Abstract
The ionophore 1,4,7,10,13-penta(n-octyl)-1,4,7,10,13-pentaazacyclopentadecane (L1) was used for the development of miniaturised perchlorate-selective electrodes in thick-film technology. Different PVC membranes containing L1 and the plasticizers o-nitrophenyl octyl ether (NPOE), dibutyl phthalate (DBP), bis(2-ethylhexyl)sebacate (DOS) and dibutyl sebacate (DBS) were prepared and placed on a graphite working electrode manufactured by using thick film serigraphic technology. The perchlorate selective electrode containing DBS as plasticizer showed a potentiometric Nernstian response of -57 mV per decade in a range of perchlorate concentration from 1 × 10-4 to 1 × 10-1 M with a detection limit of 5 × 10-5 M. The ion selective electrodes containing DBP and NPOE as plasticizers exhibit a working range from 6.3 × 10-5 to 1 × 10-1 M and 7.4 × 10-5 to 1 × 10-1 M for perchlorate, respectively, with a detection limit of ca. 2.2 × 10-5 M. For all three electrodes a response time of ca. 5 s was found. The prepared electrodes do not show appreciable decay of the slope for at least 25 days. Potentiometric selectivity coefficients (log KpotClO4-,X-) with respect to the primary anion perchlorate were evaluated using the fixed interference method. These coefficients are of the order of 10-1.7 or smaller, indicating the relatively poor interference of the different anions studied.
1. Introduction
Ion-Selective Electrodes (ISEs) have been widely used for more than thirty years in a wide range of applications for determining the concentration of certain ions in aqueous solution [1]. Among different fields of application of ISEs, we are especially interested in the development of ion-selective electrodes for anionic species [2-7]. Anions play fundamental roles in a wide number of environmental and biological processes and due to their importance the development of anion sensors and chemosensors has grown in importance in recent years and has become an important sub-area within the field of anion chemistry [8-11]. Anions show a wide range of shapes and geometries and a lot of them are pH-sensitive, factors that have to be taken into account when designing a specific anion host. Anion receptors thus use binding forces that typically involve hydrogen bonding, electrostatic interactions and coordination to suitable metal centres [12]. In this context, perhaps the most widely used scaffoldings for anion receptors are polyamines [13]. They are partially protonated in aqueous environments and usually display electrostatic and/or hydrogen bonding interactions with anionic chemical species. Despite this, there are a relatively low number of ion-selective electrodes containing polyamines as ionophores [14-15]. These are usually of the conventional PVC-membrane type, containing an ion-selective membrane attached to the end of a tube that contains an internal liquid reference solution and a reference electrode. An alternative to this system is the use of all solid state PVC electrodes in which the membrane is directly in contact with a conducting surface connected to the milivolt measuring device. One advantage of the latter approach is the lack of an internal standard solution, which leads to the potential development of miniaturised ion-selective electrodes. Among the different technologies that can be used for the fabrication of solid state PVC electrodes we are especially interested in thick film technology [16-18]. This technology has found an important application in electrochemical sensors and biosensors, as it offers a number of advantages such as simple construction, large-scale production and low cost.
As part of our investigation of the development of ion selective sensors for anions, we report here the design and development of a miniaturised perchlorate ion selective electrode using thick-film technology incorporating a PVC membrane containing a polyazacyaloalkane as selective ionophore.
2. Experimental Section
2.1. Reagents
Reagents grade o-nitrophenyl octyl ether (NPOE), dibutyl phthalate (DBP), bis(2-ethylhexyl)sebacate (DOS) and dibutyl sebacate (DBS), tetrahydrofuran (THF) and high molecular weight poly(vinylchloride) powder (PVC) were supplied by Aldrich. Perchlorate, thiocyanate, carbonate, nitrate, bromide, chloride, iodide, acetate, oxalate, sulfate and phosphate solutions were prepared from sodium or potassium salts and were obtained from Panreac. All the aqueous solutions were prepared with de-ionised distilled water (Milli-Q water purification system). The ionophore 1,4,7,10,13-penta(n-octyl)-1,4,7,10,13-pentaazacyclopentadecane was synthesised following literature procedures [4].
2.2. Materials and electrode preparation
The perchlorate sensor consists of a thick film ion-selective electrode and a commercial reference Ag/AgCl electrode. For the working electrode the manufacturing technology was based on serigraphic technology on thick film. The screen printed process consists of forcing pastes of different characteristics over a substrate through screens using scrapers. Openings in the screen define the motif that will be printed on the substrate by serigraphy. The final thickness of the pastes can be adjusted by varying the thickness of the screens. The production of the electrodes was based on alumina substrate RUBALIT 708S (CeramTec, UK) of 2 in. × 2 in. × 0.635 mm pre-cut in 12 units of 0.5 in. × 0.66 in. In order to build the above indicated electrode, three screens were made, corresponding to three defined layers: namely, the conductive layer working as a conductor of the signal, the active layer working as active surface and the upper protection layer. A drop of the PVC membrane, containing the corresponding plasticizer and the ionophore dissolved in THF, was deposited on the surface of the active layer and allowed to dry.
