Study of Swift Heavy Ion Modified Conducting Polymer Composites for Application as Gas Sensor
Abstract
:Introduction
Experimental
Materials
Polymerization of aniline in presence of PVC powder
Preparation of PANI-PVC films
Irradiation
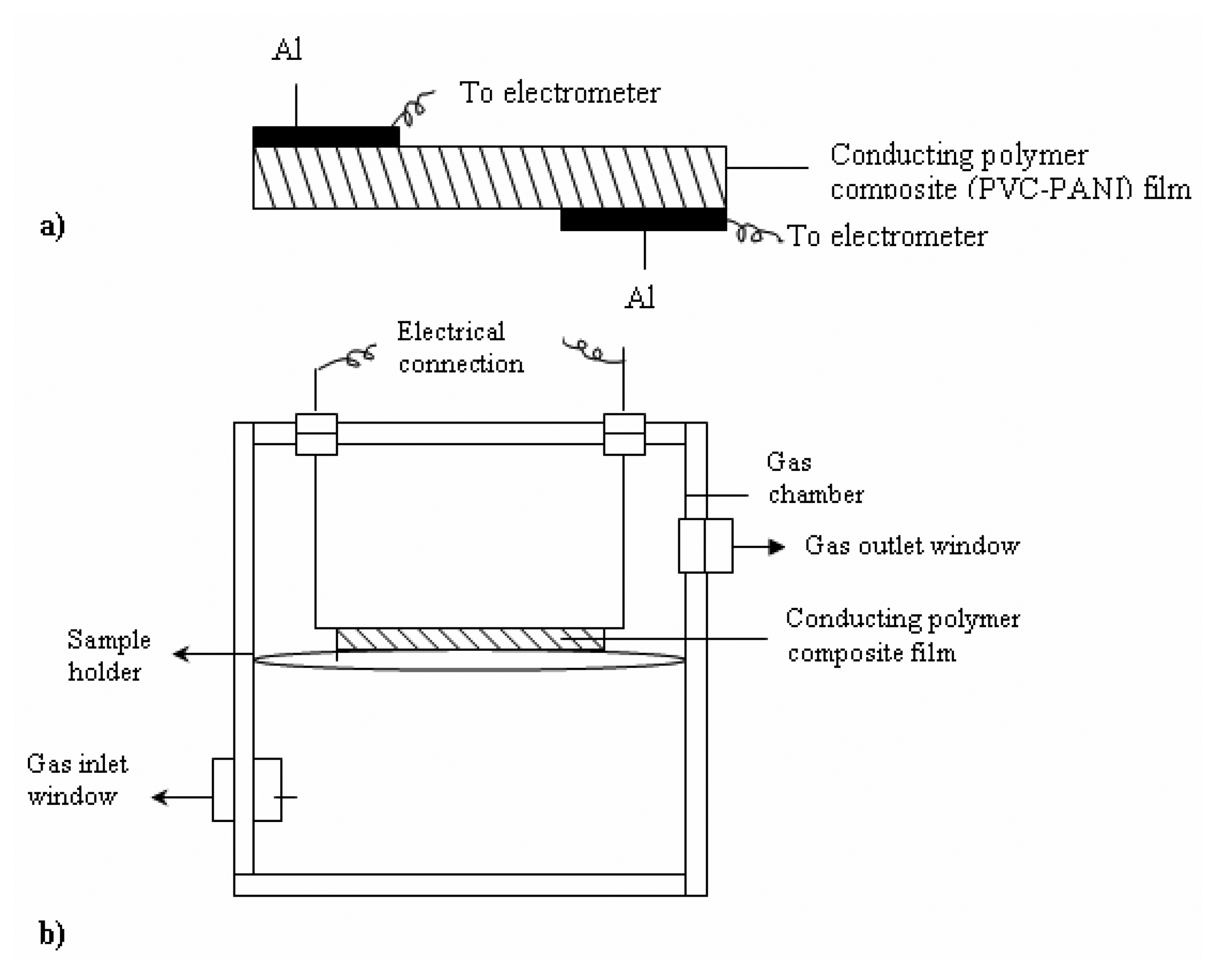
Electrode preparation and gas sensing
Results and discussion
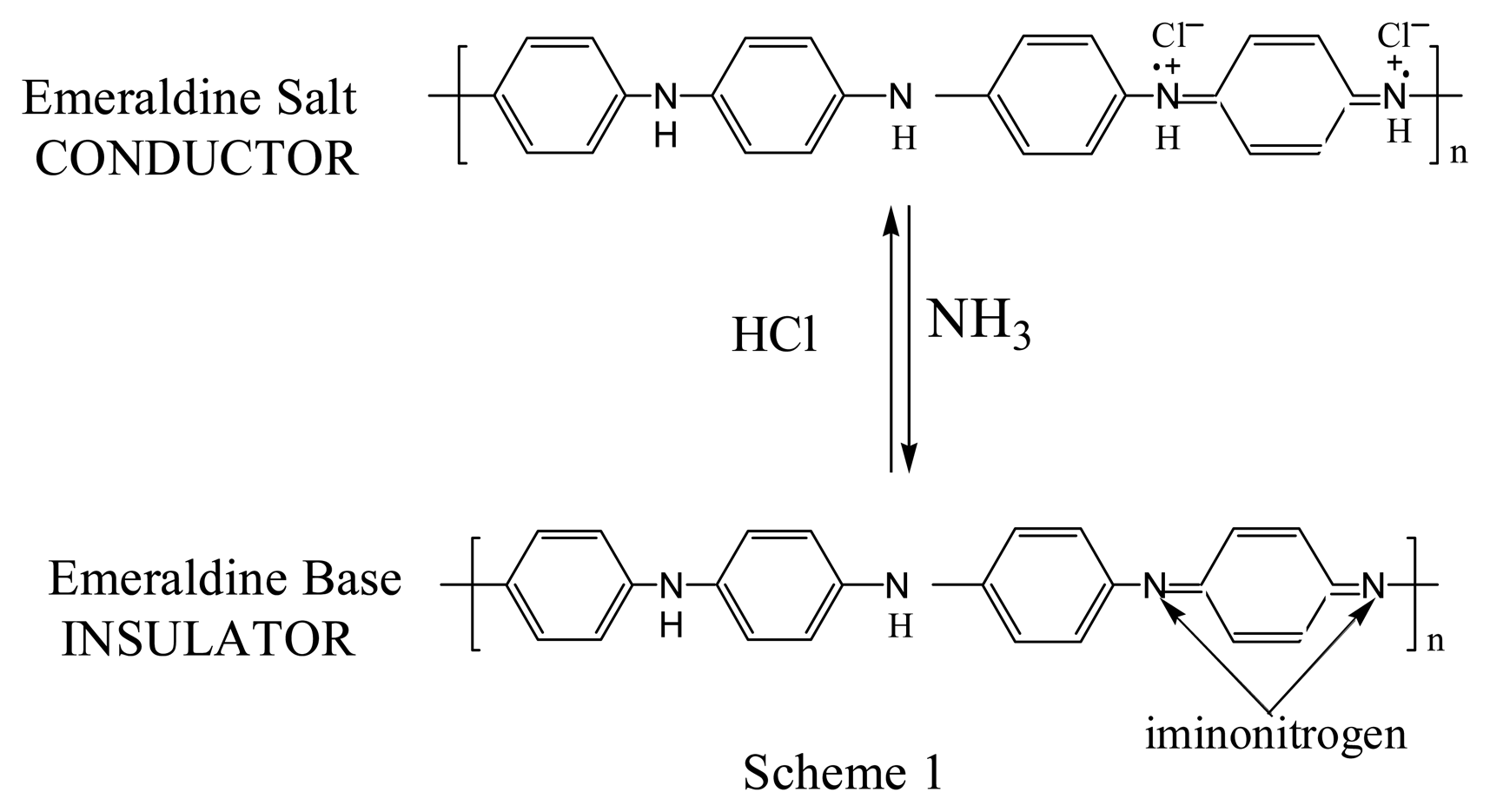
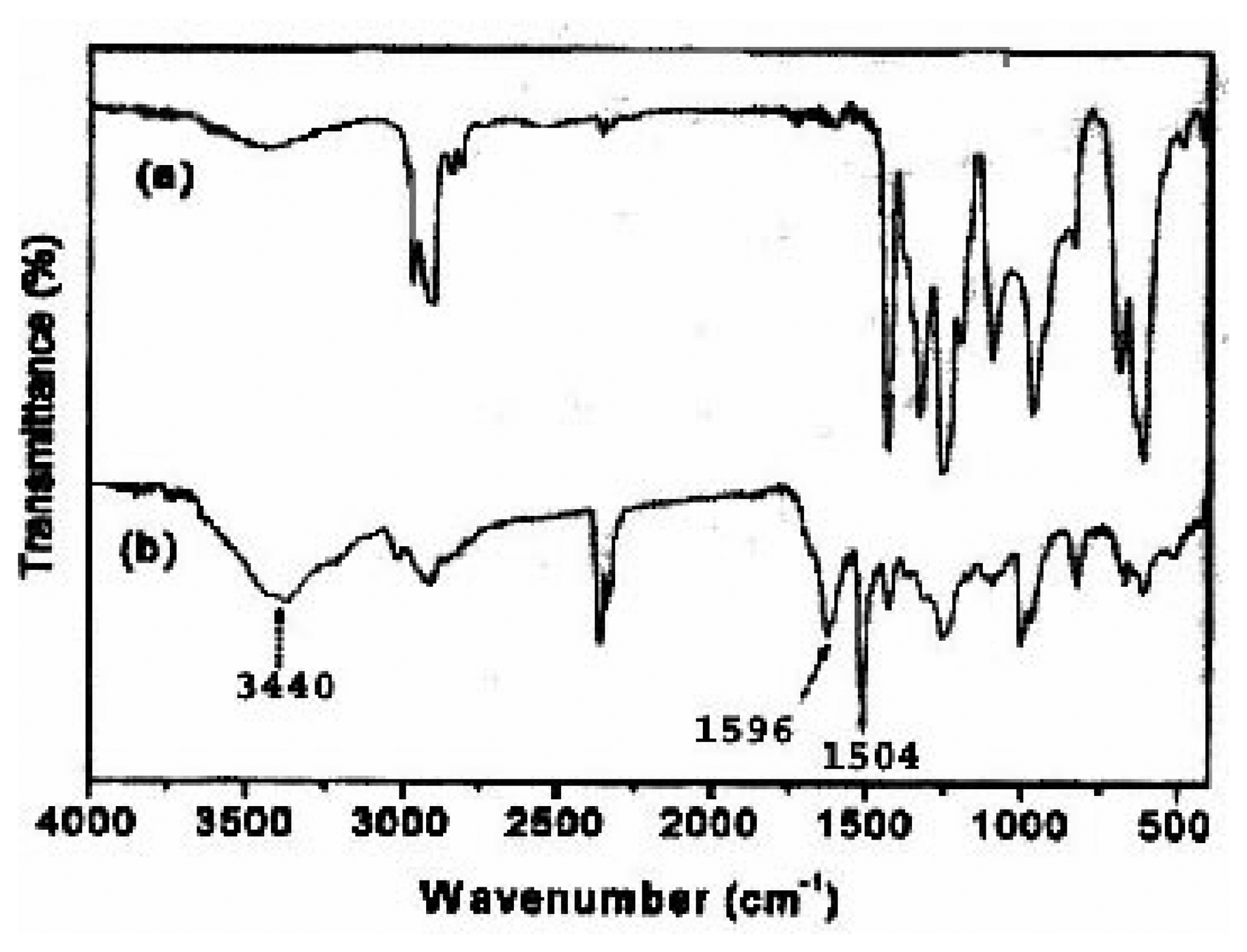
Spectroscopic investigations
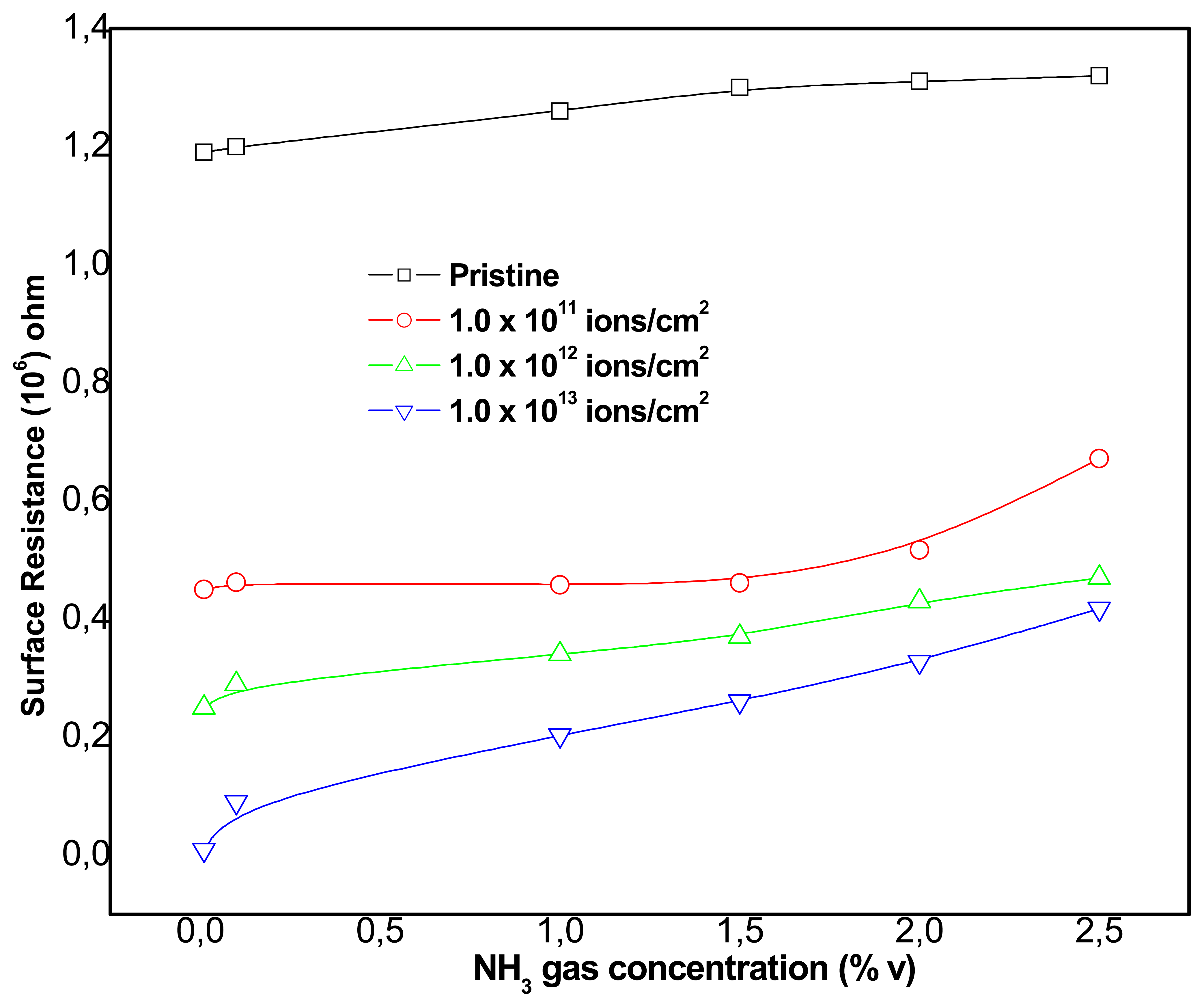
Sensing activity
Conclusions
Acknowledgments
References
- Schultze, J.W.; Karabulut, H. Application potential of conducting polymers. Electrochimica Acta 2005, 50, 1739. [Google Scholar]
- Asmus, T.; Wolf, G. K. Modification and structuring of conducting polymer films on insulating substrates by ion beam treatment. Nucl. Instr. and Meth. in Phy. Res. B. 2000, 166, 732. [Google Scholar]
- Mastragostino, M.; Arbizzani, C.; Soavi, F. Conducting polymers as electrode materials in super capacitors. Solid State Ionics 2002, 148, 493. [Google Scholar]
