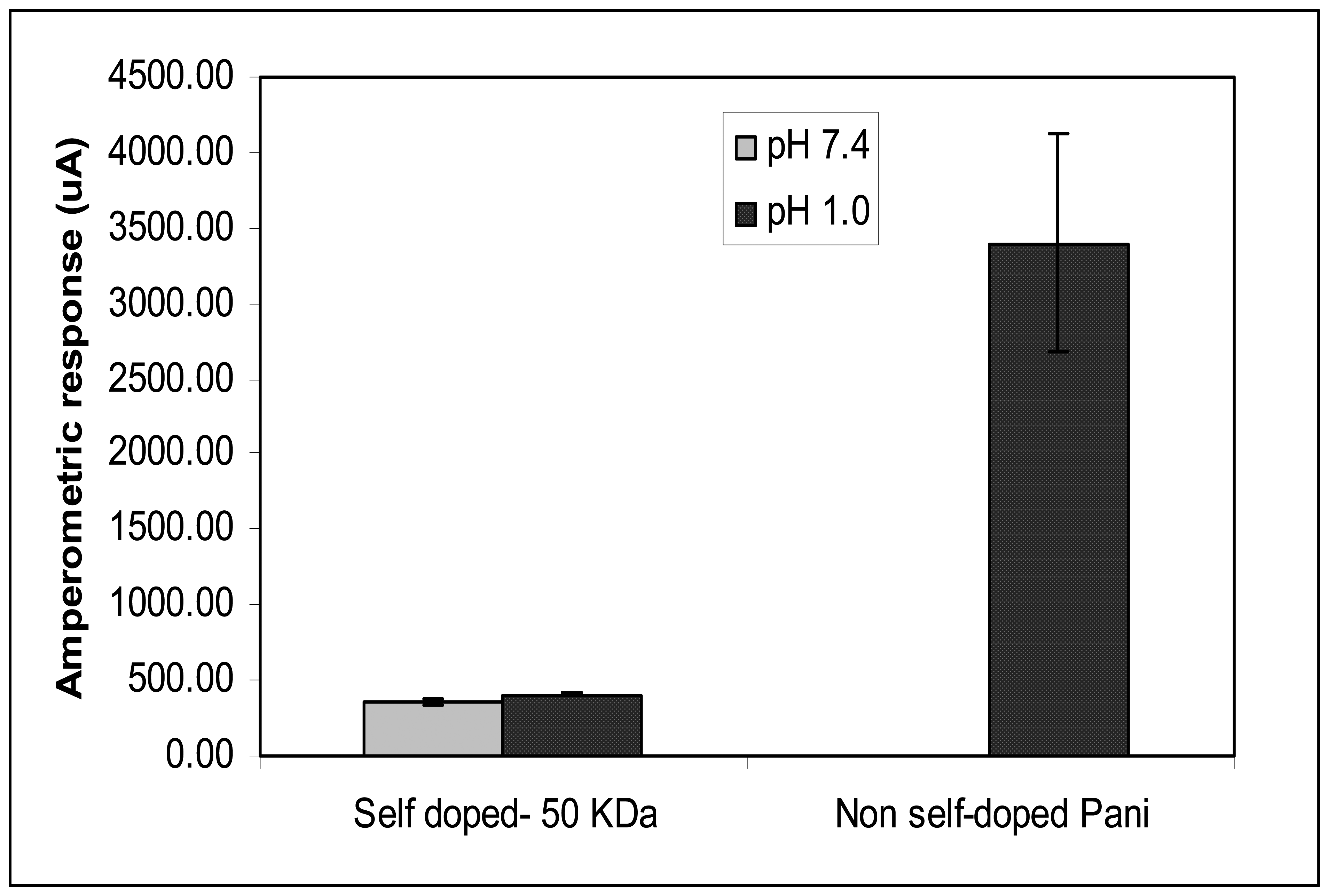
The pH-dependency of Pani compounds was also conducted in this study by testing the self and non-self doped Pani compounds in electrolytes with different pH levels: PB at pH 7.4 and 1 M hydrochloric acid at pH 1.0 (
Figure 6). Results show no statistical difference in amperometric responses between the ITO-self doped Pani (50 KDa) tested in PB, and that in 1 M hydrochloric acid. This finding suggests that there are no changes in the conductivity level of the self doped Pani from a highly acidic to a near neutral pH environment. A significant difference in responses however, was observed between the ITO-non self doped Pani in PB and that in 1 M hydrochloric acid. Though the above substrate resulted in a high amperometric response (3400.16 μA) in 1M hydrochloric acid, the ITO-non self doped Pani was observed to be not conductive in PB (
Figure 6). Additionally, the amperometric response of the ITO-non self doped Pani substrate was observed to be 10 times higher than that of the 50 KDa self doped Pani (355.78 μA) in 1 M hydrochloric acid. Though the reason for this is not yet stipulated, the difference in types of acids used in the doping process could be a factor for the difference in the conductivity level of the two tested Pani compounds. A study reported that different anions present in a doping acid influenced the conductivity, solubility, and other chemical characteristics of Pani (
21,
22). This finding confirms that the conductivity of the non-self doped Pani dependTherefore, in this study, a constant s highly on pH levels and therefore, is not suitable to be used in a neutral pH environment where most immunological reaction occurs optimally (
23). Due to this finding, the self doped Pani compounds were chosen to be incorporated in the subsequent biosensor fabrication.