The conductive paste used was Ag/Pd/Pt C4081T (supplied by HERAEUS). The layout of the tracks was made to join the board to a flat cable connector with a separation of 3 mm between terminals. The active C paste was supplied by GEM. The protective paste was the 240-SB model supplied by ESL. The thick-film electrodes were made using serigraphic methods, using polyester screens with a density of 230 mesh (Saatilane Hitech 90/48 supplied by Saatline) and films of 50 μm thickness (Ulano CDF5 supplied by Ulano). The pastes were applied by a semiautomatic serigraphic machine (AUREL model C1010). A final thickness of 30 mm after firing was achieved. The conductive paste firing process was carried out at 850 °C in a cycle of 60 min with a peak of 10 min. A similar cycle, but with heating at 150 °C in a cycle of 60 min, was used to prepare the C active surface. The firing process of the protective paste was carried out at a temperature of 150 °C in a cycle of 60 min.
2.3. Measurements
Measurements were carried out using the operational amplifier OPA129P with high input impedance (1015 Ω) in buffer configuration and an active pass-bass filter to avoid interference from the electrical network. An acquisition card ADLINK, model PCI9112 inserted into a personal computer. A program developed with VEE-Pro software was used to acquire the data. The resolution in measure acquisition was ± 0.61 mV. Potentiometric measurements were performed with a two-electrode system where the working electrode was the thick film ion-selective electrode and the reference electrode was an Ag/AgCl electrode with 3M internal KCl solution.
The detection limit was defined as the intersection of the extrapolated linear regions of the calibration graph. Potentiometric selectivity coefficients were determined according to the fixed interference method using a 0.1 M solution of the corresponding interfering ion [19-20]. Activity coefficients were calculated according to the Debye-Hückel approximation. Calibration curves were constructed by plotting the potential, E, vs the logarithm of the potassium perchlorate activity at pH 7.0. All the measurements were carried out at room temperature.
3. Results and Discussion
3.1. The emf response characteristics
The source of perchlorates in water and the soil is mainly anthropogenic as a consequence of their use in a wide range of different applications. Thus for instance, perchlorate is used as an oxidant in solid propellant for rockets and missiles, in certain fireworks, in the manufacture of matches, in nuclear reactors and electronic tubes, in tanning and finishing leather, in electroplating, etc. The toxicity of perchlorate is an active area of research [21-22], mainly focussed on the potential of perchlorate to hamper the synthesis of thyroid hormones and the potential consequences. Accordingly, the need for analytical methods for the detection of perchlorate might be of interest, especially in situations where conventional techniques are nor suitable, as is the case in many on-site analyses and rapid screening applications. In these fields, optical and electrochemical sensing devices play an important role, for instance using ion-selective electrodes.
With these ideas in mind we approached the potential development of miniaturised perchlorate electrodes using thick-film technology. Figure 1 displays the scheme and transversal view of the potentiometric electrodes shown in this paper. They consist of (1) a substrate made of isolating material, (2) a layer of conductive material over the substrate, (2a) a terminal area as a part of the conductive material, (3) a layer of active material (graphite), (4) a layer of the PVC membrane containing the ionophore and (5) an insulating layer which covers the whole electrode except for the active and terminal areas. The synthesis of the ionophore used (1,4,7,10,13-penta(n-octyl)-1,4,7,10,13-pentaazacyclopentadecane, L1, (see Scheme 1)) was carried out following previously reported procedures [4]. The ion selective membranes were prepared by mixing in 5 ml of THF, the PVC, the plasticizer and the ionophore. The mixture was used to coat the graphite working electrode and the solvent was allowed to evaporate overnight. The final electrode was finally prepared for three days by soaking it in a 0.1 M potassium perchlorate solution-HEPES at pH 7. When not in use the electrode was kept immersed in the same solution. L1 was introduced in the membrane in its unprotonated form, however the membrane made with L1 gave a clear and stable response to perchlorate, suggesting that L1 is finally protonated after the stabilisation of the membrane. This argument is based in the general idea that ISEs only function when a charge opposite to the charge of the analyte is present in the membrane.
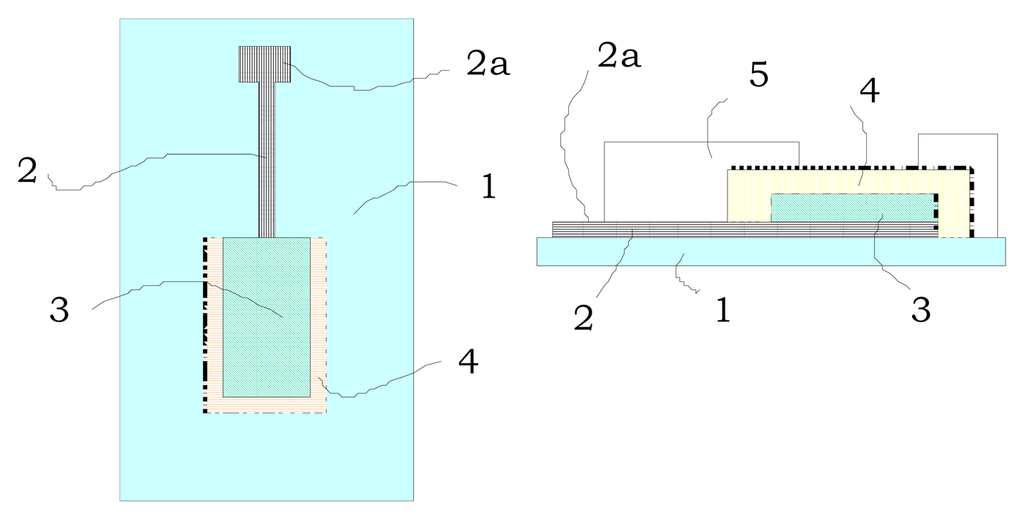
Figure 1.