- Dhawan, S.K.; Singh, N.; Rodrigues, D. Electromagnetic shielding behaviour of conducting polyaniline composites. Science and Technology of Advanced Materials 2003, 4, 105. [Google Scholar]
- Koul, S.; Chandra, R.; Dhawan, S.K. Conducting polyaniline composite: a reusable sensor material for aqueous ammonia. Sensors and Actuators B: Chemical 2001, 75, 151. [Google Scholar]
- Jain, S.; Chakane, S.; Samui, A.B.; Krishnamurthy, V.N.; Bhoraskar, S.V. Humidity sensing with weak acid-doped polyaniline and its composites. Sensors and Actuators B: Chemical 2003, 96, 124. [Google Scholar]
- Joon-Boo, Yu.; Hyung-Gi, B.; Myung-Suk, So.; Jeung-Soo, H. Analysis of diabetic patient's breath with conducting polymer sensor array. Sensors and Actuators B: Chemical 2005, 108, 305. [Google Scholar]
- Gangopadhay, R.; De, A. Conducting polymer composites: novel materials for gas sensing. Sensors and Actuators B: Chemical 2001, 77, 326. [Google Scholar]
- Amrani, M.E.H.; Dowdeswell, R.M.; Payne, P.A.; Persaud, K.C. An intelligent gas sensing system. Sensors and Actuators B: Chemical 1997, 44, 512. [Google Scholar]
