Determination of Trace Antimony (III) by Adsorption Voltammetry at Carbon Paste Electrode
Abstract
:Introduction
Experimental
Reagents and solutions
Apparatus
Procedure
Results and Discussion
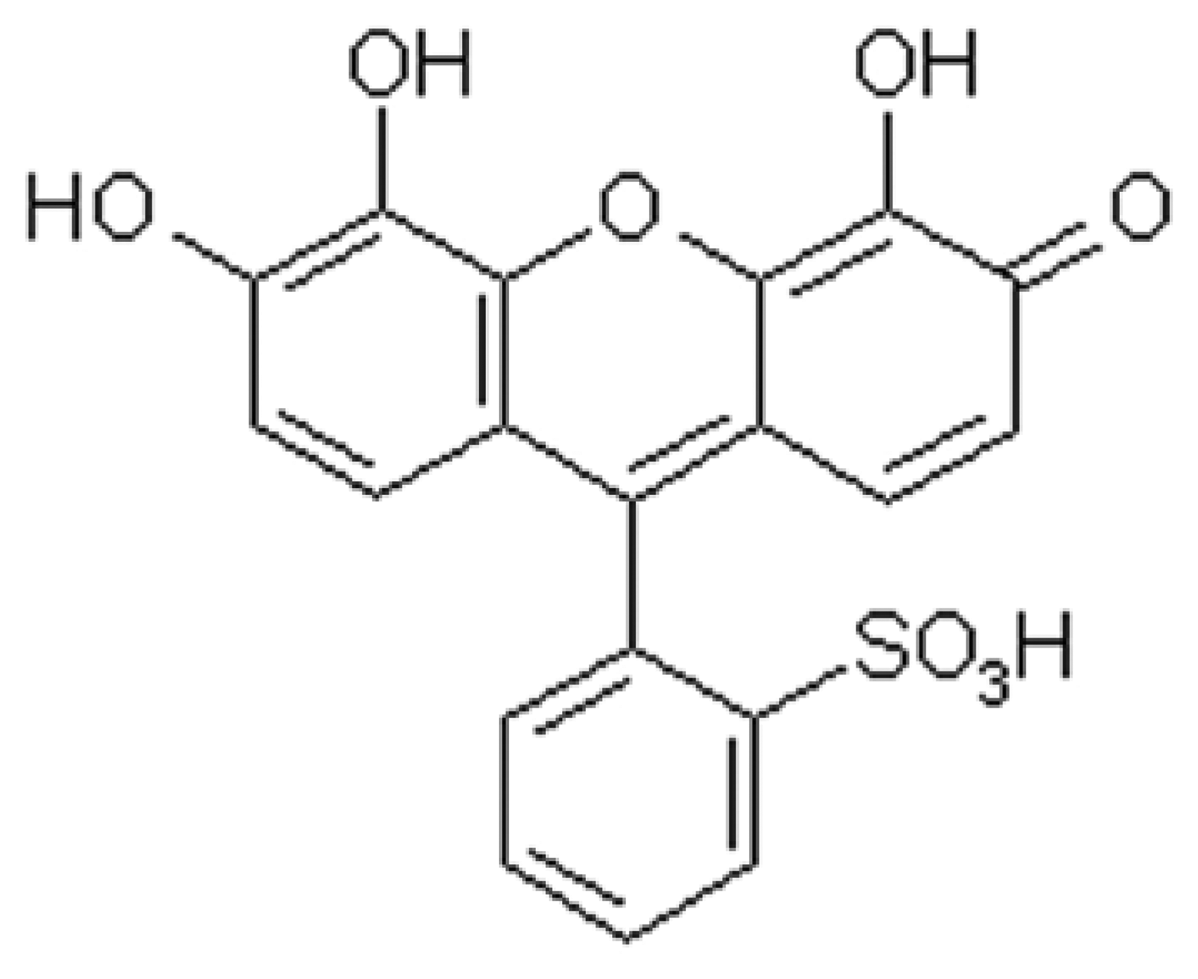
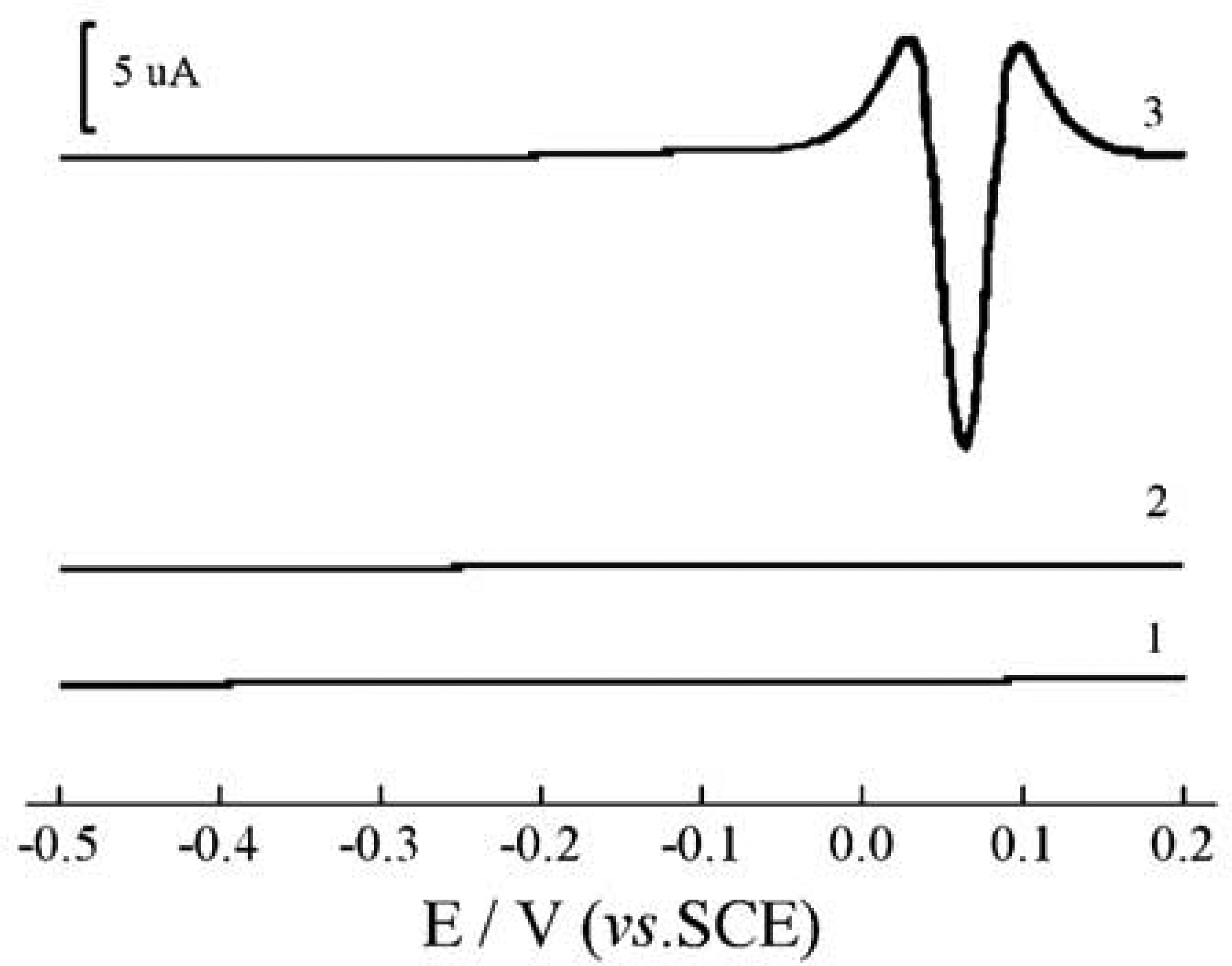
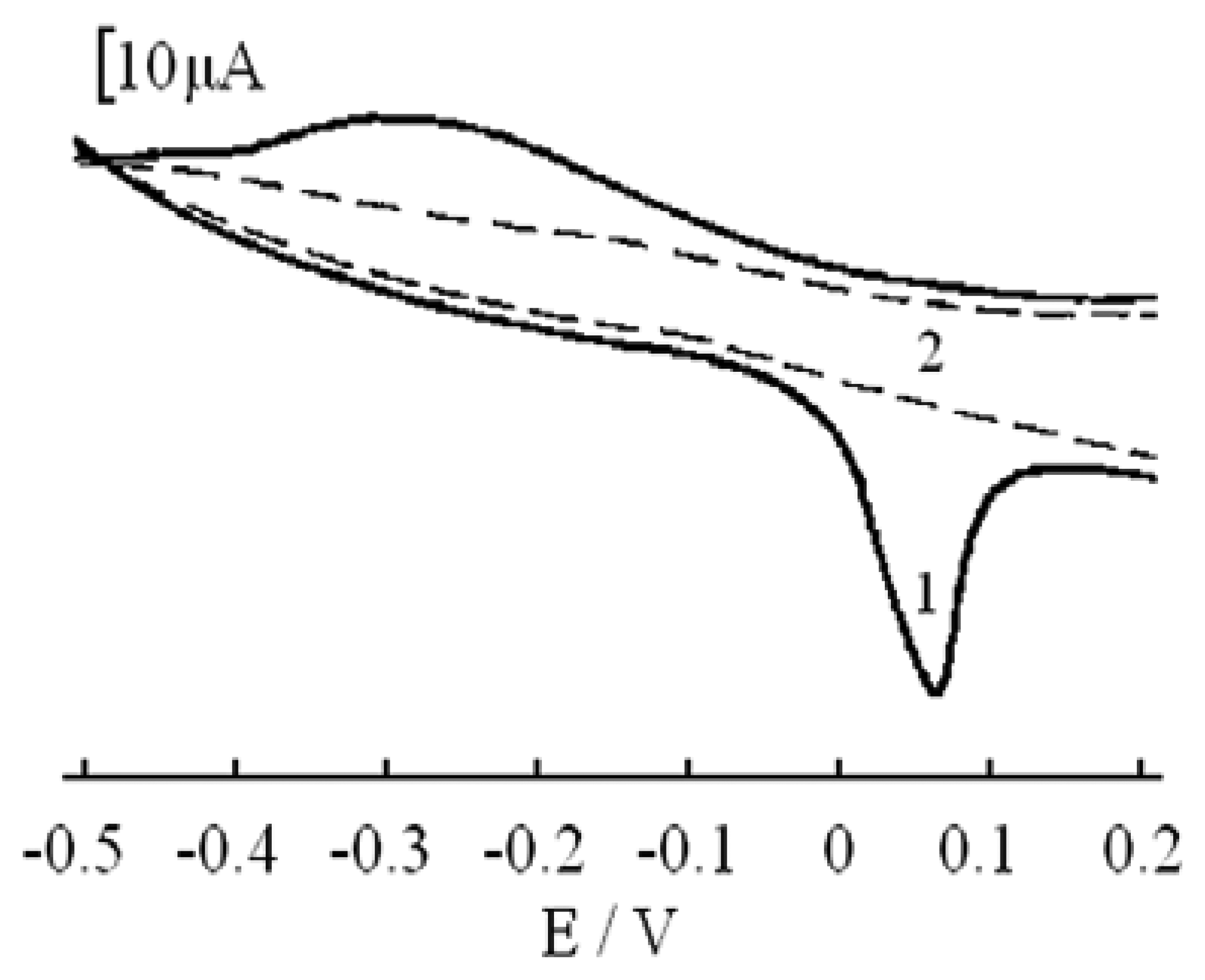
Adsorptive and voltammetric characteristics of the Sb (III)-PGR complex on CPE
Selection of the adhesive in the electrode
Effect of the supporting medium
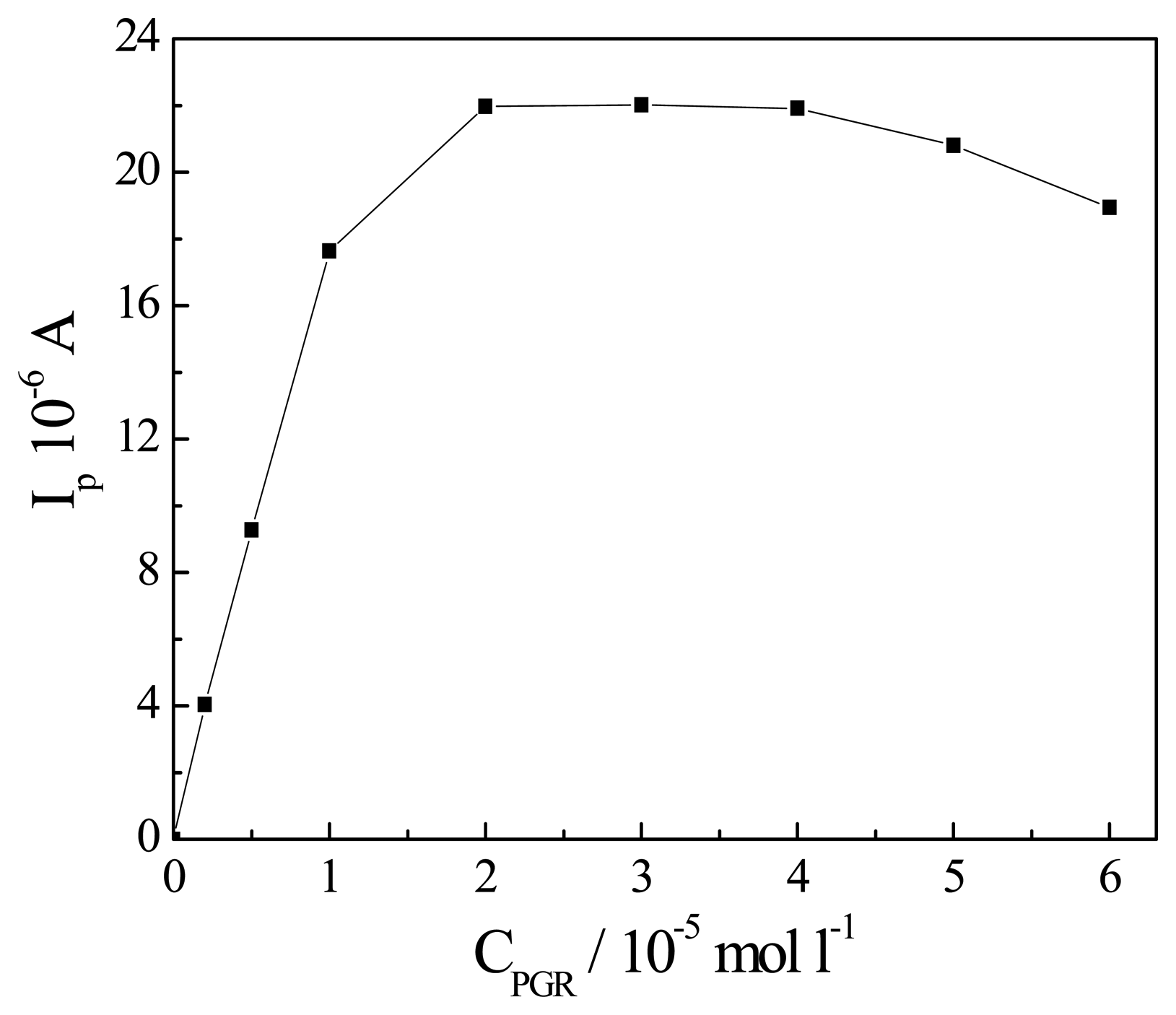
Effect of the concentration of PGR
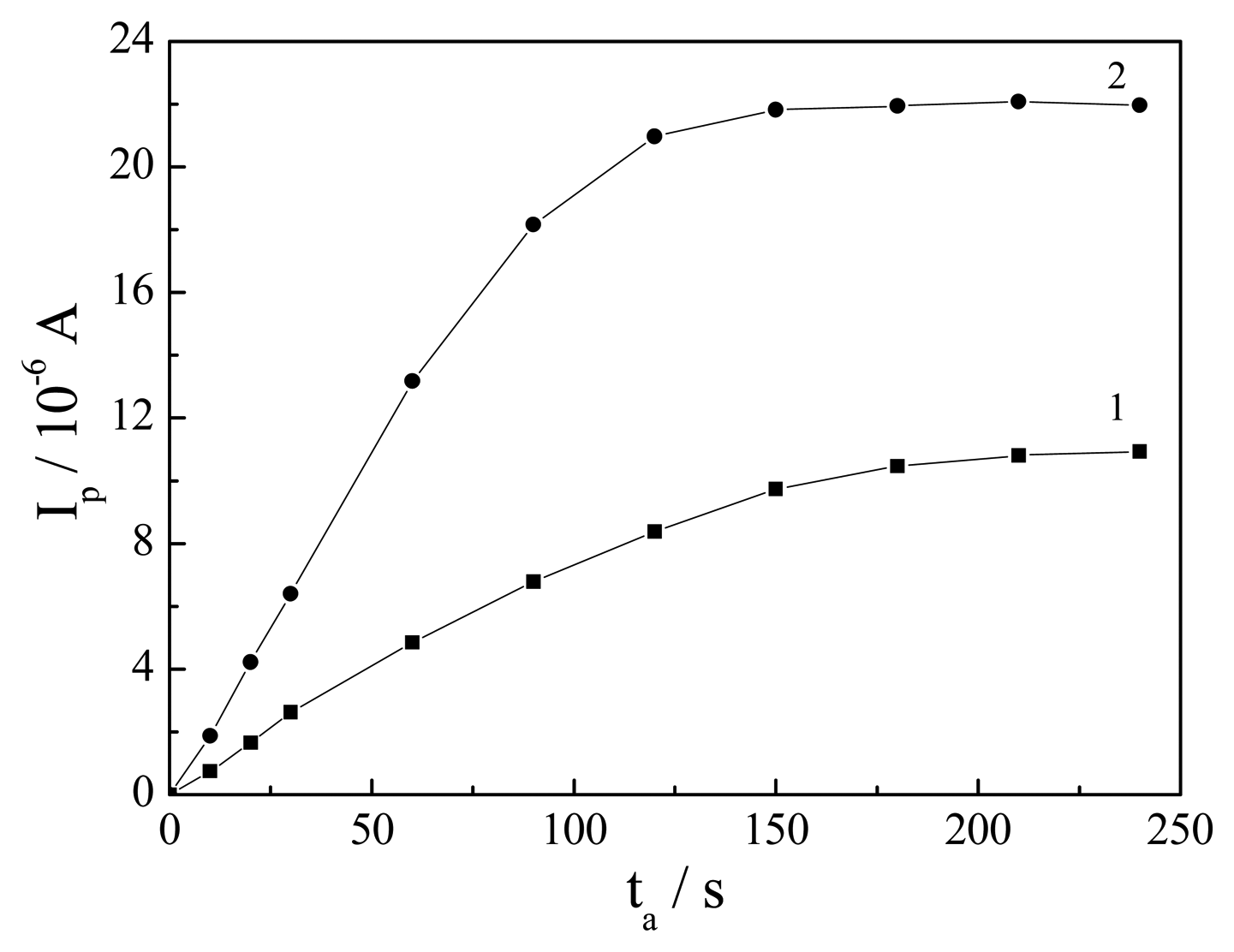