Schematic representation and transversal view of the electrode.
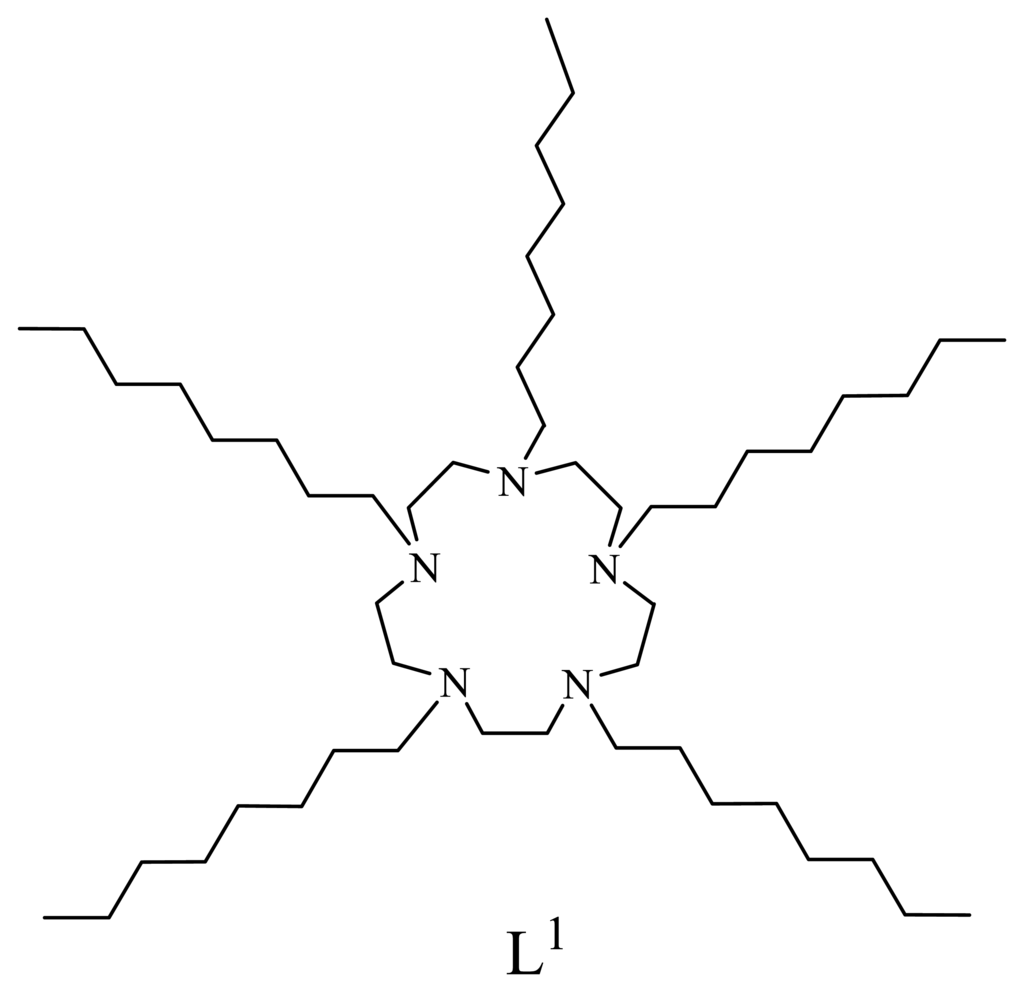
Scheme 1.
Structure of the ionophore 1,4,7,10,13-penta(n-octyl)-1,4,7,10,13-pentaazacyclopentadecane.
As is well-known the final response of ion-selective electrodes in relation to selectivity, stability, etc. depends not only on the ionophore, but also on the use of a certain plasticizer and in the proportion of the different membrane ingredients. Because of the number of these factors, in the first step several proportions of the membrane components (ionophore, plasticizer and PVC) were tested. Also, different membranes were prepared using four plasticizers; namely o-nitrophenyl octyl ether (NPOE), dibutyl phthalate (DBP), bis(2-ethylhexyl)sebacate (DOS) and dibutyl sebacate (DBS). Additionally, the membranes were prepared using different proportions of the ionophore in the 1 – 10 wt.% range. All the membranes set were tested against perchlorate and other anions. The best results in terms of stability and selectivity against perchlorate were observed when the membrane was prepared with a proportion of ca 2.8 wt.% of the ionophore and the final composition for the membranes was 2.8 wt % of ionophore, 56.0 wt % PVC and 41.2 wt % of the corresponding plasticizer. The different responses in terms of slope, detection limit and linear range of the prepared electrodes in relation to the use of different plasticizers are reported in Table 1.