- Jouve, C.; Jullien, D.; Remaki, B. Conductive polyethylene as sensitive layer for gas detection. Sensors and Actuators B: Chemical 1995, 28, 75. [Google Scholar]
- Dogan, S.; Akbulut, U.; Yalcin, T.; Suzer, S.; Toppare, L. Conducting polymers of aniline. II. A composite as a gas sensor. Synthetic Metal 1993, 60, 27. [Google Scholar]
- Ram, M.K.; Yavuz, O.; Aldissi, M. NO2 gas sensing based on ordered ultrathin films of conducting polymer and its nanocomposite. Synthetic Metal 2005, 151, 77. [Google Scholar]
- Amado, F.D.R.; Gondran, E.; Ferreira, J.Z.; Rodrigues, M.A.S.; Ferreira, CA. Synthesis and characterization of high impact polystyrene/polyaniline composite membranes for electrodialysis. Journal of Membrane Science 2004, 234, 139. [Google Scholar]
- Gupta, R.K.; Singh, R.A. Preparation and characterization of polymer composites of polyaniline with polyvinylchloride and polystyrene. Journal of Non-Crystalline Solids 2005, 351, 2022. [Google Scholar]
- Srivastava, A.; Singh, T.V.; Mule, S.; Rajan, C.R.; Ponrathnam, S. Study of chemical, optical and thermal modifications induced by 100 MeV silicon ions in a polycarbonate film. Nucl. Instr. Meth. B 2002, 192, 402. [Google Scholar]