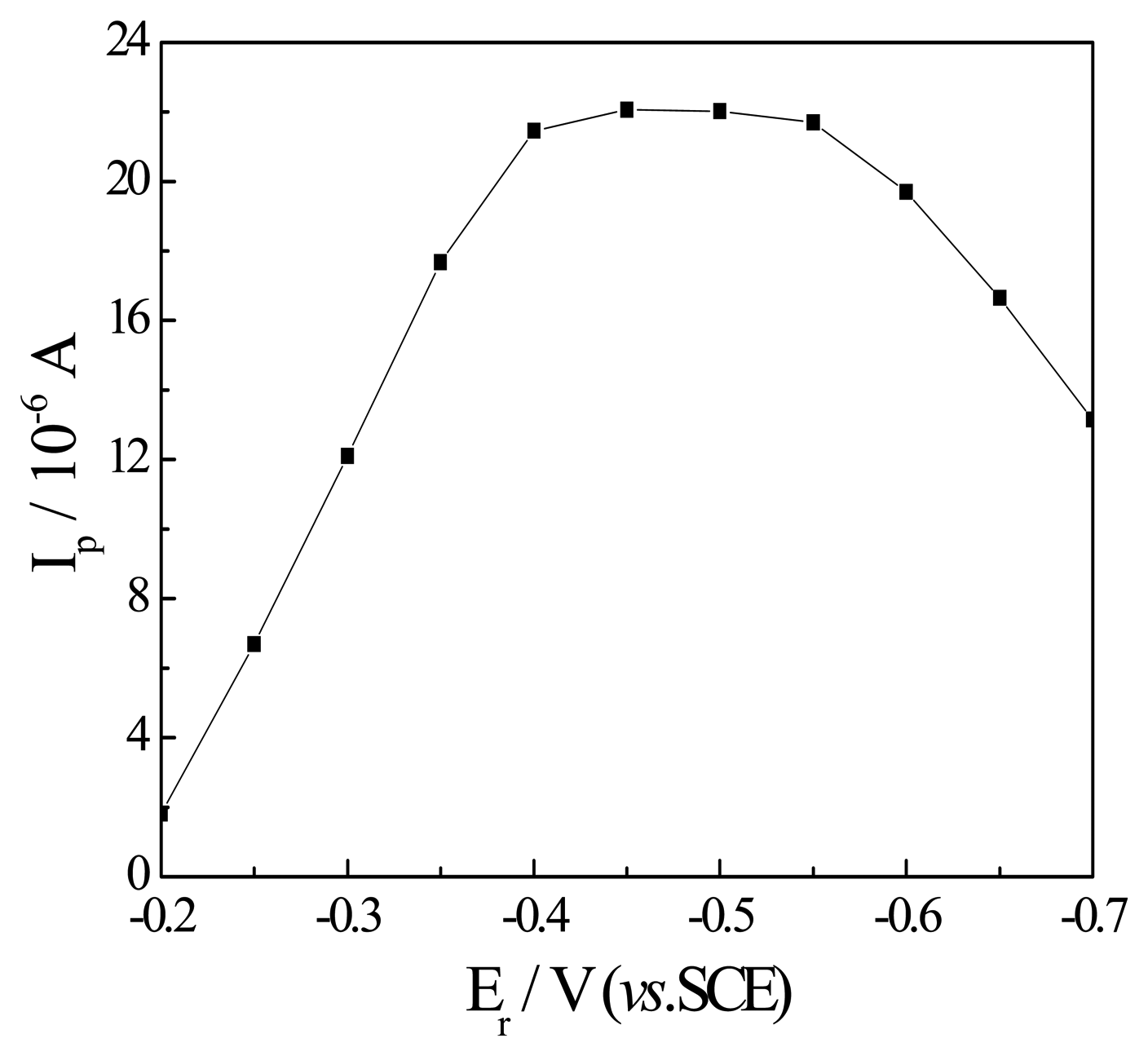
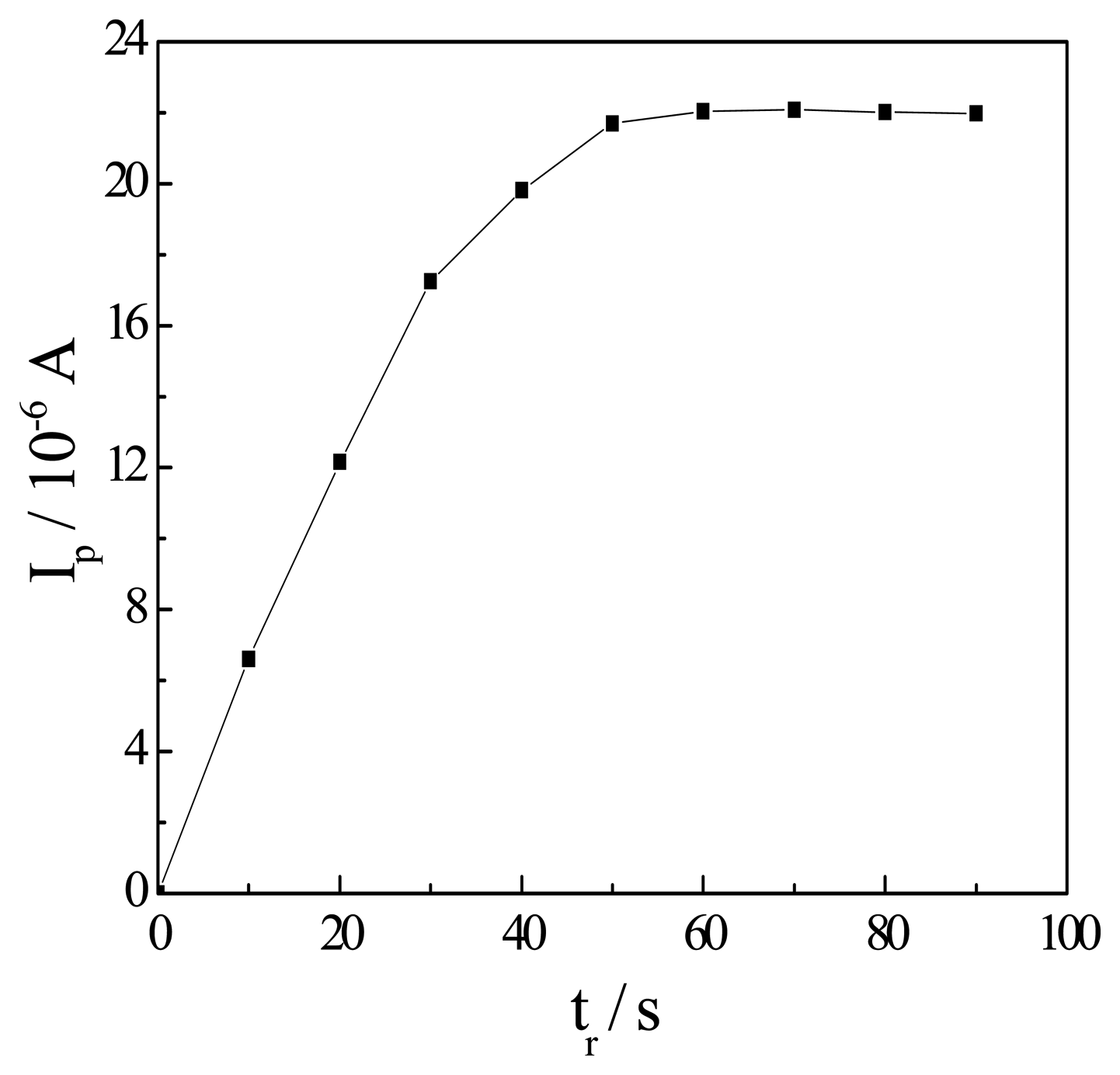
Effect of the reduction potential and time
Linear range and detection limit of the method
Interference studies
Analytical applications
Conclusions
Acknowledgments
References
- Kubota, T.; Kawakami, A.; Sagara, T.; Ookubo, N.; Okutani, T. Determination of antimony content in natural water by graphite furnace atomic absorption spectrometry after collection as antimony (III)-pyrogallol complex on activated carbon. Talanta 2001, 53, 1117. [Google Scholar]
