Distance-Based Paper Device Combined with Headspace Extraction for Determination of Cyanide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Synthesis of Gold, Silver and Gold@silver Core-Shell Nanoparticles
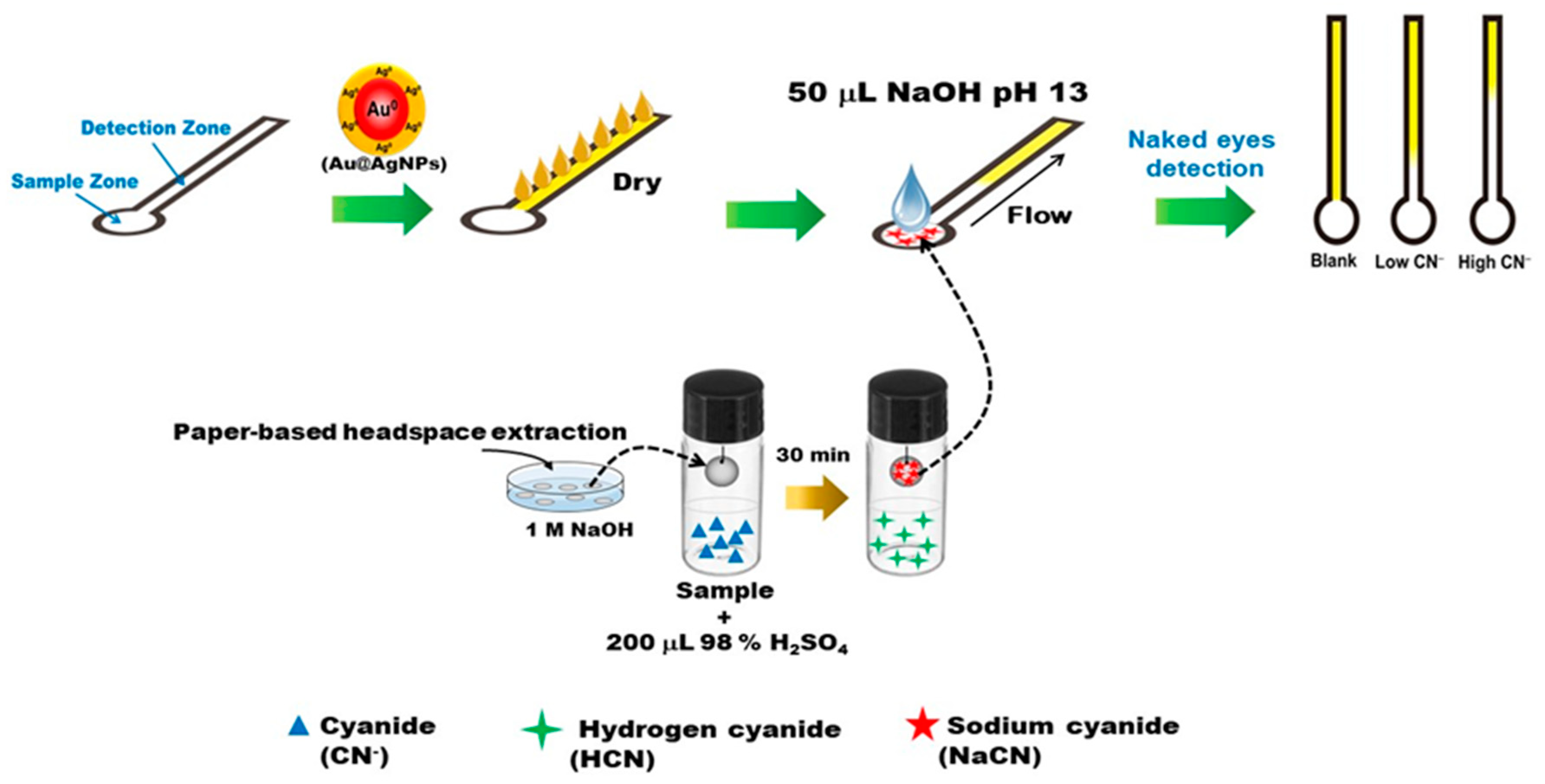