Table 1.
Response characteristics of the different perchlorate-selective electrodes in thick-film technology.
The electrode containing DOS as plasticizer shows a very poor reproducibility and stability and was not studied. In contrast, the perchlorate selective electrode containing DBS as plasticizer showed a potentiometric Nernstian response of -57 mV per decade in a range of perchlorate concentration from 1 × 10-4 to 1 × 10-1 M with a detection limit of 5 × 10-5 M. The response of the electrode to perchlorate and other inorganic anions is shown in Figure 2. The ion selective electrode containing DBP and NPOE as plasticizers exhibits a working range from 6.3 × 10-5 to 1 × 10-1 M and 7.4 × 10-5 to 1 × 10-1 M for perchlorate respectively with a detection limit of ca. 2.2 × 10-5 M. For all three electrodes a response time of ca. 5 s was found as the time required for the membrane electrode to reach a potential within ± 1mV of the final equilibrium when the measurements were performed alternatively in 10-2 and 10-4 mol l-1 solutions of perchlorate. The prepared electrodes do not show appreciable decay of the slope for at least 25 days. After this period of time it was quite common to observe loss of adherence of the membrane to the alumina and graphite support.
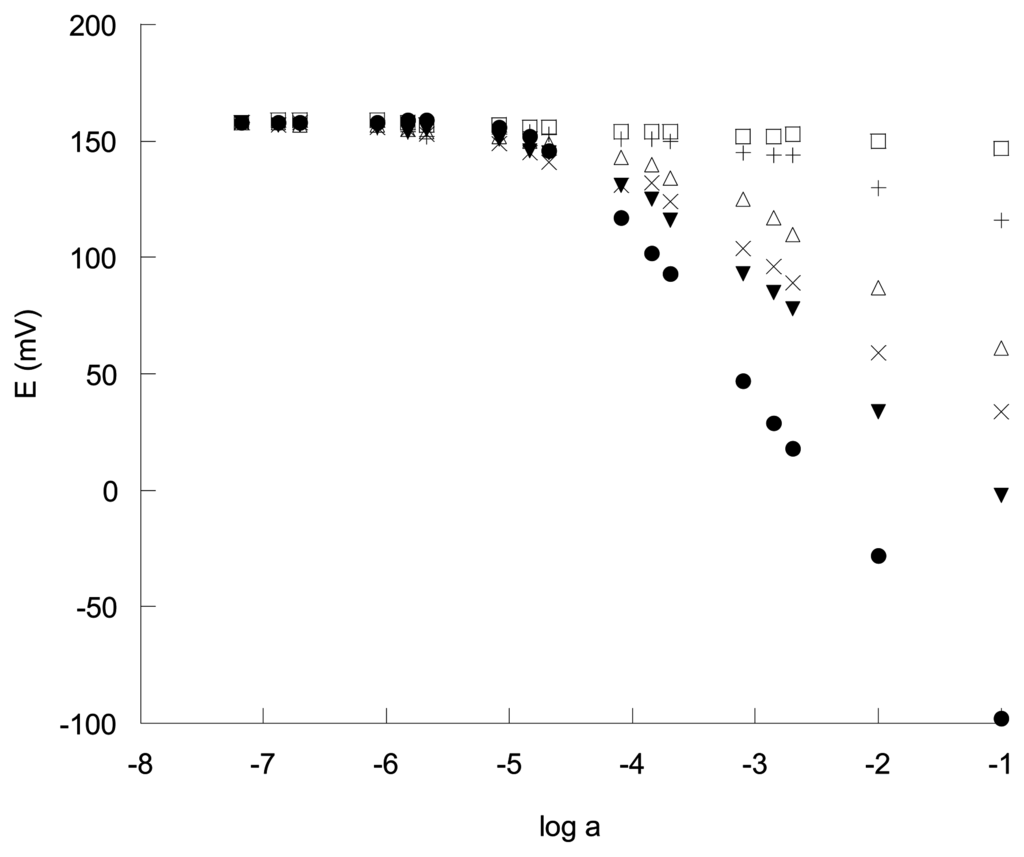
Figure 2.
Potentiometric anion response to perchlorate (●) of the electrode L1 containing DBP as plasticizer at pH 7 in the presence of certain anions, (▾) thiocyanate, (X) bromide, (Δ) nitrate, (+) chloride, (□) sulfate.
3.2. The effect of pH
The effect of pH on the response of the membranes containing L1 was tested over the pH range of ca. 5-13 at a fixed concentration of perchlorate of 1 × 10-4 M. As an example of the general observed behaviour the Figure 3 shows the results for the membrane containing L1 and DBP as plasticizer. The pH was adjusted by using HCl or KOH. As can be observed (see Figure 3), there is a slight variation of the potential as a function of pH over the pH range 5-9. In highly alkaline media the potential decreases more significantly probably due to de-protonation of the ligand or due to membrane response to OH-. A very similar behaviour was observed for the membranes containing L1 as ionophore with the plasticizers o-nitrophenyl octyl ether or dibutyl sebacate.
