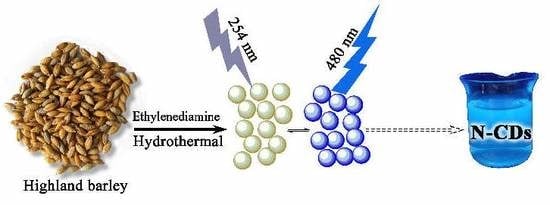
Green Hydrothermal Synthesis of N-doped Carbon Dots from Biomass Highland Barley for the Detection of Hg2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Preparation of N-CDs
2.4. Detection of Hg2+
2.5. Investigation of Selectivity and Competitiveness
3. Results and Discussion
3.1. Synthesis and Characterization of the N-CDs
3.2. Optical Properties of the N-CDs
3.3. Fluorescence Detection of Hg2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.B.; Qin, X.Y.; Liu, S.; Chang, G.H.; Zhang, Y.W.; Luo, Y.L.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.P. Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Cui, J.H.; Zheng, M.T.; Hu, C.F.; Tan, S.Z.; Xiao, Y.; Yang, Q.; Liu, Y.L. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Diao, H.P.; Chang, H.H.; Wang, H.J.; Li, T.T.; Wei, W.L. Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging. Sens. Actuators B 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Xu, Q.; Li, W.J.; Ding, L.; Yang, W.; Xiao, H.H.; Ong, W.J. Function-driven engineering of 1D carbon nanotubes and 0D carbon dots: Mechanism, properties and applications. Nanoscale. 2019, 11, 1475–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.W.; Hou, Y.L.; Kang, F.; Lyu, F.C.; Xiong, Y.; Chen, W.C.; Lee, C.S.; Xu, Z.T.; Rogach, A.L.; Lu, J.; et al. Rare earth-free composites of carbon dots/metal–organic frameworks as white light emitting phosphors. J. Mater. Chem. C 2019, 7, 2207–2211. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Hu, S.; Niu, K.; Sun, J.; Yang, J.; Zhao, N.; Du, X.J. One-step synthesis of fluorescent carbon nanoparticlesby laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar]
- Xue, M.Y.; Zhan, Z.H.; Zou, M.B.; Zhang, L.L.; Zhao, S.L. Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. New J. Chem. 2016, 40, 1698–1703. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.H.; Niu, F.S.; Gooding, J.J.; Liu, J.Q. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, Y.Q.; Zhao, J.P.; Miao, M.; Chao, S.M.; Fang, J.H.; Shi, L.Y. Easy synthesis of photoluminescent N-doped carbon dots from winter melon for bio-imaging. RSC Adv. 2015, 5, 31250–31254. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots:Properties, Syntheses, and Biological Applications. Small 2015, 14, 1620–1636. [Google Scholar]
- Wu, Z.L.; Zhang, P.; Gao, M.X.; Liu, C.F.; Wang, W.; Leng, F.; Huang, C.Z. One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from Bombyx mori silk—Natural proteins. J. Mater. Chem. B 2013, 1, 2868–2873. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kuang, T.R.; Liu, Y.; Cai, L.L.; Peng, X.F.; Sreeprasad, T.S.; Zhao, P.; Yu, Z.Q.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar]
- Zhai, Y.L.; Zhu, Z.J.; Zhu, C.Z.; Ren, J.T.; Wang, E.K.; Dong, S.J. Multifunctional water-soluble luminescent carbon dots for imaging and Hg2+ sensing. J. Mater. Chem. B 2014, 2, 6995–6999. [Google Scholar] [CrossRef]
- Li, Z.; Yu, H.J.; Bian, T.; Zhao, Y.F.; Zhou, C.; Shang, L.; Liu, Y.H.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Highly luminescent nitrogen-doped carbon quantum dots as effective fluorescent probes for mercuric and iodide ions. J. Mater. Chem. C 2015, 3, 1922–1928. [Google Scholar] [CrossRef]
- Gao, Z.H.; Lin, Z.Z.; Chen, X.M.; Zhong, H.P.; Huang, Z.Y. A fluorescent probe based on N-doped carbon dots for highly sensitive detection of Hg2+ in aqueous solutions. Anal. Methods 2016, 8, 2297–2304. [Google Scholar] [CrossRef]