2.2 Concept of detection
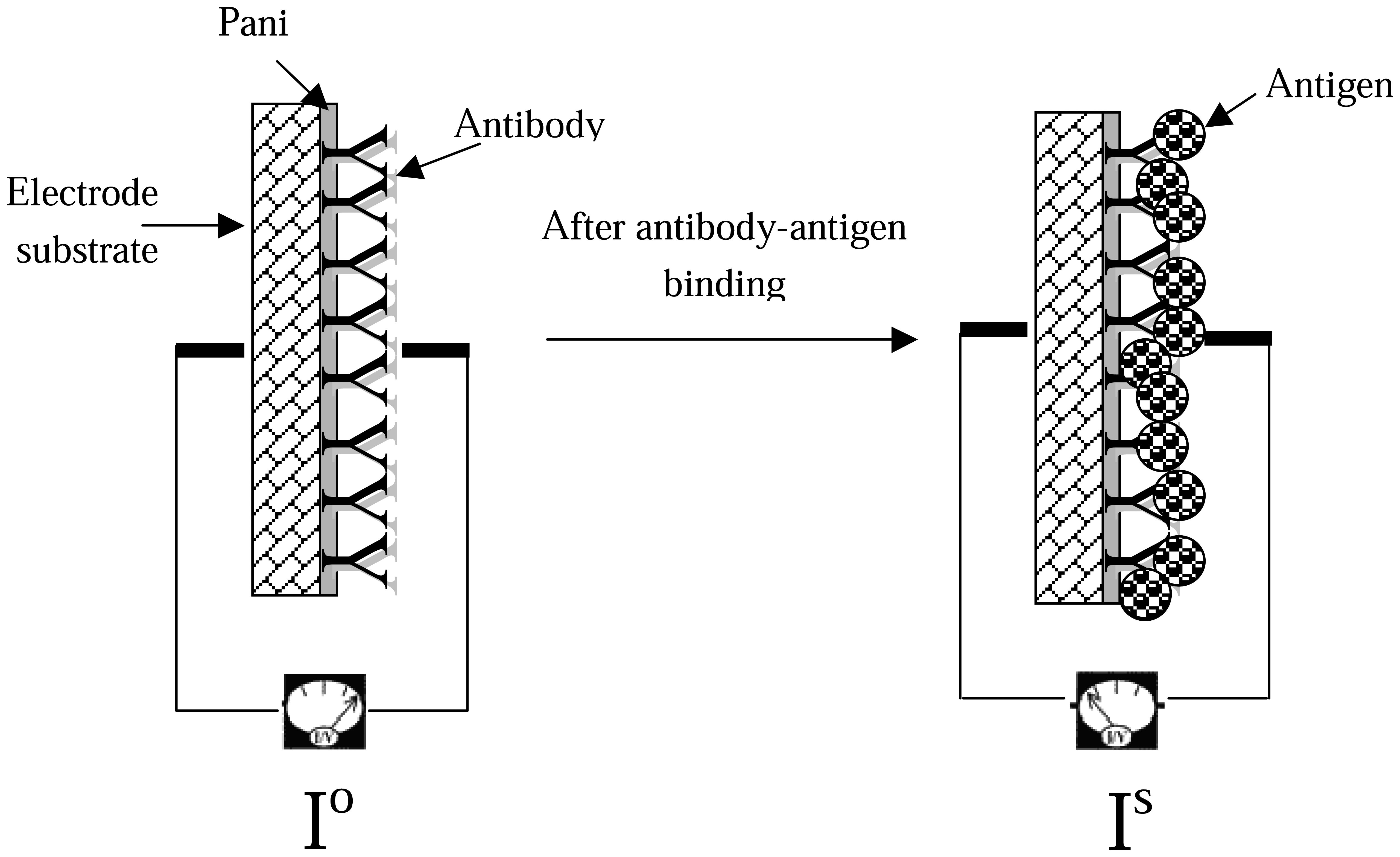
The ITO-Pani biosensor uses a direct antibody-antigen binding format with the self doped Pani as the transducer. By using the three-electrode electrochemical set-up, the input signal is transferred from the auxiliary electrode to the working electrode by the ionic charges forming in the electrolyte solution. When a fixed potential is applied, electrons are allowed to flow freely from the auxiliary electrode to the ITO-Pani substrate (working electrode) due to the conductive property of Pani and ITO substrate. When proteins (e.g., antibodies with a molecular weight of 150 KDa), are immobilized within the polymer backbones, electron flows are restricted. This phenomenon could be caused by the insulating protein membrane, interfering the transfer of electrons within the polymer π-backbone (24). The electron flow is restricted even more when a bigger antigen-antibody complex (molecular weight of BVDV is at least 4 MDa) present within the Pani backbone. It is here hypothesized that the bigger the protein complex present in the Pani backbone, the more restricted is the flow of electron.
A potential of between 0.2 V and 0.8 V has been demonstrated in previous studies to be a sufficient input signal, especially when dealing with whole cells or biological elements (25-27). Therefore, in this study, a constant potential of 0.5 V was chosen arbitrarily as the input signal.
The biosensor detection concept is based on the difference between the signal before (I
o) and the signal after (I
s) antibody-antigen binding (
Figure 8). This current drop (ΔI) is expressed mathematically as Δ I= I
o – I
s. Theoretically, the higher the current drop between I
o and I
s, the more antibody-antigen complexes are formed on the biosensor surface, blocking the transfer of electrons. Therefore, the value of current drop (ΔI) should be increasing with increasing antigen concentration.