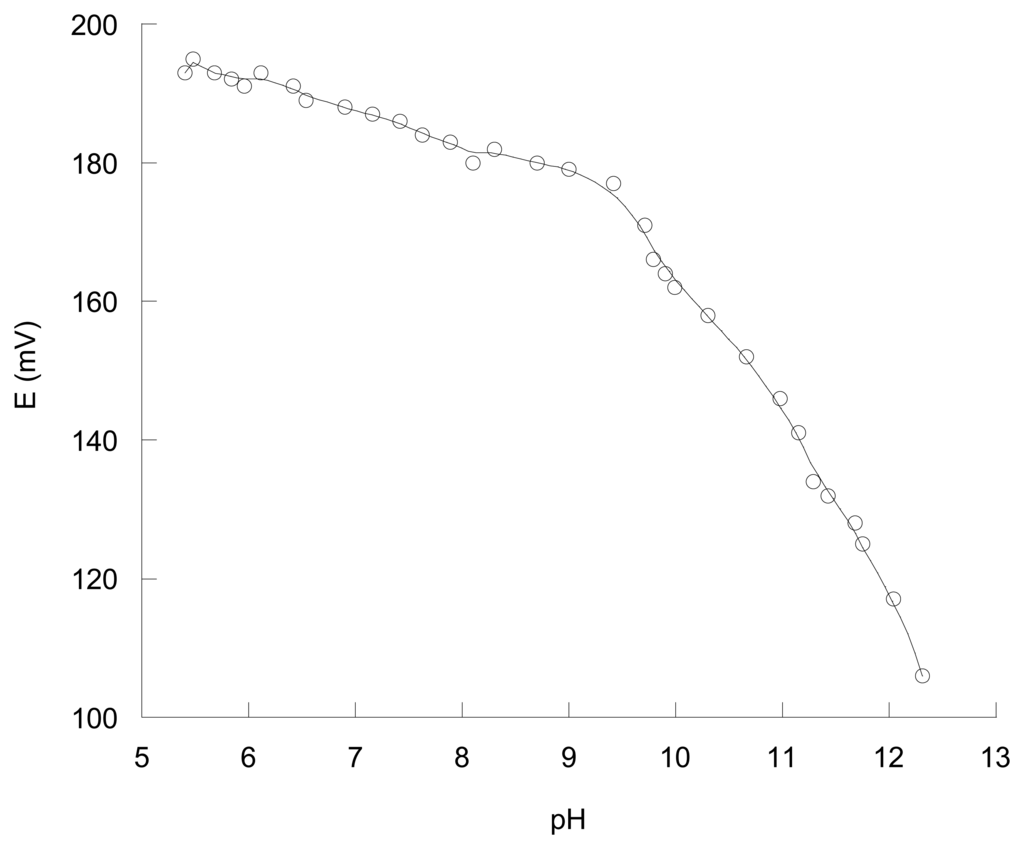
Figure 3.
The pH effect on the potential of the membrane electrode containing L1, PVC and DBP.
3.3. Selectivity
Selectivity is one of the most important characteristic of a sensor, as it helps to determine whether a reliable measurement of a target analyte is possible in a certain sample. Selectivity can be measured in terms of potentiometric selectivity coefficients (log KpotClO4-,X-) that evaluates the response of the electrode to the primary anion in the presence of a certain interfering chemical species. log KpotClO4-,X- were evaluated using the fixed interference method at a concentration of interfering anions of 1.0 × 10-2 M.
Somewhat slightly better results in relation to the selectivity coefficients were obtained for the electrode containing L1 and NPOE as plasticizer which exhibited a selectivity sequence of anion in the following order: ClO4- > SCN- > I- > NO3- > Br- > HCO3- > CH3COO- = Cl- > phosphate > SO42- > C2O42-, whereas similar selectivity coefficients values were found for the membranes L1-DBP and L1-DBS. The selectivity sequence obtained for electrode L1-DBP was ClO4- > SCN- > I- > HCO3- ≅ NO3- > Br- > CH3COO- ≅ Cl- > phosphate > C2O42- > SO42-, whereas that for L1-DBS was ClO4- > SCN- ≅ HCO3- > I- > NO3- > Br- ≅ CH3COO- > Cl- > phosphate > SO42- ≅ C2O42-. The selectivity sequences basically follows the Hofmeister Series (ClO4- > SCN- > I- > NO3- > Br- > Cl- > HCO3- > CH3COO- > SO42- > HPO42-) although some slight deviations might be interpreted on the basis of some preferential coordination of the ionophore L1 with certain anions. Table 2 and Figure 4 show the potentiometric selectivity coefficients for interfering anions relative to perchlorate for the electrodes L1-NPOE, L1-DBP and L1-DBS.

Table 2.
Potentiometric selectivity for perchlorate selective PVC membranes containing L1 as ionophore and NPOE, DBP and DBS as plasticizers.