2.4. Device Fabrication
2.5. Optimization of Detection Conditions on Distance–Based Paper Devices
2.6. Applications for Real Samples
3. Results and discussion
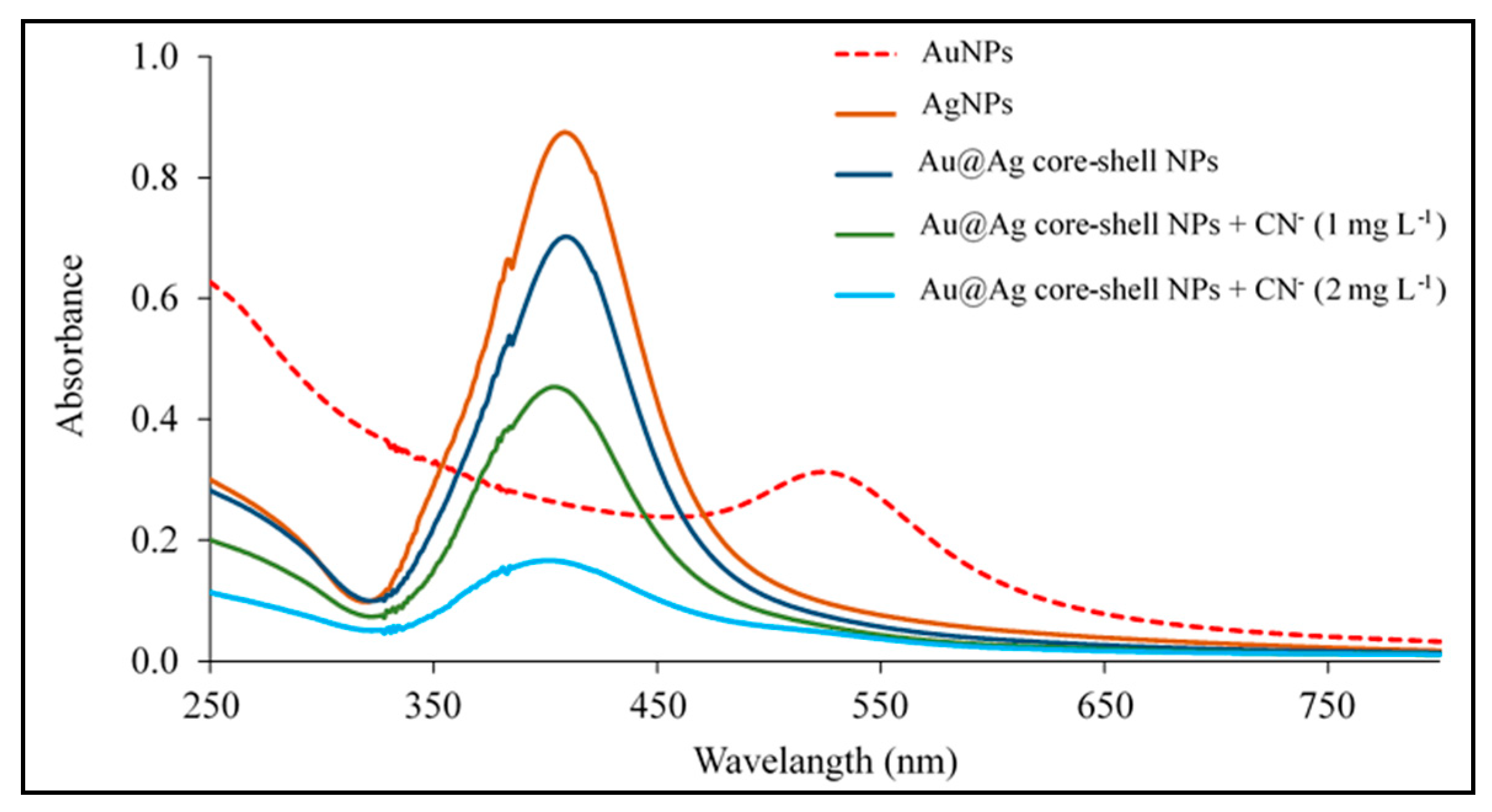
3.1. Characterization of Au@Ag NPs