2.3 Fabrication of ITO-Pani biosensor
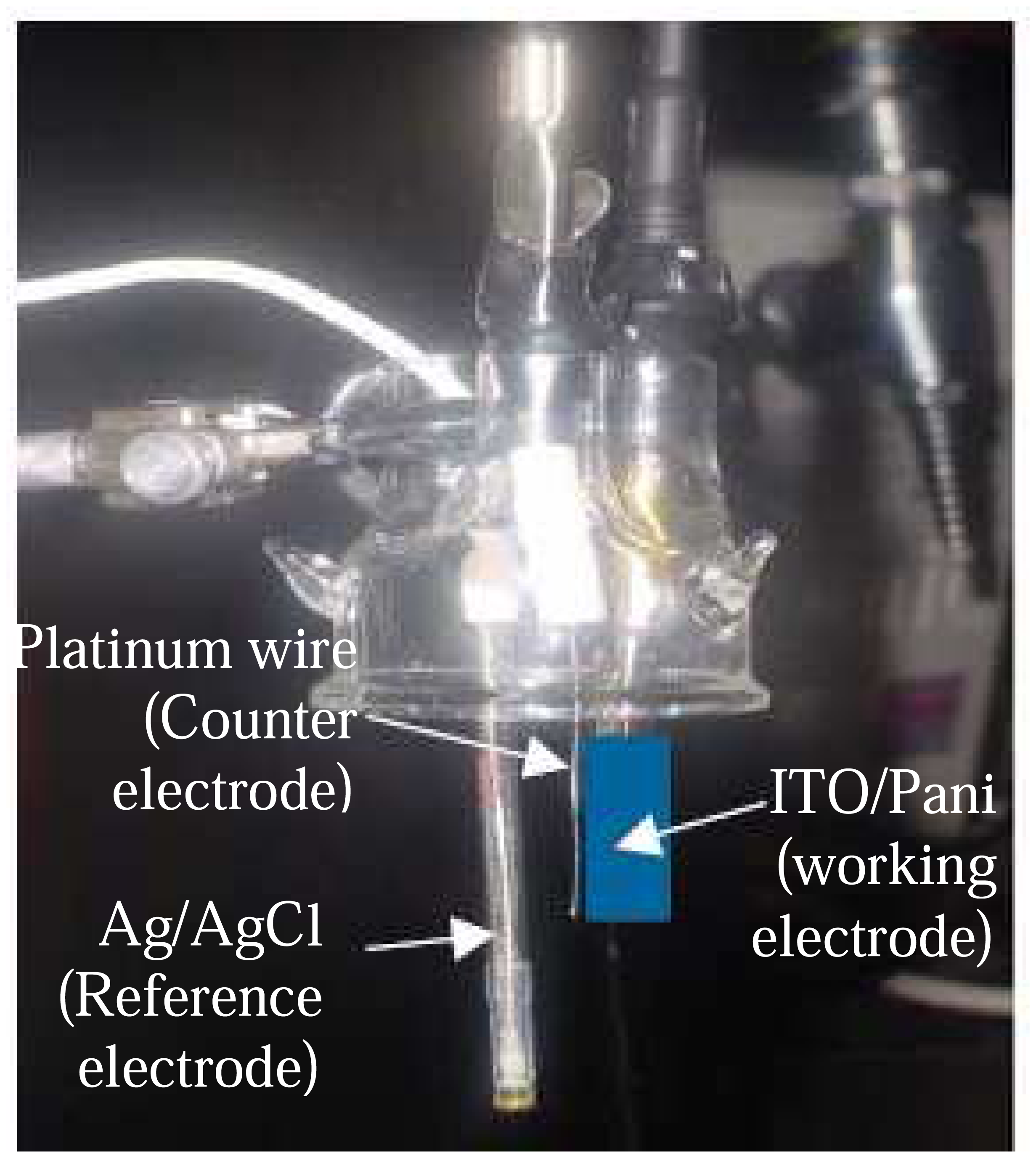
The ITO-Pani biosensor consisted of two components: immunosensor and an amperometric measuring device (
Figure 8). The immunosensor was constructed from an ITO glass, and layered with Pani and antibodies. The amperometric measuring device was a three electrode electrochemical cell that was connected to a potentiostat and described in detail in the Experimental Section (section 3). The ITO glass is a common substrate used in an amperometric biosensor due to its structural flexibility to bind directly with biological elements (
28), stable electrical property with high charge-carrier density (
29), and inexpensive production cost. In this study, an ITO glass substrate with a dimension of 1.27 × 2.54 × 0.1 cm
3 and a resistance value ranging from 15 to 25 ohms were prepared for biosensor fabrication.
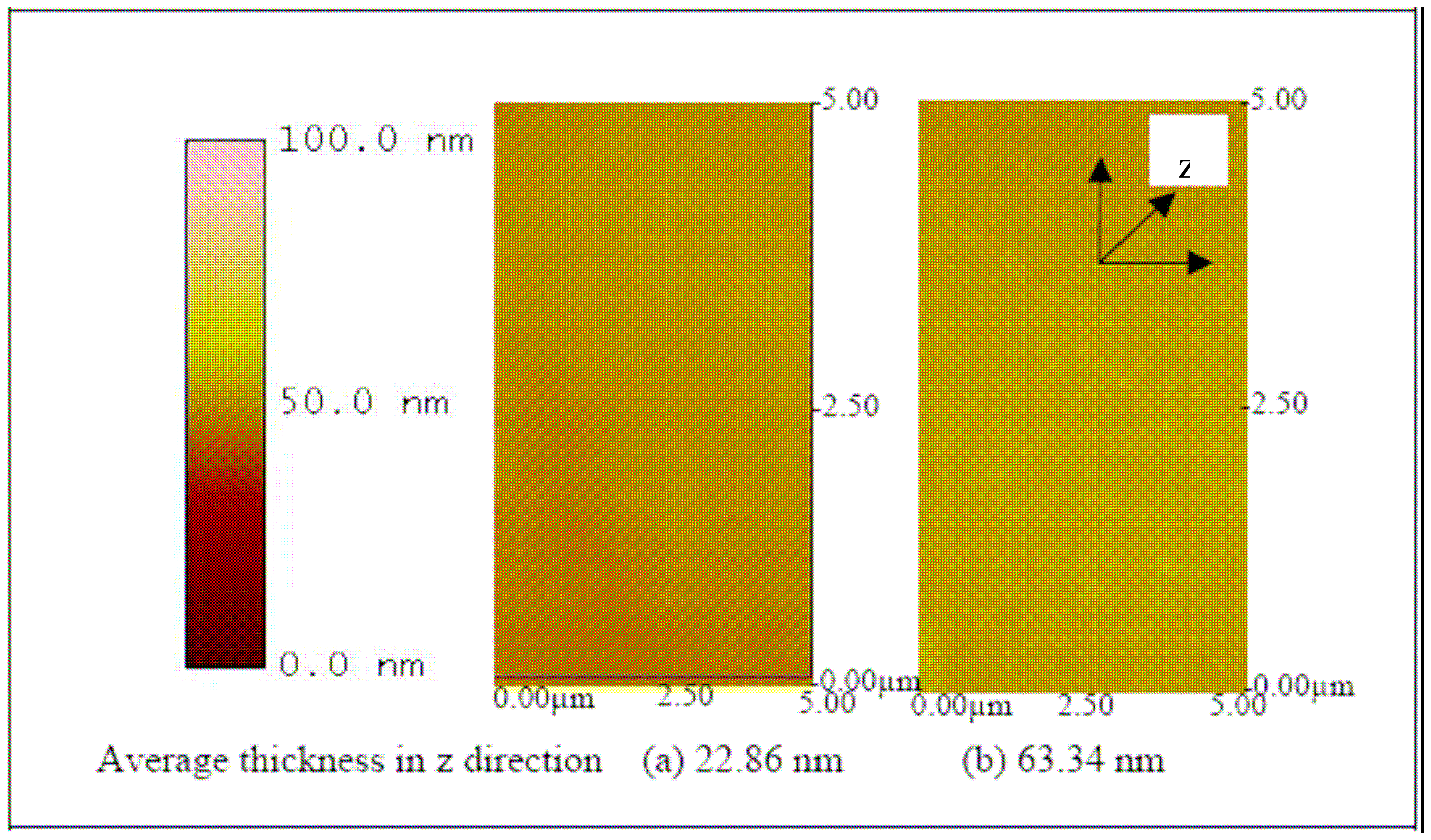
To fabricate the biosensor, the ITO substrate was first cleaned and treated with a strong oxidizing agent, ammonium hydroxide, to enable the adherence of Pani to the ITO substrate (
30).
