Improvement of Left Ventricular Ejection Time Measurement in the Impedance Cardiography Combined with the Reflection Photoplethysmography
Abstract
1. Introduction
2. Materials and Methods
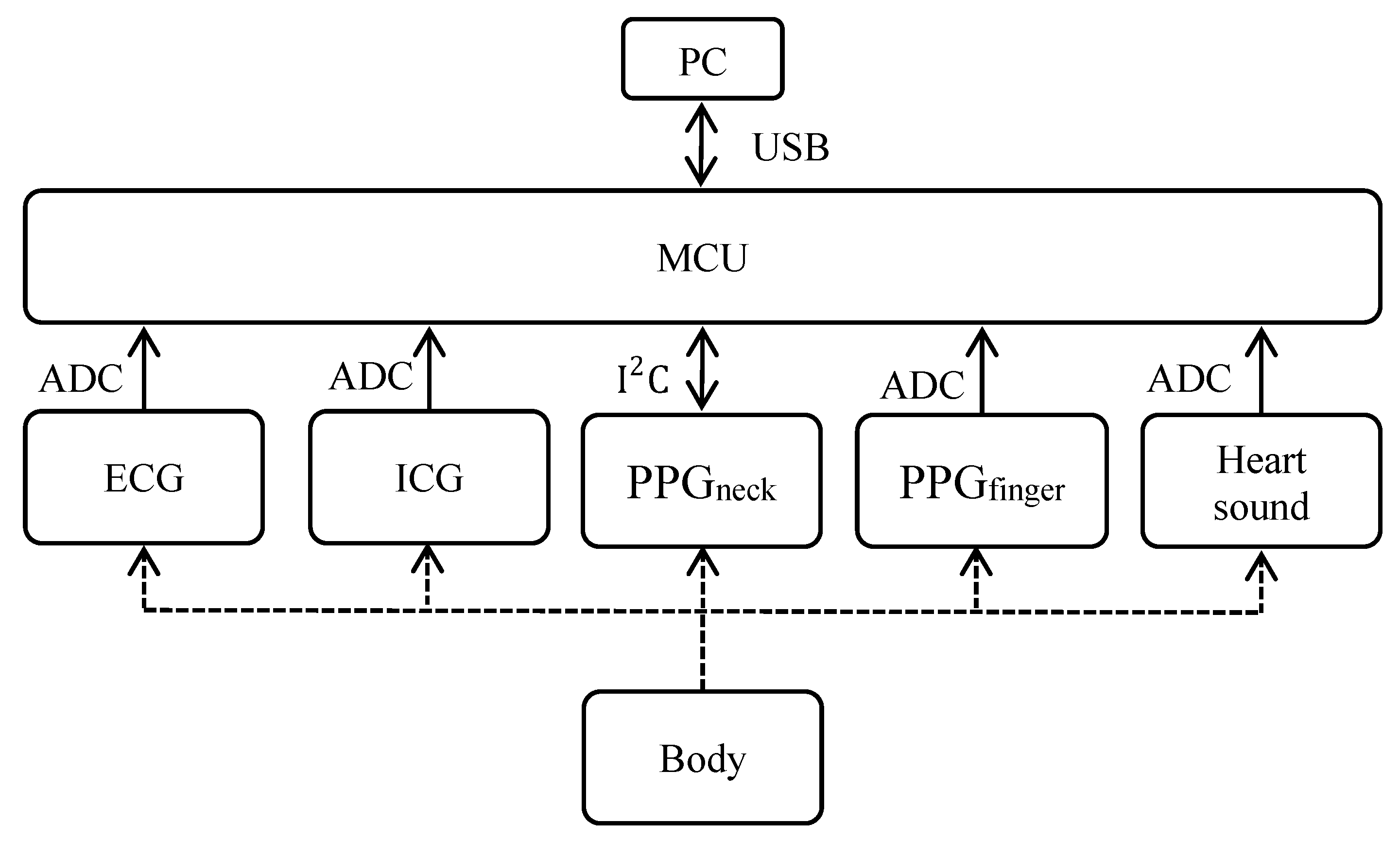