- Xiong, Y.; Schneider, J.; Reckmeier, C.J.; Huang, H.; Kasák, P.; Rogach, A.L. Carbonization conditions influence the emission characteristics and the stability against photobleaching of nitrogen doped carbon dots. Nanoscale 2017, 9, 11730–11738. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.Q.; Wang, L.; Zhang, Y.W.; Qin, X.Y.; Luo, Y.L.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.P. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu(II) Ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhang, L.L.; Li, X.F.; Liu, R.J.; Lin, L.Y.; Zhao, S.L. Green Preparation of S and N Co-Doped Carbon Dots from Water Chestnut and Onion as Well as Their Use as an Off–On Fluorescent Probe for the Quantification and Imaging of Coenzyme A. ACS Sustain. Chem. Eng. 2017, 5, 4992–5000. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.J.; Zhou, D.L.; Bao, N.; Xu, Y.; Wang, A.J.; Feng, J.J. One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC Adv. 2013, 3, 21691–21696. [Google Scholar] [CrossRef]
- He, S.J.; Li, D.; Zhu, C.F.; Song, S.P.; Wang, L.H.; Long, Y.T.; Fan, C.H. Design of a gold nanoprobe for rapid and portable mercury detection with the naked eye. Chem. Commun. 2008, 40, 4885–4887. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhai, J.F.; Tian, J.Q.; Luo, Y.L.; Sun, X.P. Carbon nanoparticle for highly sensitive and selective fluorescent detection of mercury(II) ion in aqueous solution. Biosens. Bioelectron. 2011, 26, 4656–4660. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.D.; Liu, X.L.; Xie, Y.D.; Lv, H.T.; Wang, Z.Q.; Yang, H.Z.; Han, A.X.; Yang, X.M.; Zang, L. A Ratiometric Fluorescent Sensor for Cd2+ Based on Internal Charge Transfer. Sensors 2017, 17, 2517. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Ghanemi, K.J. Determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry after solid phase extraction on agar modified with 2-mercaptobenzimidazole. Hazard. Mater. 2009, 161, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, C.; Li, B.; Sun, J.; Wang, J.; Gao, Y.; Zhao, Y.; Chai, Z.J. Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS. Anal. At. Spectrom. 2006, 21, 94–96. [Google Scholar] [CrossRef]
- Yan, F.Y.; Zou, Y.; Wang, M.; Mu, X.L.; Yang, N.; Chen, L. Highly photoluminescent carbon dots-based fluorescent chemosensors for sensitive and selective detection of mercury ions and application of imaging in living cells. Sens. Actuators B 2014, 192, 488–495. [Google Scholar] [CrossRef]
- Li, L.B.; Yu, B.; You, T.Y. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (Ⅱ) ions. Biosens. Bioelectron. 2015, 74, 263–269. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.W.; Jeong, S.W.; Kim, C.H.; Lee, Y.C.; Huh, Y.S.; Lee, J. Photoluminescent Green Carbon Nanodots from Food-Waste-Derived Sources: Large-Scale Synthesis, Properties, and Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef]
- Mohapatra, S.; Sahu, S.; Sinha, N.; Bhutia, S.K. Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg2+ in water and living cells. Analyst 2015, 140, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.H.; Ma, W.J.; Shi, Y.; Li, Z.; Yang, X.M. Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. CARBON 2013, 60, 421–428. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhuang, Y.; Zhang, X.; Chen, W.; Hong, L.L.; Yan, Q.X.; Yu, K.; Huang, S.M. One-pot synthesis of N-doped carbon dots with tunable luminescence properties. J. Mater. Chem. 2012, 22, 16714–16718. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhou, Y.; Li, S.X.; Dong, M.T.; Huang, C.B. Low-cost synthesis of carbon nanodots from natural products used as a fluorescent probe for the detection of ferrum(III) ions in lake water. Anal. Methods 2014, 6, 2086–2090. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Luo, Y.; Lu, W.; Sun, X.P. Acid-driven, microwave-assisted production of photoluminescent carbon nitride dots from N,N-dimethylformamide. RSC Adv. 2011, 1, 951–953. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, M.; Liu, Y.; He, F.; Gao, F.; Su, Y.; Wei, H.; Zhang, Y. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 2014, 6, 1890–1895. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Luo, Y.; Zhai, J.; Sun, X.P. Preparation of photoluminescent carbon nitride dots from CCl4 and 1,2-ethylenediamine: A heat-treatment-based strategy. J. Mater. Chem. 2011, 21, 11726–11729. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label-free detection of Fe (III) in aqueous media. Biosens. Bioelectron. 2014, 58, 219–225. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green Synthesis of Luminescent Nitrogen-Doped Carbon Dots from Milk and Its Imaging Application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Sheng, Z.H.; Han, H.Y.; Zou, M.Q.; Li, C.X. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhou, Q.X.; Yuan, Y.Y.; Wu, Y.L. Hydrothermal synthesis of fluorescent carbon dots from sodium citrate and polyacrylamide and their highly selective detection of lead and pyrophosphate. Carbon 2017, 115, 550–560. [Google Scholar] [CrossRef]
- Wang, K.J.; Guan, F.; Li, H.X.; Li, M.L.; Feng, H.X.; Fan, H.V. One-step synthesis of carbon nanodots for sensitive detection of cephalexin. RSC Adv. 2015, 5, 20511–20515. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, J.; Lim, B.; Rhee, S.W. Improving the functionality of carbon nanodots: Doping and surface functionalization. J. Mater. Chem. A 2016, 4, 11582–11603. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.D.; Guo, Y.L.; Liu, W.S.; Qin, W.W. Luminescent properties of milk carbon dots and their sulphur and nitrogen doped analogues. RSC Adv. 2014, 4, 51658–51665. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, C.F.; Xu, P.P.; Li, A.Q.; Chen, Y.J.; Zhuo, K.L. Synthesis of cellulose-derived carbon dots using acidic ionic liquid as a catalyst and its application for detection of Hg2+. J. Mater. Sci. 2016, 51, 861–867. [Google Scholar] [CrossRef]
- Gao, X.H.; Du, C.; Zhuang, Z.H.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Cao, B.M.; Yuan, C.; Liu, B.H.; Jiang, C.L.; Guan, G.J.; Han, M.Y. Ratiometric fluorescence detection of mercuric ion based on the nanohybrid of fluorescence carbon dots and quantum dots. Anal. Chim. Acta 2013, 786, 146–152. [Google Scholar] [CrossRef]
- Liu, R.H.; Li, H.T.; Kong, W.Q.; Liu, J.; Liu, Y.; Tong, C.Y.; Zhang, X.; Kang, Z.H. Ultra-sensitive and selective Hg2+ detection based on fluorescent carbon dots. Mater. Res. Bull. 2013, 48, 2529–2534. [Google Scholar] [CrossRef]







| FL Probe | Precursor | QY (%) | LOD (μM) | LinearR (nM) | Ref. |
|---|---|---|---|---|---|
| CPs | Pomelo peel | 6.9 | 0.00023 | 0.5–10 and 500–4 × 104 | 2 |
| N-CQD | folic acid | 15.7 | 0.23 | 0–2.5 × 104 | 14 |
| FNCPs | strawberry juice | 6.88 | 0.003 | 10–5 × 104 | 22 |
| N,S/C-dots | Citric acid/urea/L-cysteine | 25.2 | 2 | 0–4 × 104 | 29 |
| N-CDs | Highland barley/ethanediamine | 14.4 | 0.48 | 1× 103–16 × 104 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Cheng, D.; Liu, X.; Han, A. Green Hydrothermal Synthesis of N-doped Carbon Dots from Biomass Highland Barley for the Detection of Hg2+. Sensors 2019, 19, 3169. https://doi.org/10.3390/s19143169
Xie Y, Cheng D, Liu X, Han A. Green Hydrothermal Synthesis of N-doped Carbon Dots from Biomass Highland Barley for the Detection of Hg2+. Sensors. 2019; 19(14):3169. https://doi.org/10.3390/s19143169
Chicago/Turabian StyleXie, Yadian, Dandan Cheng, Xingliang Liu, and Aixia Han. 2019. "Green Hydrothermal Synthesis of N-doped Carbon Dots from Biomass Highland Barley for the Detection of Hg2+" Sensors 19, no. 14: 3169. https://doi.org/10.3390/s19143169