- Deng, T.L.; Chen, Y.W.; Belzile, N. Antimony speciation at ultra trace levels using hydride generation atomic fluorescence spectrometry and 8-hydroxyquinoline as an efficient masking agent. Anal. Chim. Acta 2001, 432, 293. [Google Scholar]
- Quentel, F.; Filella, M. Determination of inorganic antimony species in seawater by differential pulse anodic stripping voltammetry: stability of the trivalent state. Anal. Chim. Acta 2002, 452, 237. [Google Scholar]
- Tanaka, T.; Ishiyama, T.; Okamoto, K. Determination of Antimony in Steel by Differential Pulse Anodic Stripping Voltammetry at a Rotating Gold Film Electrode. Anal. Sci. 2000, 16, 19. [Google Scholar]
- Long, H.; Hou, X.F.; Li, Y.H. Polarographic Adsorptive Catalytic Wave of Tin (IV)-Pyrogallol Red-Vanadium (IV)-Sodium Dodecyl Sulfonate System. Chin. J. Anal. Chem. 1999, 27, 316. [Google Scholar]
- Safavi, A.; Shams, E. Determination of trace amounts of copper (II) by adsorptive stripping voltammetry of its complex with pyrogallol red. Anal. Chim. Acta 1999, 385, 265. [Google Scholar]
- Kazemzadeh, A.; Ensafi, A.A. Simultaneous determination of nitrite and nitrate in various samples using flow-injection spectrophotometric detection. Microchem. J. 2001, 69, 159. [Google Scholar]