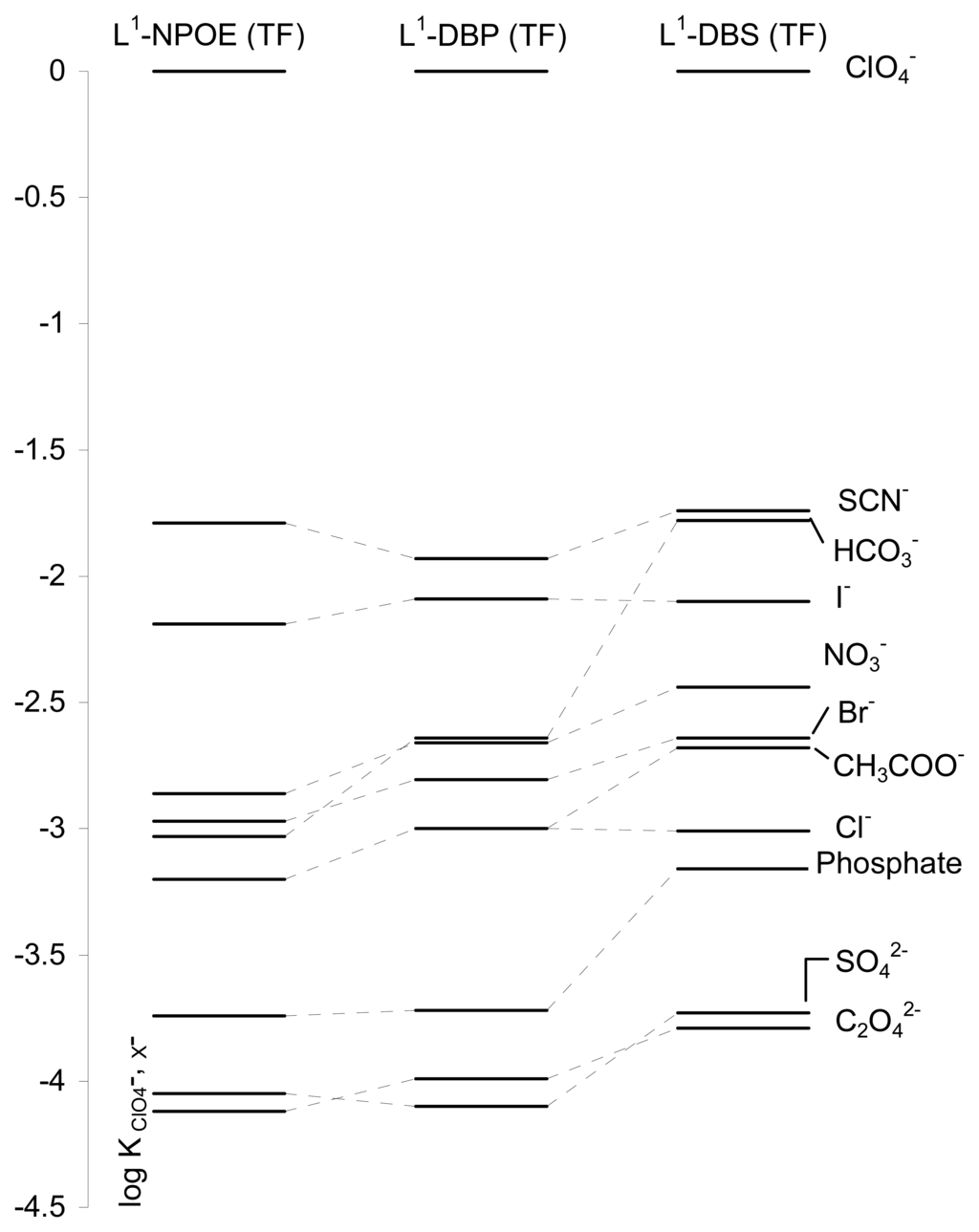
Figure 4.
Potentiometric anion selectivity coefficients of electrodes containing the ionophore L1.
Since the development of the first perchlorate selective electrode [23-24] in 1969 a number of different perchlorate electrodes have been developed using both liquid membranes [25-30] and polymeric membranes [31-35]. Table 3 shows the response characteristics of some reported perchlorate-selective electrodes. As can be seen, the linear range and detection limit for the electrode we report here is on average slightly larger than those reported for similar perchlorate electrodes. However the detection limit of the thick-film electrode is still low enough for many applications. Additionally, if we take into account the selectivity coefficients, the prepared electrodes L1-NPOE, L1-DBP and L1-DBS displayed a quite selective response to perchlorate. Thus for instance, in these electrodes, the logarithm of the selectivity coefficients is lower than -2.5 for most of the tested anions (except for thiocyanate and iodide), indicating that these anions would not significantly interfere in the determination of perchlorate. In our case, if we compare the selectivity coefficients in Table 2, the best response is found for L1 with the plasticizer NPOE (see Figure 4). These results compare well with those recently obtained by us using PVC membrane perchlorate-selective electrodes containing polyazacycloalkanes as carriers [4]. Besides the quite selective behaviour found in the use of polyamines as ionophores, in this case, we have the additional advantages of the potential for mass production and low-cost thick-film technology. In general the response found in terms of potentiometric selectivity coefficients for the three prepared electrodes, L1-NPOE, L1-DBP and L1-DBS is similar or lower than that reported when using a simple quaternary ammonium salt (tetraoctylammonium chloride) as carrier [36-38], although the response is not as selective as that reported recently using a phosphadithia macrocycle [31] and a gold(I) complex [34].