3.2. Study of Reagents Influence on the Length of Au@Ag NPs Color Band on dPADs
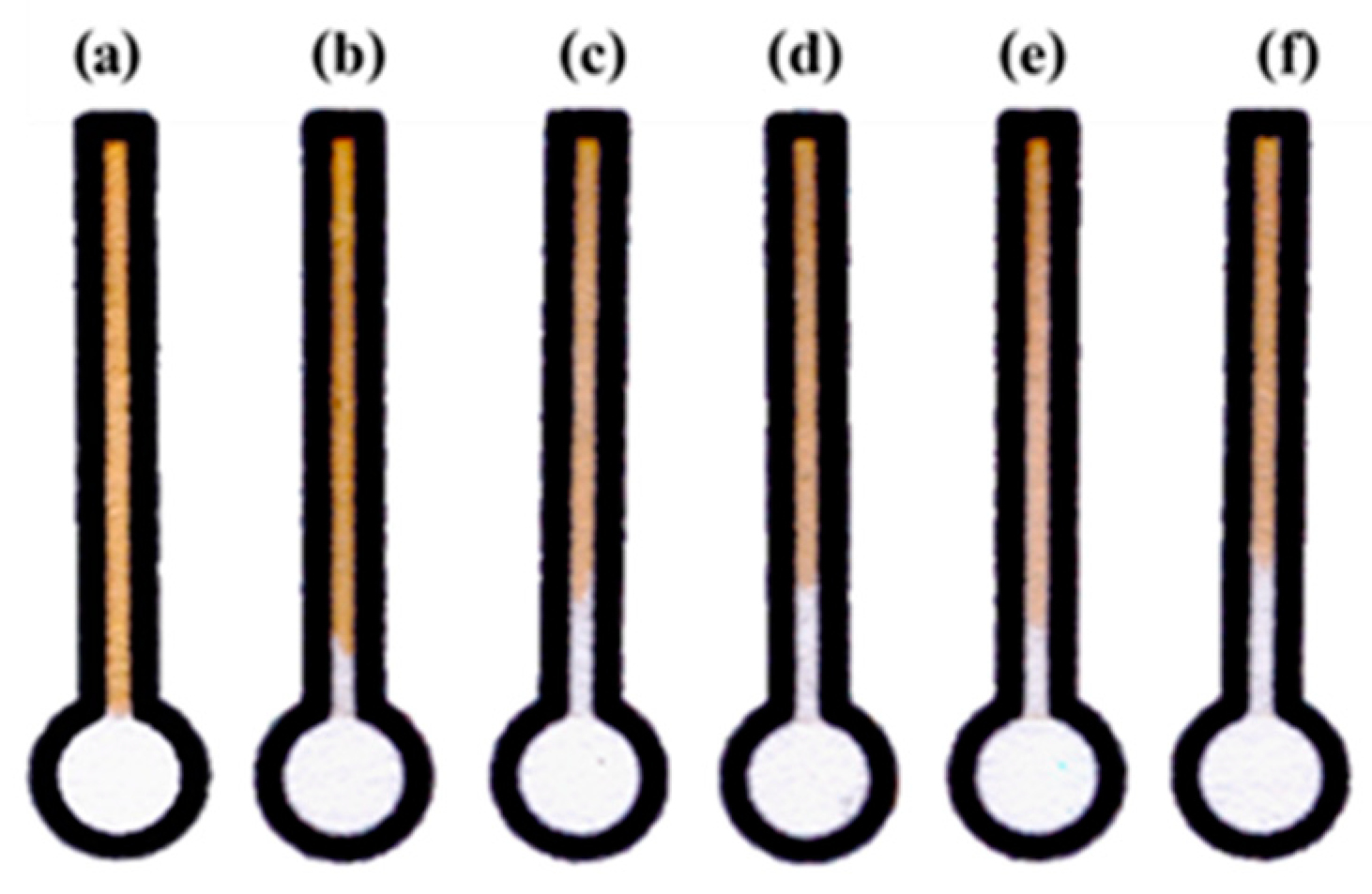
3.3. Optimization of Detection Conditions on dPADs
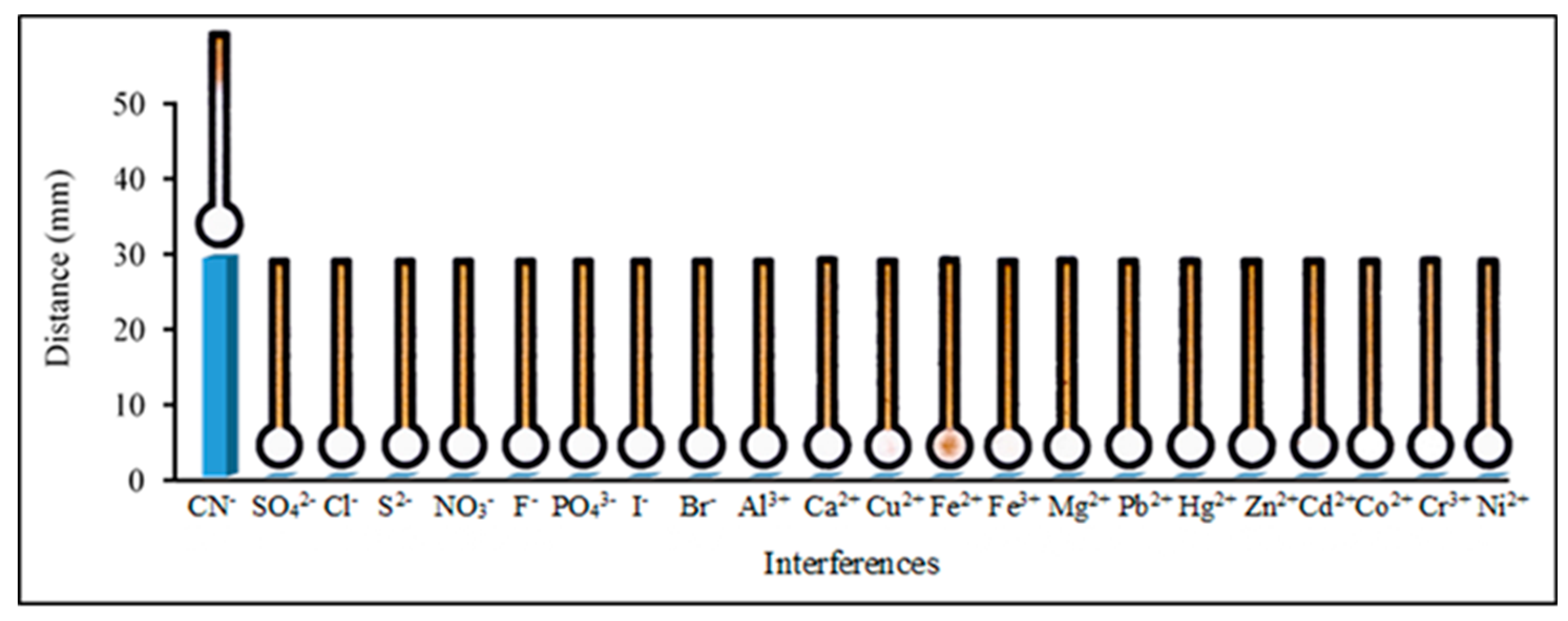
3.4. Analytical Performance
3.5. Applications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Code, International Cyanide Management. Cyanide Chemistry. Available online: https://www.cyanidecode.org/cyanide-facts/cyanide-chemistry (accessed on 18 February 2019).