Figure 9 shows the increase in thickness with NH
4OH. Then, the self doped Pani was layered onto the ITO substrate using a spin coating method. Spin coating method was chosen because of its ease of use, rapid processing time, reproducibility and is inexpensive compared to other types of polymer coating mechanism, such as Langmuir-Bloggert technique (
31) or layer by layer molecular deposition (
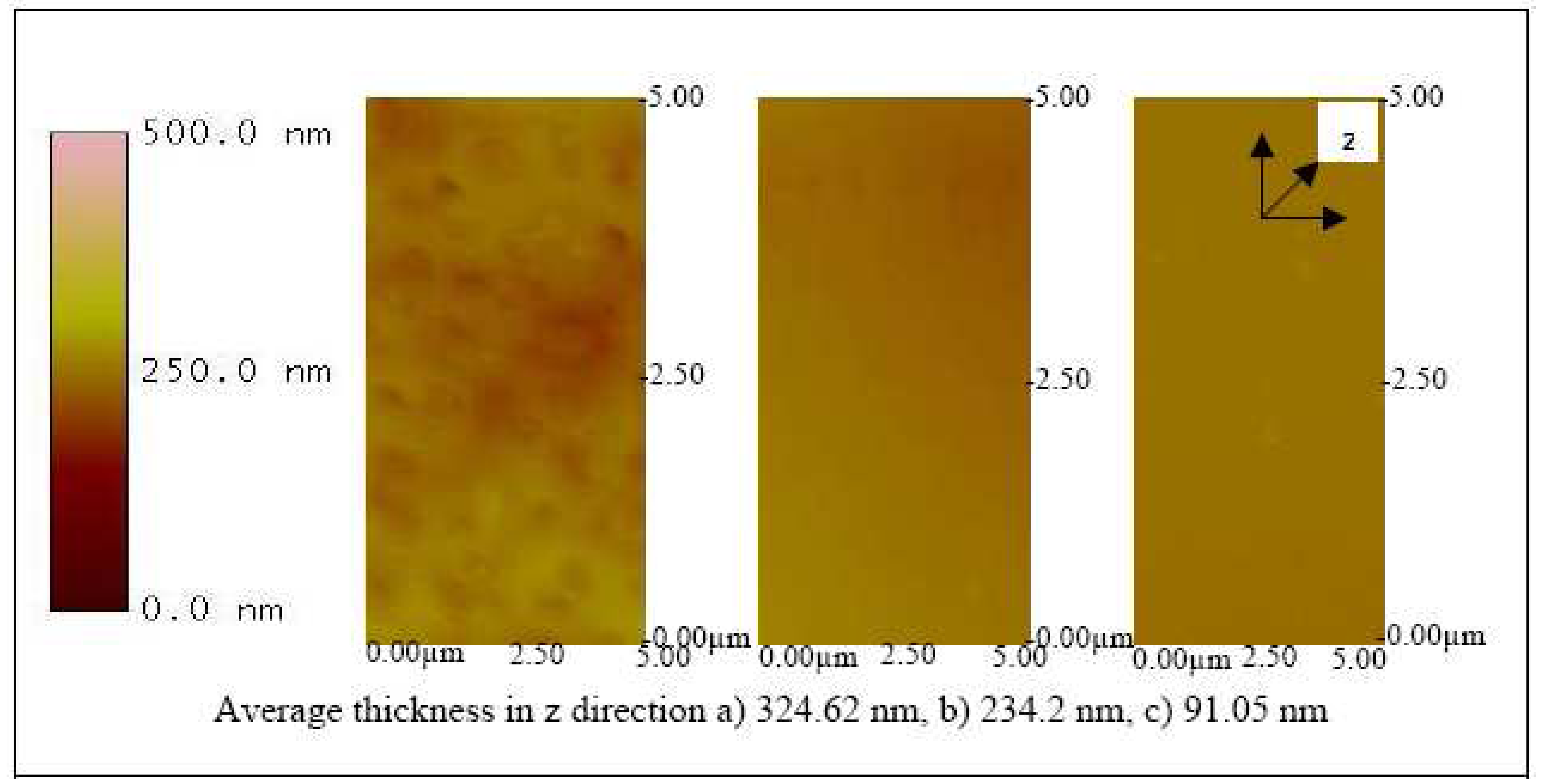
32). With a speed of 500 rpm for 6 seconds, a thickness of 324.62, 234.2, and 91.05 nm were observed on the ITO substrates coated with 65, 50 and 20 KDa Pani, respectively (
Figure 10). After the coating procedure, the substrate was then functionalized into a biosensor by immobilizing antibodies onto the surface. A three-electrode electrochemical cell was used to charge the substrate by applying a small (0.5 V) negative potential. This step was essential to promote electrostatic bonding between the negatively charged substrate and the NH
+ site of the antibodies (
33). The use of a divalent crosslinker, glutaraldehyde, also helped facilitate the antibody binding mechanism (
34,
35).