- Williams, K.M.; Marshall, T. Protein concentration of cerebrospinal fluid by precipitation with Pyrogallol Red prior to sodium dodecyl sulphate-polyacrylamide gel electrophoresis. J. Biochem. Biophys. Methods 2001, 47, 197. [Google Scholar]
- Mousavi, M.F.; Rahmani, A.; Golabi, S.M.; Shamsipur, M.; Sharghi, H. Differential pulse anodic stripping voltammetric determination of lead (II) with a 1,4-bis(prop-2′-enyloxy)-9,10-anthra- quinone modified carbon paste electrode. Talanta 2001, 55, 305. [Google Scholar]
- Abbaspour, A.; Moosavi, S.M.M. Chemically modified carbon paste electrode for determination of copper (II) by potentiometric method. Talanta 2002, 56, 91. [Google Scholar]
- Guo, H.S.; Li, Y.H.; Xie, H.Q. Determination of Trace Antimony by Adsorptive Stripping Voltammetry with Pyrogallol Red Modified Carbon Paste Electrode. Nat. Sci. J. of Xiangtan Univ. 2001, 23, 68. [Google Scholar]
- Wei, X.Y.; Tan, J.X. Polarographic Adsorptive Complex Catalytic Wave of Antimony (III) and Tin (IV) with 7-Iodo-8-hydroxyquinolin-5-sulfonic Acid (Ferron) and Its Application. Fenxi Shiyanshi 1996, 15, 58. [Google Scholar]
- Zhang, X.L.; Ma, C.S.; Wang, L.Z.; Zhang, J.G. Trace adsorptive voltammetric determination of antimony in hair. Talanta 1995, 42, 897. [Google Scholar]