- (EPA), United States Environmental Protection Agency. Ambient Water Quality Criteria for Cyanide; EPA: Washington, DC, USA, 1984. [Google Scholar]
- Taylor, J. Toxicological Profile for Cyanide: Agency for Toxic Substances and Disease Registry; ATSDR: Atlanta, GA, USA, 2010. [Google Scholar]
- Guojie, L.; Liu, J.; Hara, K.; Wang, Y.; Yu, Y.; Gao, L.; Li, L. Rapid Determination of Cyanide in Human Plasma and Urine by Gas Chromatography–Mass Spectrometry with Two-Step Derivatization. J. Chromatogr. B 2009, 877, 3054–3058. [Google Scholar]
- Desharnais, B.; Huppé, G.; Lamarche, M.; Mireault, P.; Skinner, C.D. Cyanide Quantification in Post-Mortem Biological Matrices by Headspace GC–MS. Forensic Sci. Int. 2012, 222, 346–351. [Google Scholar] [CrossRef]
- Blaedal, W.J.; Easty, D.B.; Anderson, L.; Farrell, T.R. Potentiometric Determination of Cyanide with an Ion Selective Electrode. Application to Cyanogenic Glycosides in Sudan Grasses. Anal. Chem. 1971, 43, 890–894. [Google Scholar] [CrossRef]
- Lindsay, A.E.; O’Hare, D. The Development of an Electrochemical Sensor for the Determination of Cyanide in Physiological Solutions. Anal. Chim. Acta 2006, 558, 158–163. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Z.; Li, J.; Luo, L. A Micro-Chemiluminescence Determination of Cyanide in Whole Blood. Forensic Sci. Int. 2005, 148, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Maddukuri, N.; Gong, M. A Direct and Rapid Method to Determine Cyanide in Urine by Capillary Electrophoresis. J. Chromatogr. A 2015, 1414, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, S.; Farhadi, K.; Forough, M.; Sabzi, R.E. Silver Nanoparticles as a Cyanide Colorimetric Sensor in Aqueous Media. Anal. Methods 2011, 3, 2599–2603. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, H.Y.; Wu, C.S.; Meena, J.S.; Simon, T.; Ko, F.H. A Highly Sensitive and Selective Cyanide Detection Using a Gold Nanoparticle-Based Dual Fluorescence–Colorimetric Sensor with a Wide Concentration Range. Sens. Actuators B Chem. 2016, 227, 283–290. [Google Scholar] [CrossRef]
- Zeng, J.B.; Cao, Y.Y.; Chen, J.J.; Wang, X.D.; Yu, J.F.; Yu, B.B.; Yan, Z.F.; Chen, X. Au@Ag Core/Shell Nanoparticles as Colorimetric Probes for Cyanide Sensing. Nanoscale 2017, 6, 9939–9943. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Zhou, X.; Wen, C.Y.; Yu, J.; Han, X.; Li, X.; Yan, Z.F.; Zeng, J. A Convenient Colorimetric Method for Sensitive and Specific Detection of Cyanide Using Ag@Au Core–Shell Nanoparticles. Sens. Actuators B Chem. 2016, 228, 366–372. [Google Scholar] [CrossRef]
- Saraji, M.; Bagheri, N. Paper-Based Headspace Extraction Combined with Digital Image Analysis for Trace Determination of Cyanide in Water Samples. Sens. Actuators B Chem. 2018, 270, 28–34. [Google Scholar] [CrossRef]
- İncel, A.; Akın, O.; Çağır, A.; Yıldız, Ü.H.; Demir, M.M. Smart Phone Assisted Detection and Quantification of Cyanide in Drinking Water by Paper Based Sensing Platform. Sens. Actuators B Chem. 2017, 252, 886–893. [Google Scholar] [CrossRef]
- Cate, D.M.; Noblitt, S.D.; Volckens, J.; Henry, C.S. Multiplexed Paper Analytical Device for Quantification of Metals Using Distance-Based Detection. Lab Chip 2015, 15, 2808–2818. [Google Scholar] [CrossRef]
- Dungchai, W.; Sameenoi, Y.; Chailapakul, O.; Volckens, J.; Henry, C.S. Determination of Aerosol Oxidative Activity Using Silver Nanoparticle Aggregation on Paper-Based Analytical Devices. Analyst 2013, 138, 6766–6773. [Google Scholar] [CrossRef]