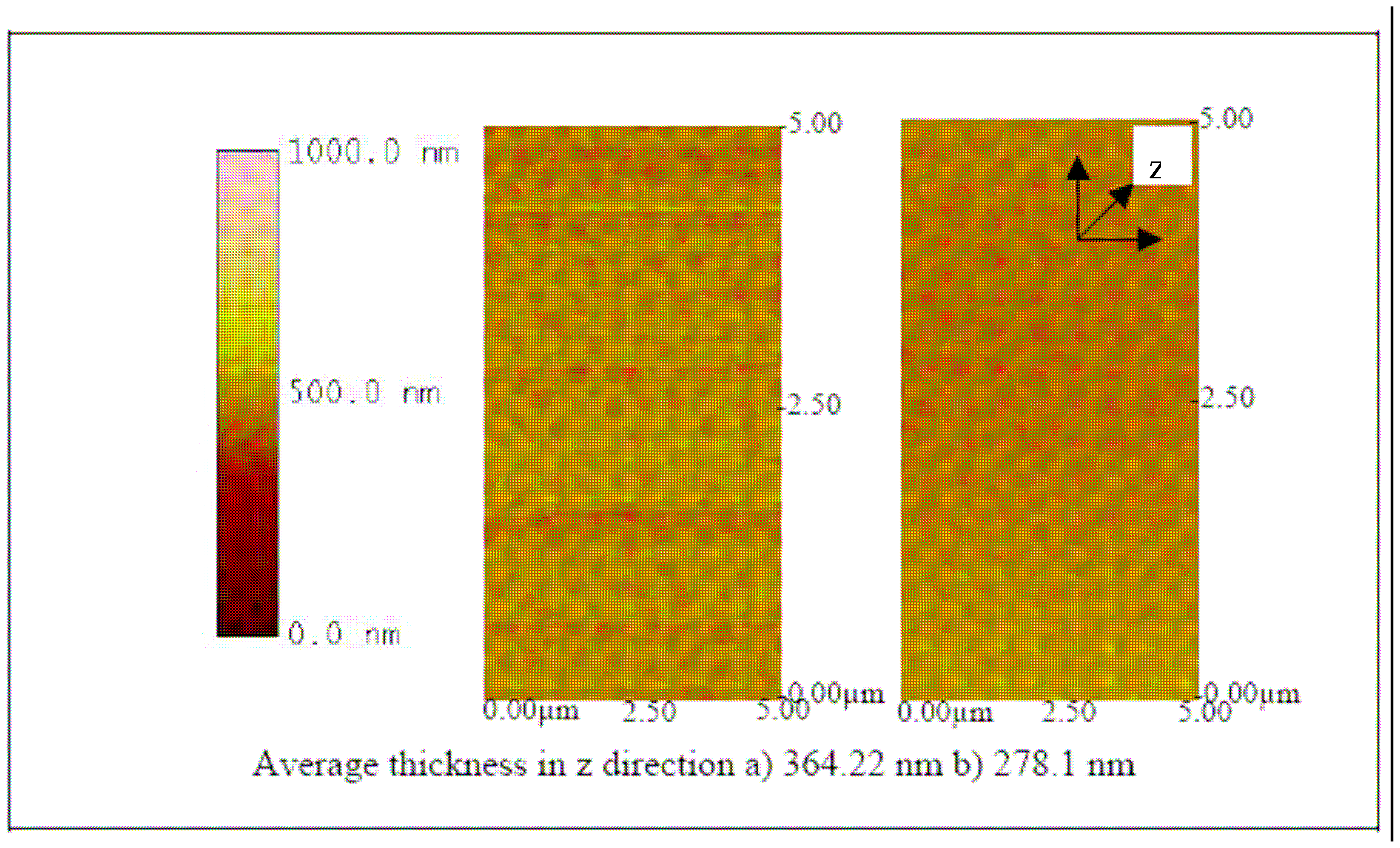
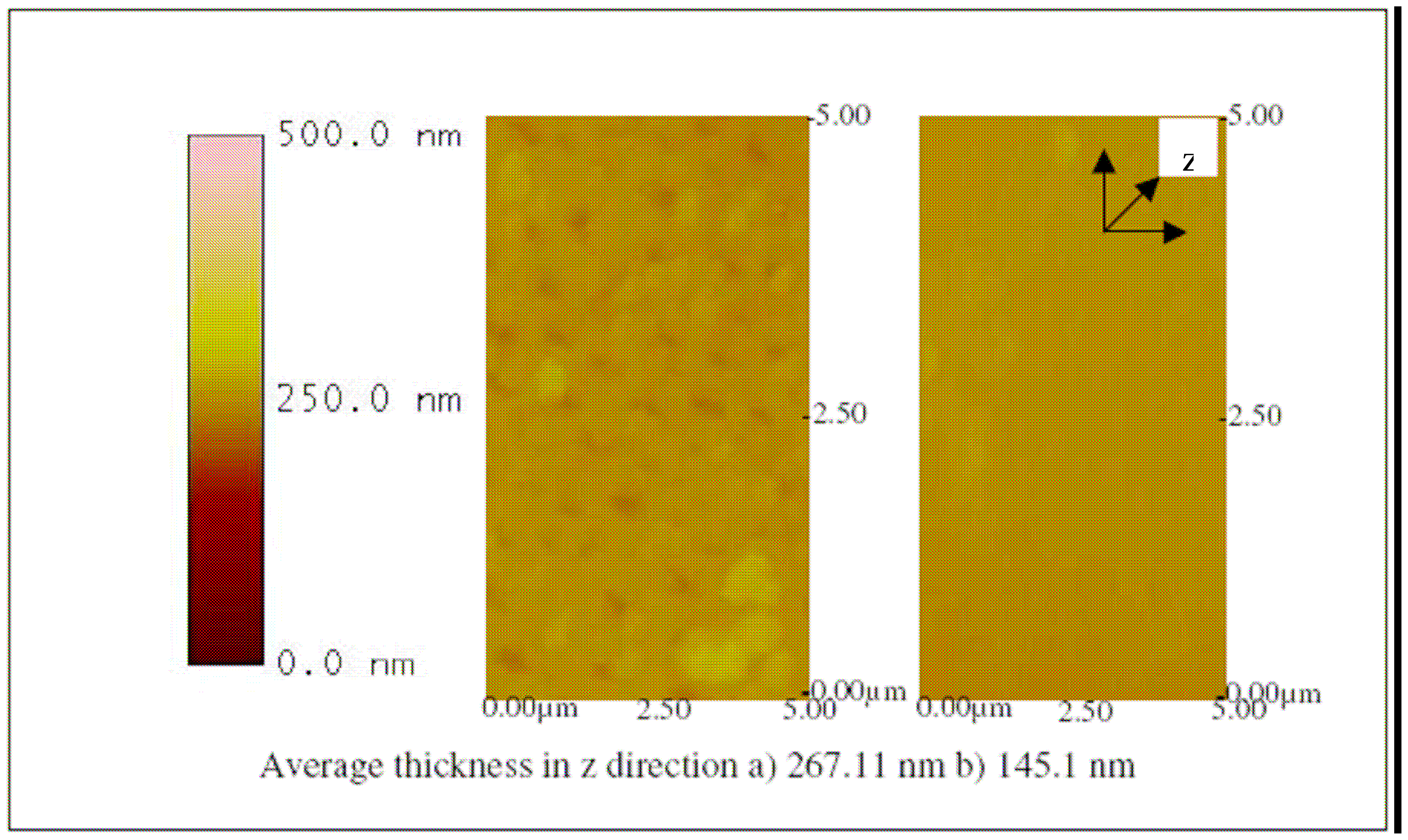
The successful fabrication of the biosensor was evaluated by using an atomic force microscope (AFM).
Figures 9,
10,
11,
12,
13 and
14 show AFM images of an ITO-Pani biosensor prepared with 20, 50, and 65 KDa Pani, functionalized with antibodies, and incubated with 10
4 and 10
6 CCID/ ml of BVDV. An increase in height in the z direction (thickness) between plain ITO (
Figure 9) and ITO-Pani substrates (
Figure 10) indicates successful polymer coating.
