Magneto-controlled Quantized Electron Transfer to Surface-confined Redox Units and Metal Nanoparticles
Abstract
:Introduction
Experimental section
Chemicals and materials
Chemical modification of electrodes
Electrochemical and microgravimetric measurements
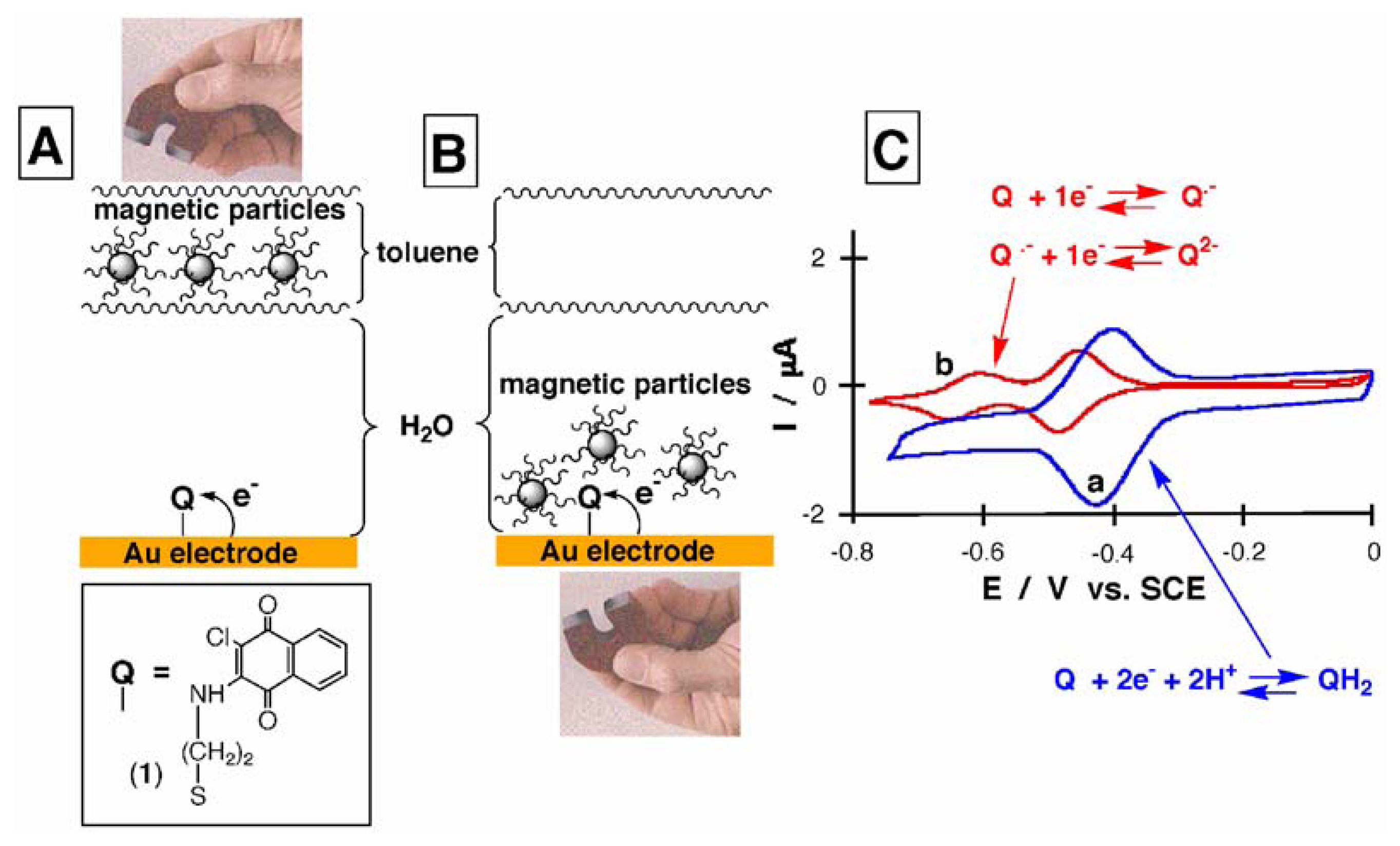
Results and discussion
Conclusions
References
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle arrays on surfaces for electronic, optical and sensoric applications. ChemPhysChem. 2000, 1, 18. [Google Scholar]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties and applications. Angew. Chem. Int. Ed. 2004, 43, 6042. [Google Scholar]
- Zhao, X.J.; Tapec-Dytioco, R.; Wang, K.M.; Tan, W.H. Collection of trace amounts of DNA/mRNA molecules using genomagnetic nanocapturers. Anal. Chem. 2003, 75, 3476. [Google Scholar]
- Wilson, R. Haptenylated mercaptodextran-coated gold nanoparticles for biomolecular assays. Chem. Commun. 2003, 108. [Google Scholar]
- Lee, H.; Purdon, A.M.; Chu, V.; Westervelt, R.M. Controlled assembly of magnetic nanoparticles from magnetotactic bacteria using microelectromagnets arrays. Nano Lett. 2004, 4, 995. [Google Scholar]
- Wang, J.; Kawde, A.-N. Magnetic-field stimulated DNA oxidation. Electrochem. Commun. 2002, 4, 349. [Google Scholar]
- Wang, J.; Xu, D.K.; Polsky, R. Magnetically-induced solid-state electrochemical detection of DNA hybridization. J. Am. Chem. Soc. 2002, 124, 4208. [Google Scholar]
- Li, J.S.; He, X.X.; Wu, Z.Y.; Wang, K.M.; Shen, G.L.; Yu, R.Q. Piezoelectric immunosensor based on magnetic nanoparticles with simple immobilization procedures. Anal. Chim. Acta. 2003, 481, 191. [Google Scholar]
- Kourilov, V.; Steinitz, M. Magnetic-bead enzyme-linked immunosorbent assay verifies adsorption of ligand and epitope accessibility. Anal. Biochem. 2002, 311, 166. [Google Scholar]
- Katz, E.; Sheeney-Haj-Ichia, L.; Willner, I. Magneto-switchable electrocatalytic and bioelectrocatalytic transformations. Chem. Eur. J. 2002, 8, 4138. [Google Scholar]
- Willner, I.; Katz, E. Magnetic control of electrocatalytic and bioelectrocatalytic processes. Angew. Chem. Int. Ed. 2003, 42, 4576. [Google Scholar]
- Katz, E.; Willner, I. Magneto-stimulated hydrodynamic control of electrocatalytic and bioelectrocatalytic processes. J. Am. Chem. Soc. 2002, 124, 10290. [Google Scholar]