- Hioki, T.; Noda, S.; Sugiura, M.; Kakaeno, M.; Yamada, K.; Kawamoto, J. Electrical and optical properties of ion-irradiated organic polymer Kapton H. Appl. Phys. Lett. 1983, 43, 30. [Google Scholar]
- Sevil, U.A.; Güven, O.; Kovács, A.; Slezsák, I. Gamma and electron dose response of the electrical conductivity of polyaniline based polymer composites. Radiation Physics and Chemistry 2003, 67, 575. [Google Scholar]
- Sonar, P.; Sharma, A.L.; Chandra, A.; Muellen, K.; Srivastava, A. Synthesis and study of conductivity behavior of blended conducting polymer films irradiated with swift heavy ions of silicon. Current Applied Physics 2003, 3, 247. [Google Scholar]
- Singh, N.L.; Shrinet, V.; Pandaya, N.R.; Sharma, A.; Patel, N.V.; Avasthi, D.K. Effect of ion irradiation on hydrogen gas sensitivity of polymer. Nucl. Instr. Meth. B. 1999, 156, 191. [Google Scholar]
- Ziegler, J.F.; Biersack, J.P. The stopping ranges of ions in matter, SRIM-2003; IBM Research: New York, USA, 2000; p. 1. [Google Scholar]
- Meixiang, W.; Ming, Li.; Junchao, L.; Zhenxing, L. Structure and electrical properties of the oriented polyaniline films. J. Applied Poly Science. 1994, 53, 131. [Google Scholar]

© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for non-commercial purposes.
Share and Cite
Srivastava, A.; Singh, V.; Dhand, C.; Kaur, M.; Singh, T.; Witte, K.; Scherer, U.W. Study of Swift Heavy Ion Modified Conducting Polymer Composites for Application as Gas Sensor. Sensors 2006, 6, 262-269. https://doi.org/10.3390/s6040262
Srivastava A, Singh V, Dhand C, Kaur M, Singh T, Witte K, Scherer UW. Study of Swift Heavy Ion Modified Conducting Polymer Composites for Application as Gas Sensor. Sensors. 2006; 6(4):262-269. https://doi.org/10.3390/s6040262
Chicago/Turabian StyleSrivastava, Alok, Virendra Singh, Chetna Dhand, Manindar Kaur, Tejvir Singh, Karin Witte, and Ulrich W. Scherer. 2006. "Study of Swift Heavy Ion Modified Conducting Polymer Composites for Application as Gas Sensor" Sensors 6, no. 4: 262-269. https://doi.org/10.3390/s6040262