| Sample | Found (μg/L) | R.S.D. (%) | Added Sb3+ (μg/L) | Total found (μg/L) | Recovery (%) |
|---|---|---|---|---|---|
| Tap water | 0.24 | 3.8 | 0.50 | 0.72 | 96 |
| River water | 0.32 | 4.2 | 0.50 | 0.83 | 102 |
| Waste water | 1.68 | 4.7 | 0.50 | 2.17 | 98 |
| Sample | Found (μg/g) | R.S.D. (%) | Added Sb3+ (μg/g) | Total found (μg/g) | Recovery (%) |
|---|---|---|---|---|---|
| Human hair 1 | 6.57 | 3.4 | 5.00 | 11.49 | 98.4 |
| Human hair 2 | 5.88 | 5.3 | 5.00 | 11.06 | 103.6 |
© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Guo, H.; Li, Y.; Chen, X.; Nie, L.; He, N. Determination of Trace Antimony (III) by Adsorption Voltammetry at Carbon Paste Electrode. Sensors 2005, 5, 284-292. https://doi.org/10.3390/s5040284
Guo H, Li Y, Chen X, Nie L, He N. Determination of Trace Antimony (III) by Adsorption Voltammetry at Carbon Paste Electrode. Sensors. 2005; 5(4):284-292. https://doi.org/10.3390/s5040284
Chicago/Turabian StyleGuo, Huishi, Yiheng Li, Xiaokang Chen, Libo Nie, and Nongyue He. 2005. "Determination of Trace Antimony (III) by Adsorption Voltammetry at Carbon Paste Electrode" Sensors 5, no. 4: 284-292. https://doi.org/10.3390/s5040284