Table 3.
Characteristics of some reported perchlorate selective electrodes.
Conclusions
In this work miniaturised PVC-membrane perchlorate-selective electrodes in thick-film technology were designed and developed. A selective response to perchlorate was found, with a fairly good detection limit, liner range and response time, Nernstian behaviour and relatively good potentiometric selectivity coefficients. We are currently developing new miniaturised electrodes for target anions using mass production and low-cost thick-film technology.
Acknowledgments
We would like to thank the Ministerio de Ciencia y Tecnología (projects MAT2003-08568-C03). F.S. thanks the Ministerio de Educación y Ciencia for a Ramón y Cajal contract. Mª J.S. thanks the Universidad Politécnica de Valencia for a Doctoral Fellowship. We would like to thank the R+D+i Linguistic Assistance Office at the Universidad Politécnica de Valencia for their help in revising this paper.
References and Notes
- Antonisse, M.M.G.; Reinhoudt, D.N. Potentiometric anion selective sensors. Electroanalysis 1999, 11, 1035–1048. [Google Scholar]
- Seguí, M.-J.; Lizondo-Sabater, J.; Martínez-Máñez, R.; Sancenón, F.; Soto, J. A perchlorate-seelctive membrane electrode based on a Cu(II) complex of the ligand 1,4,8,11-tetra(n-octyl)-1,4,8,11-tetraazacyclotetradecane. Analyst 2002, 127, 387–390. [Google Scholar]
- Lizondo-Sabater, J.; Martínez-Máñez, R.; Sancenón, F.; Seguí, M.-J.; Soto, J. Cobalt(II) and nickel(II) complexes of a cyclam derivative as carriers in iodide-selective electrodes. Anal. Chim. Acta 2002, 459, 229–234. [Google Scholar]
- Lizondo-Sabater, J.; Seguí, M.-J.; Lloris, J.M.; Martínez-Máñez, R.; Pardo, T.; Sancenón, F.; Soto, J. New membrane perchlorate-selective electrodes containing polyazacycloalkanes as carriers. Sens. Actuators B 2004, 101, 20–27. [Google Scholar]
- Seguí, M.-J.; Lizondo-Sabater, J.; Martínez-Máñez, R.; Pardo, T.; Sancenón, F.; Soto, J. Ion-selective electrodes for anonic surfactants using a new aza-oxa-cycloalkane as active ionophore. Anal. Chim. Acta 2004, 525, 83–90. [Google Scholar]
- Coll, C.; Labrador, R.H.; Martínez-Máñez, R.; Soto, J.; Sancenón, F.; Seguí, M.-J.; Sánchez, E. Ionic liquids promote selective responses towards the highly hydrophilic anion sulfate in PVC membrane ion-selective electrodes. Chem. Commun 2005, 3033–3035. [Google Scholar]
- Seguí, M.J.; Lizondo-Sabater, J.; Martínez-Máñez, R.; Sancenón, F.; Soto, J. Linear polyamines as carriers in thiocyanate-selective membrane electrodes. Talanta 2006, 68, 1182–1189. [Google Scholar]
- Martínez-Máñez, R.; Sancenón, F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar]
- Gupta, V.K.; Ludwig, R.; Agarwal, S. Anion recognition through modified calixarenes; a highly selective sensor for HPO42-. Anal. Chim. Acta 2005, 538, 213. [Google Scholar]
- Jain, A.K.; Gupta, V.K.; Singh, L.P.; Srivastava, P.; Raisoni, J.R. Anion recognition through novel C-thiophenecalix[4]resorcinarene: PVC based sensor for chromate ions. Talanta 2005, 65, 716–721. [Google Scholar]
- Gupta, V.K.; Agarwal, S. PVC based 5,10,15,20-tetrakis (4-methoxyphenyl) porphyrinatocobalt(II) membrane potentiometric sensor for arsenite. Talanta 2005, 65, 730–734. [Google Scholar]
- Beer, P.D.; Gale, P.A. Anion recognition and sensing: the state of the art and future perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar]
- Descalzo, A.B.; Marcos, M.D.; Martínez-Máñez, R.; Soto, J.; Beltrán, D.; Amorós, P. Anthrylmethylamine functionalised mesoporous silica-based materials as hyrbid fluorescent chemosensors for ATP. J. Mater. Chem. 2005, 15, 2721–2731. [Google Scholar]
- Carey, C.M.; Riggan, W.B., Jr. Cyclic polyamine ionophore for use in a dibasic phosphate-selective electrode. Anal. Chem. 1994, 66, 3587–3591. [Google Scholar]
- Hartley, A.M.; House, W.A.; Callow, M.E.; Leadbeater, B.S.C. Application of a cyclic polyamine ionophore in phosphate selective electrodes for environmental analysis. Int. J. Environ. Anal. Chem. 2000, 76, 199–214. [Google Scholar]
- Martínez-Máñez, R.; Soto, J.; Lizondo-Sabater, J.; García-Breijo, E.; Gil, L.; Ibáñez, J.; Alcaina, I.; Alvarez, S. New potentiometric dissolved oxygen sensors in thick film technology. Sens. Actuators B 2004, 101, 295–301. [Google Scholar]
- Martínez-Máñez, R.; Soto, J.; García-Breijo, E.; Gil, L.; Ibáñez, J.; Llobet, E. An “electronic tongue” design for the qualitative analysis of natural waters. Sens. Actuators B 2005, 104, 302–307. [Google Scholar]
- Martínez-Máñez, R.; Soto, J.; García-Breijo, E.; Gil, L.; Ibáñez, J.; Gadea, E. A multisensor in thick-film technology for water quality control. Sens. Actuators A 2005, 120, 589–595. [Google Scholar]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar]
- IUPAC, Selectivity coefficients for ion-selective electrodes: recommended methods for reporting KA,BPOT values. Pure Appl. Chem. 1995, 67, 507–518.