Figure 10 also shows that the higher molecular weight Pani formed a thicker layer on the ITO substrate. Similarly, an increase in thickness was observed in
Figure 11 where each of the ITO-Pani substrate was immobilized with antibodies, suggesting successful antibody immobilization. More importantly,
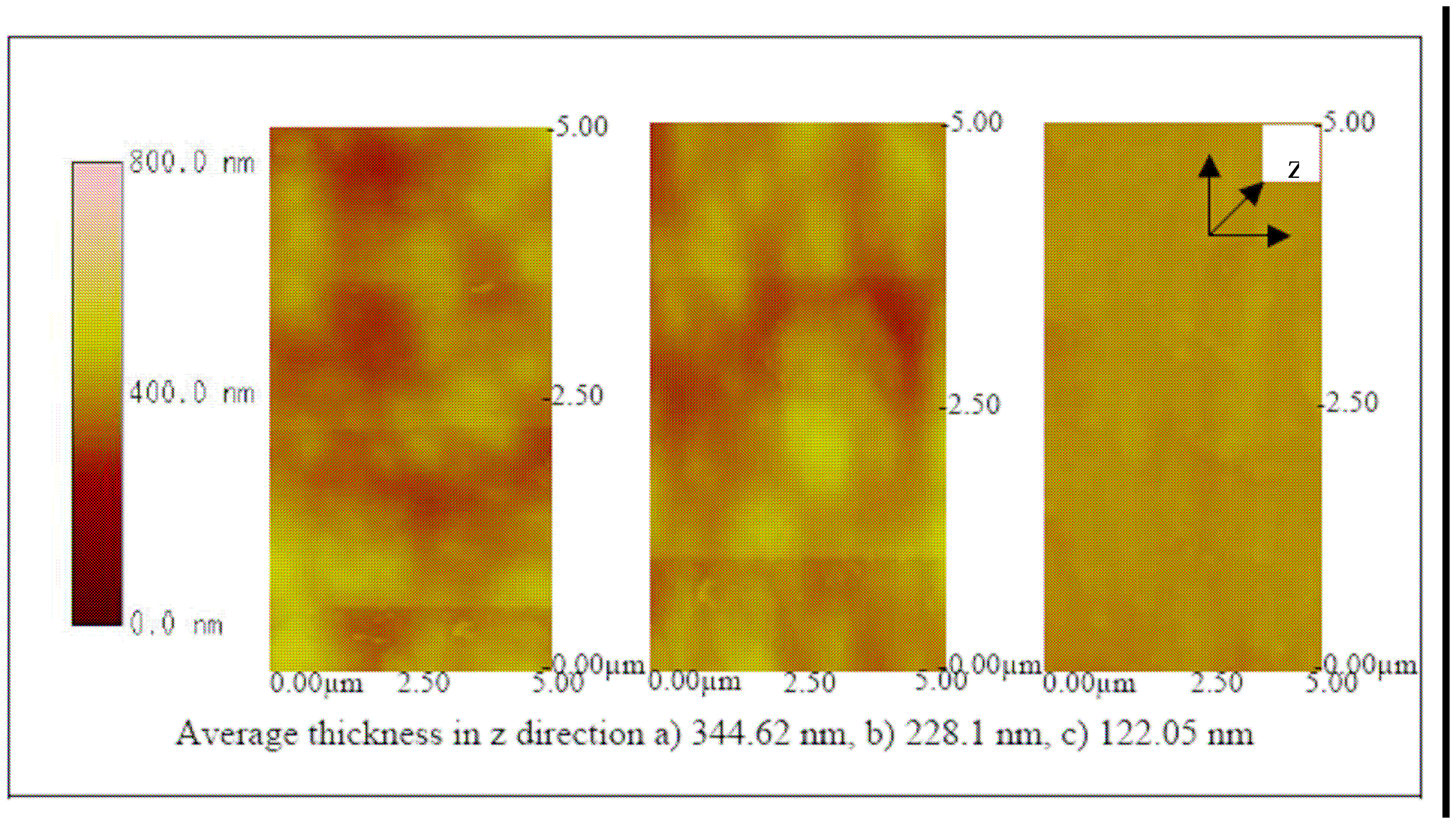
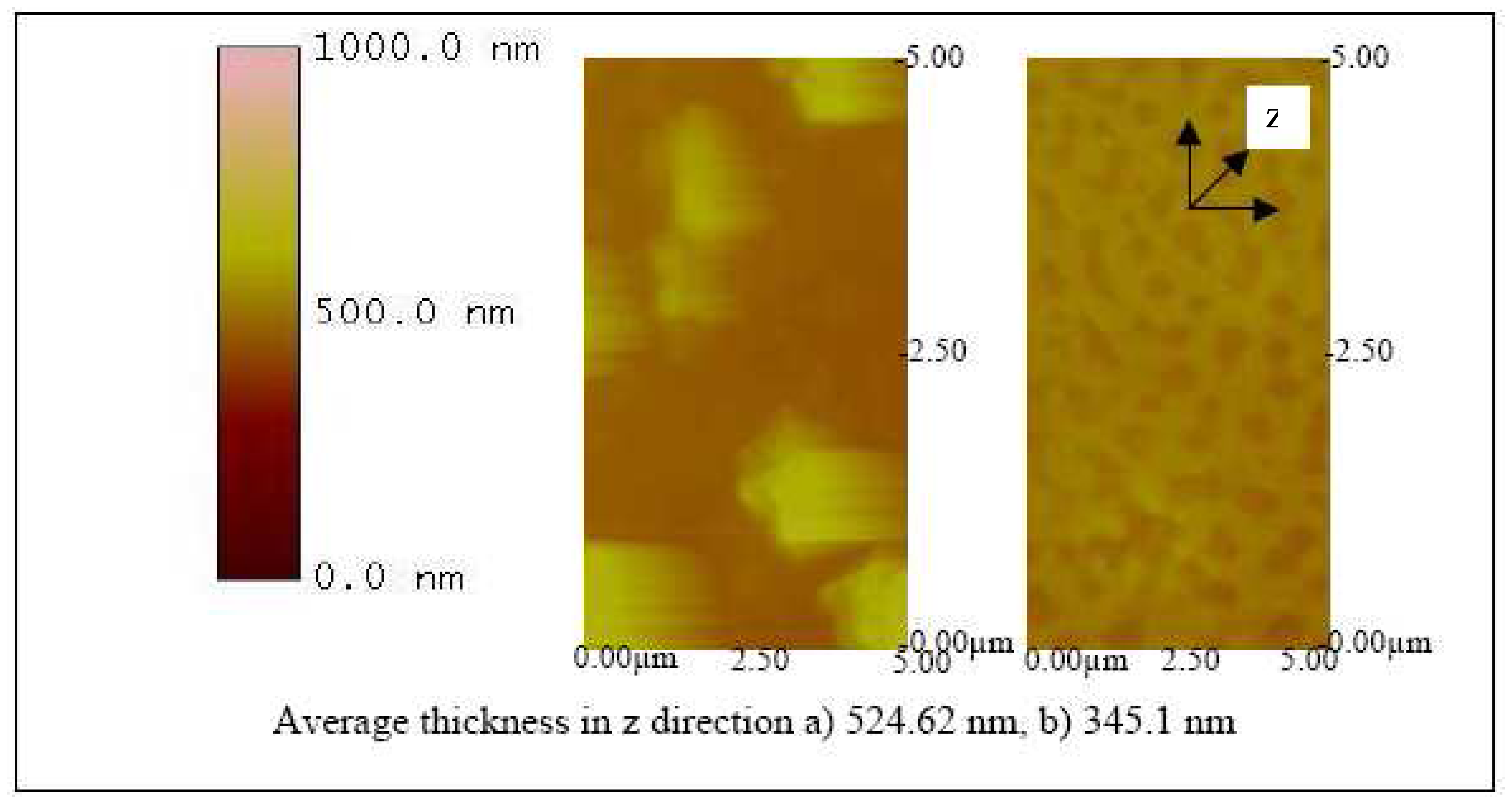
Figures 12,
13 and
14 show the AFM images of the biosensor surface after incubating them in various concentrations of BVDV cultures. A thicker substrate was observed when incubating the biosensor with the higher concentration of BVDV, supporting the logical phenomenon that the higher the antigen concentration, the more antibody-antigen complex occurs. When the biosensors were tested with the same level of BVDV concentration (e.g., 10
6 CCID/ml), biosensors coated with the higher molecular weight Pani (e.g., 65 KDa) were observed to be thicker than that with a lower molecular weight (e.g., 20 KDa) (
Figures 12,
13 and
14). This finding shows that the higher the molecular weight of Pani, the more antibody binding sites is available. This finding is further confirmed in the subsequent experiment.