- Phoonsawat, K.; Ratnarathorn, N.; Henry, C.S.; Dungchai, W. A Distance-Based Paper Sensor for the Determination of Chloride Ions Using Silver Nanoparticles. Analyst 2018, 143, 3867–3873. [Google Scholar] [CrossRef]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Distance-Based Tear Lactoferrin Assay on Microfluidic Paper Device Using Interfacial Interactions on Surface-Modified Cellulose. ACS Appl. Mater. Interfaces 2015, 7, 24864–24875. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nguyen, M.P.; Ibrahim, S.; Yoshioka, N.; Henry, C.S.; Tjahjono, D.H. A Selective Distance-Based Paper Analytical Device for Copper (II) Determination Using a Porphyrin Derivative. Talanta 2017, 174, 493–499. [Google Scholar] [CrossRef]
- Shahvar, A.; Saraji, M.; Gordan, H.; Shamsaei, D. Combination of Paper-based thin film microextraction with smartphone-based sensing for sulfite assay in food samples. Talanta 2019, 197, 578–583. [Google Scholar] [CrossRef]
- Li, D.; Duan, H.; Ma, Y.; Deng, W. Headspace-sampling paper-based analytical device for colorimetric/surface-enhanced raman scattering dual sensing of sulfur dioxide in wine. Anal. Chem. 2018, 90, 5719–5727. [Google Scholar] [CrossRef]
- Lim, S.H.; Martino, R.; Anikst, V.; Xu, Z.; Mix, S.; Benjamin, R.; Schub, H.; Eiden, M.; Rhodes, P.A.; Banaei, N. Rapid diagnosis of tuberculosis from analysis of urine volatile organic compounds. ACS Sens. 2016, 1, 852–856. [Google Scholar] [CrossRef]
- Tang, S.; Qi, T.; Xia, D.; Xu, M.; Xu, M.; Zhu, A.; Shen, W.; Lee, H.K. Smartphone nano-colorimetric determination of hydrogen sulfide in bio samples after silver-sold core-shell nanoprism-based headspace single-drop microextraction. Anal. Chem. 2019, 91, 5888–5895. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Villar-Blanco, L.; Lavilla, I.; Bendicho, C. Test for arsenic speciation in waters based on a paper-based analytical device with scanometric detection. Anal. Chim. Acta 2018, 1011, 1–10. [Google Scholar] [CrossRef]
- Huang, K.; Dai, R.; Deng, W.; Guo, S.; Deng, H.; Wei, Y.; Zhou, F.; Long, Y.; Li, J.; Yuan, X.; et al. Gold nanoclusters immobilized paper for visual detection of zinc in whole blood and cells by coupling hydride generation with headspace solid phase extraction. Sens. Actuators B Chem. 2018, 255, 1631–1639. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.L.; Hillier, J. A study of the nucleation and growth process in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Express, Environmental. SimpledistTM System Operating & Instruction Manual; Environmental Express: Charleston, SC, USA, 2015. [Google Scholar]



| Samples | Developed Method (±SD, n = 3) Headspace Distillation | IC Method (±SD, n = 3) |
|---|---|---|
| CN− found (mg L−1) | CN− found (mg L−1) | |
| 1 | 3.96 ± 0.13 3.51 ± 0.17 | 4.06 ± 0.08 |
| 2 | 9.26 ± 0.14 8.46 ± 0.12 | 8.58 ± 0.13 |
| 3 | 9.10 ± 0.04 10.58 ± 0.81 | 9.43 ± 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatha, P.; Phutthaphongloet, T.; Timpa, P.; Ninwong, B.; Income, K.; Ratnarathorn, N.; Dungchai, W. Distance-Based Paper Device Combined with Headspace Extraction for Determination of Cyanide. Sensors 2019, 19, 2340. https://doi.org/10.3390/s19102340
Khatha P, Phutthaphongloet T, Timpa P, Ninwong B, Income K, Ratnarathorn N, Dungchai W. Distance-Based Paper Device Combined with Headspace Extraction for Determination of Cyanide. Sensors. 2019; 19(10):2340. https://doi.org/10.3390/s19102340
Chicago/Turabian StyleKhatha, Papichaya, Thanyarat Phutthaphongloet, Phenphitcha Timpa, Benjawan Ninwong, Kamolwich Income, Nalin Ratnarathorn, and Wijitar Dungchai. 2019. "Distance-Based Paper Device Combined with Headspace Extraction for Determination of Cyanide" Sensors 19, no. 10: 2340. https://doi.org/10.3390/s19102340