- Katz, E.; Willner, I. Enhancement of bioelectrocatalytic processes by the rotation of mediator-functionalized magnetic particles on electrode surfaces: Comparison with a rotating disk electrode. Electroanalysis 2005, 17, 1616. [Google Scholar]
- Patolsky, F.; Weizmann, Y.; Katz, E.; Willner, I. Magnetically amplified DNA assays (MADA): Sensing of viral DNA and single base mismatches using nucleic acid-modified magnetic particles. Angew. Chem. Int. Ed. 2003, 42, 2372. [Google Scholar]
- Weizmann, Y.; Patolsky, F.; Katz, E.; Willner, I. Amplified DNA sensing and immunosensing by the rotation of functional magnetic particles. J. Am. Chem. Soc. 2003, 125, 3452. [Google Scholar]
- Patolsky, F.; Weizmann, Y.; Katz, E.; Willner, I. Amplified telomerase analysis by using rotating magnetic particles: The rapid and sensitive detection of cancer cells. ChemBioChem 2004, 5, 943. [Google Scholar]
- Katz, E.; Sheeney-Haj-Ichia, L.; Basnar, B.; Felner, I.; Willner, I. Magnetoswitchable controlled hydrophilicity/hydrophobicity of electrode surfaces using alkyl-chain-functionalized magnetic particles: Application for switchable electrochemistry. Langmuir 2004, 20, 9714. [Google Scholar]
- Katz, E.; Baron, R.; Willner, I. Magnetoswitchable electrochemistry gated by alkyl-chain-functionalized magnetic nanoparticles: Controlling of diffusional and surface-confined electrochemical process. J. Am. Chem. Soc. 2005, 127, 4060. [Google Scholar]
- Shen, L.F.; Laibinis, P.E.; Hatton, T.A. Bilayer surfactant stabilized magnetic fluids: Synthesis and interactions at interfaces. Langmuir 1999, 15, 447. [Google Scholar]
- Katz, E.; Schlereth, D.D.; Schmidt, H.-L. Electrochemical study of pyrroloquinoline quinone covalently immobilized as monolayer onto a cystamine modified gold electrode. J. Electroanal. Chem. 1994, 367, 59. [Google Scholar]
- Katz, E.; Solov'ev, A.A. Chemical modification of platinum and gold electrodes by naphthoquinones using amines containing sulfhydryl or disulphide groups. J. Electroanal. Chem. 1990, 291, 171. [Google Scholar]
- Berkovsky, B.M.; Medvedev, V.F.; Karkov, M.S. Magnetic Fluids: Engineering Applications; Oxford University Press: New York, 1993. [Google Scholar]
- Rosensweig, R.R. Ferrohydrodynamics; Cambridge University Press: Cambridge, England, 1985. [Google Scholar]