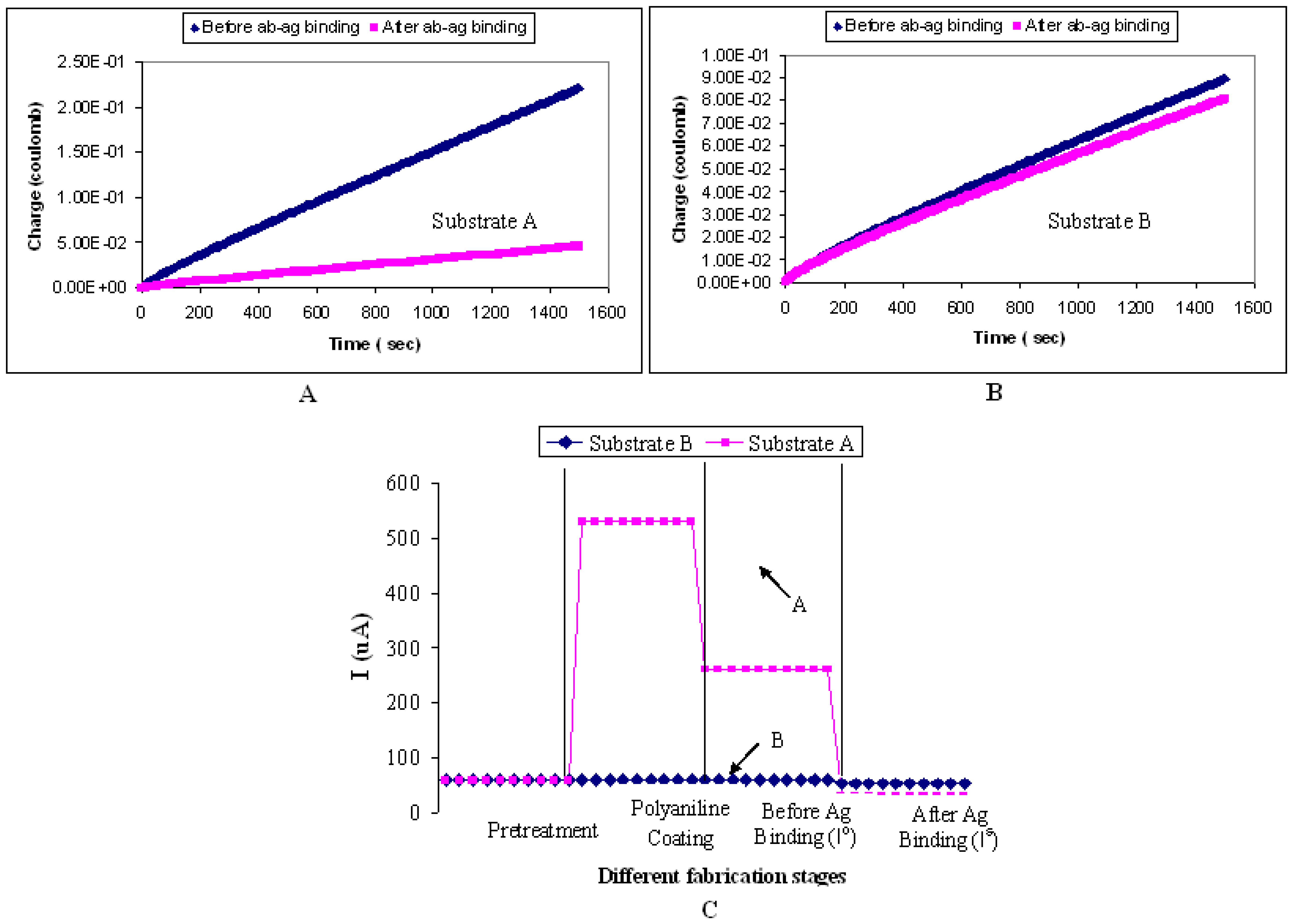
To elucidate the use of Pani as a mediator or a “glue” for the binding of antibody on the biosensor platform, ITO glasses undergoing A) the fabrication process described in sections 3.4 and 3.5, and B) the same fabrication process without the Pani spin-coating step, were tested for their amperometric responses (
Figure 15). Depending on the fabrication process, the substrate is referred to as substrate A or substrate B thereafter.
Figure 15A shows a significant difference between (I
o) and (I
s) for substrate A. The (I
o) and (I
s) responses for substrate B (
Figure 15B), on the other hand, are statistically insignificant between each other. The latter finding is proposed to be caused by the absence of Pani on the ITO substrate which contributes to the lack of antibody binding, and subsequently to the insignificant responses between (I
o) and (I
s). To investigate this theory further, the presence of antibodies on both substrates was confirmed by repeating the above experiment with flourescent-tagged antibodies. Results show that a much higher fluorescence emission level (250 out of 256-bit color mode) was observed from substrate A than that from substrate B (20 out of 256-bit color mode). This finding implies that more antibodies are present from substrate A than substrate B. Therefore, in this study, it can be concluded that Pani is not only required as the biosensor transducing system but also as a mediator for the antibody binding. Kim et al. (2000) also demonstrated the use of Pani as a mediator between the antibodies and gold electrodes in their Pani-based biosensor.
Figure 15C shows the amperometric responses of the biosensor at every fabrication stage. The amperometric responses were calculated using the method described in section 3.1.2. An increase in current response is observed after the ITO substrate is coated with Pani. Then a decrease in current response is observed after antibody immobilization, suggesting the reduction in the electron flow that could be due to the insulating property of antibodies. A much higher drop in current response is observed after the antibody-antigen binding. This result supports the proposition made earlier that the bigger the protein molecules present on the surface of the biosensor, the lower the flow of electrons and thus the smaller the signal response.
This study shows the possibility of using ITO glass, coupled with spin-coating mechanism to deposit Pani and antibodies on the biosensor surface, as a new biosensor platform. In this format, the ability of using the same biosensor for both the blank and sample testing is shown to be promising. Further study will be dedicated in analyzing the sensitivity, specificity and stability of the biosensor to detect pure culture of target organisms.