- McNabb, F.M.A.; Jang, D.A.; Larsen, C.T. Does thyroid function in developing birds adapt to sustained ammonium perchlorate exposure? Toxicol Sci. 2004, 82, 106–113. [Google Scholar]
- Miranda, L.A; Pisano, A.; Casco, V. Ultratructural study on thyroid glands of Bufo arenarum larvae kept in potassium perchlorate solution. Biocell. 1996, 20, 147–153. [Google Scholar]
- Ross, J.W. Ion Selective Electrodes; Durst, R.A., Ed.; US Government Printing Office: Washington, DC, 1969; p. p. 57. [Google Scholar]
- Orion Research. Br. Pat. 1197264, 1968.
- Rohm, T.J.; Guilbault, G.G. New methods for the preparation of perchlorate ion-selective electrodes. Anal. Chem. 1974, 46, 590–592. [Google Scholar]
- Wilson, A.C.; Pool, K.H. An improved ion-selective electrode for perchlorate. Talanta 1976, 23, 387–388. [Google Scholar]
- Hassan, S.S.M.; Elsaied, M.M. A new liquid membrane electrode for selective determination of perchlorate. Talanta 1986, 33, 679–684. [Google Scholar]
- Jain, A.K.; Jahan, M.; Tyagi, V. Construction and assessment of some perchlorate-selective liquid membrana electrodes. Anal. Chim. Acta 1990, 231, 69–75. [Google Scholar]
- Coetzee, C.J.; Freiser, H. Anion responsive electrodes based on ion-association extraction systems. Anal. Chem. 1968, 40, 2071–2071. [Google Scholar]
- Coetzee, C.J.; Freiser, H. Liquid-liquid membrane electrodes based on ion association extraction systems. Anal. Chem. 1969, 41, 1128–1130. [Google Scholar]
- Casabó, J.; Escriche, L.; Pérez-Jiménez, C.; Muñoz, J.A.; Teixidor, F.; Bausells, J.; Errachid, A. Application of a new phosphadithiamacrocycle to ClO4- selective CHEMFET and ion-selective devices. Anal. Chim. Acta 1996, 320, 63–68. [Google Scholar]
- Errachid, A.; Pérez-Jiménez, C.; Casabó, J.; Escriche, L.; Muñoz, J.A.; Bratov, A.; Bausells, J. Perchlorate-selective MEMFETs and ISEs based on a new phosphadithiamacrocycle. Sens. Actuators B 1997, 43, 206–210. [Google Scholar]
- Siswanta, D.; Takenaka, J.; Suzuki, T.; Sasakura, H.; Hisamoto, H.; Suzuki, K. Noovel neutral anion ionophores based on fluorinated polyether compounds as a sensory molecule for ion-selective electrodes. Chem. Lett. 1997, 195–196. [Google Scholar]
- Sánchez-Pedreño, C.; Ortuño, J.A.; Hernández, J. Perchlorate-selective polymeric membrana electrode base don a gold(I) complex: application to water and urine análisis. Anal. Chim. Acta 2000, 415, 159–164. [Google Scholar]
- Shamsipur, M.; Soleymanpour, A.; Akhond, M.; Sharghi, H.; Hasaninejad, A.R. Perchlorate selective membrane electrodes based on a phosphorus(V)-tetraphenylporphyrin complex. Sens. Actuators B 2003, 89, 9–14. [Google Scholar]
- Pérez-Olmos, R.; Rios, A.; Martín, M.P.; Lapa, R.A.S.; Lima, J.L.F.C. Construction and evaluation of ion selective electrodes for perchlorate with a summing operational amplifier: application to pyrotechnic mixtures analysis. Analyst 1999, 124, 97–100. [Google Scholar]
- Fogg, A.G.; Pathan, A.S.; Burns, D.T. A liquid-state perchlorate ion-selective electrode based on brilliant green perchlorate. Anal. Chim. Acta 1974, 73, 220–223. [Google Scholar]
- Kataoka, M.; Kambara, T. A liquid membrane type perchlorate ion-selective electrode. J. Electroanal. Chem. 1976, 73, 279–284. [Google Scholar]
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.