- Chambers, J.Q. The chemistry of the quinonoid compounds; Patai, S., Ed.; Interscience: New York, 1974; p. 739. [Google Scholar]
- Chen, S.W.; Ingram, R.S.; Hostetler, M.J.; Pietron, J.J.; Murray, R.W.; Schaaff, T.G.; Khoury, J.T.; Alvarez, M.M.; Whetten, R.L. Gold nanoelectrodes of varied size: Transition to molecule-like charging. Science 1998, 280, 2098. [Google Scholar]
- Chen, S.W.; Murray, R.W.; Feldberg, S.W. Quantized capacitance charging of monolayer-protected Au clusters. J. Phys. Chem B 1998, 102, 9898. [Google Scholar]
- Chen, S. Nanoparticle assemblies: “Rectified” quantized charging in aqueous media. J. Am. Chem. Soc. 2000, 122, 7420. [Google Scholar]
- Hicks, J.F.; Templeton, A.C.; Chen, S.W.; Sheran, K.M.; Jasti, R.; Murray, R.W.; Debord, J.; Schaaf, T.G.; Whetten, R.L. The monolayer thickness dependence of quantized double-layer capacitances of monolayer-protected gold clusters. Anal. Chem. 1999, 71, 3703. [Google Scholar]
- Andres, R.P.; Bein, T.; Dorogi, M.; Feng, S.; Henderson, J.I.; Kubiak, C.P.; Mahoney, W.; Osifchin, R.G.; Reifenberger, R. “Coulomb staircase” at room temperature in a self-assembled molecular nanostructures. Science 1996, 272, 1323. [Google Scholar]
- Markovich, G.; Leff, D.V.; Chung, S.-W.; Soyez, H.M.; Dunn, B.; Heath, J.R. Parallel fabrication and single-electron charging of devices based on ordered, two-dimensional phases of organically functionalized metal nanocrystals. Appl. Phys. Lett. 1997, 70, 3107. [Google Scholar]
- Green, S.J.; Stokes, J.J.; Hostetler, M.J.; Pietron, J.; Murray, R.W. Three-dimensional monolayers: Nanometer-sized electrodes of alkanethiolate-stabilized gold cluster molecules. J. Phys. Chem. B 1997, 101, 2663. [Google Scholar]

© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for non-commercial purposes.
Share and Cite
Katz, E.; Willner, I. Magneto-controlled Quantized Electron Transfer to Surface-confined Redox Units and Metal Nanoparticles. Sensors 2006, 6, 420-427. https://doi.org/10.3390/s6040420
Katz E, Willner I. Magneto-controlled Quantized Electron Transfer to Surface-confined Redox Units and Metal Nanoparticles. Sensors. 2006; 6(4):420-427. https://doi.org/10.3390/s6040420
Chicago/Turabian StyleKatz, Eugenii, and Itamar Willner. 2006. "Magneto-controlled Quantized Electron Transfer to Surface-confined Redox Units and Metal Nanoparticles" Sensors 6, no. 4: 420-427. https://doi.org/10.3390/s6040420