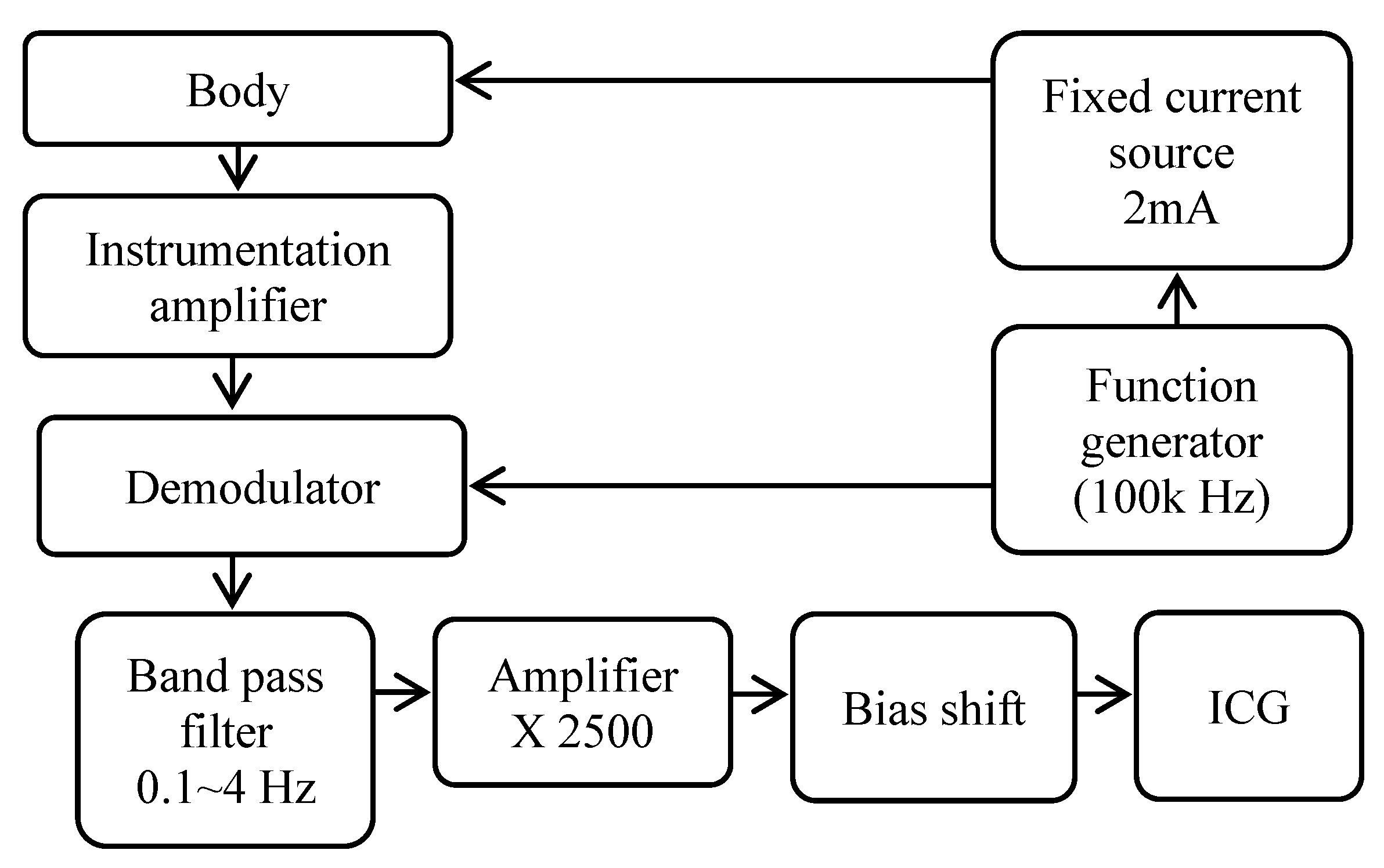
2.1. Hardware Architecture
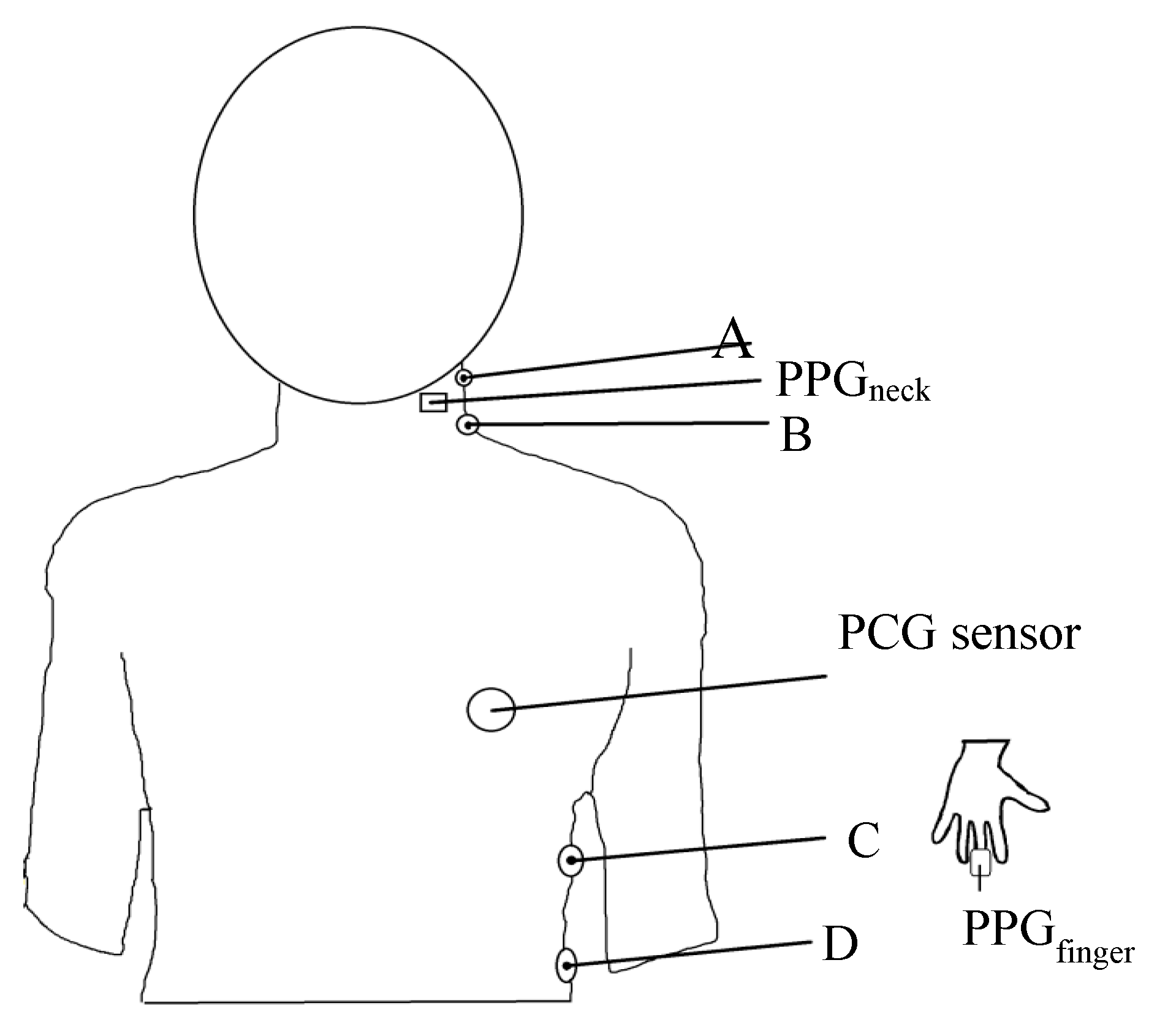
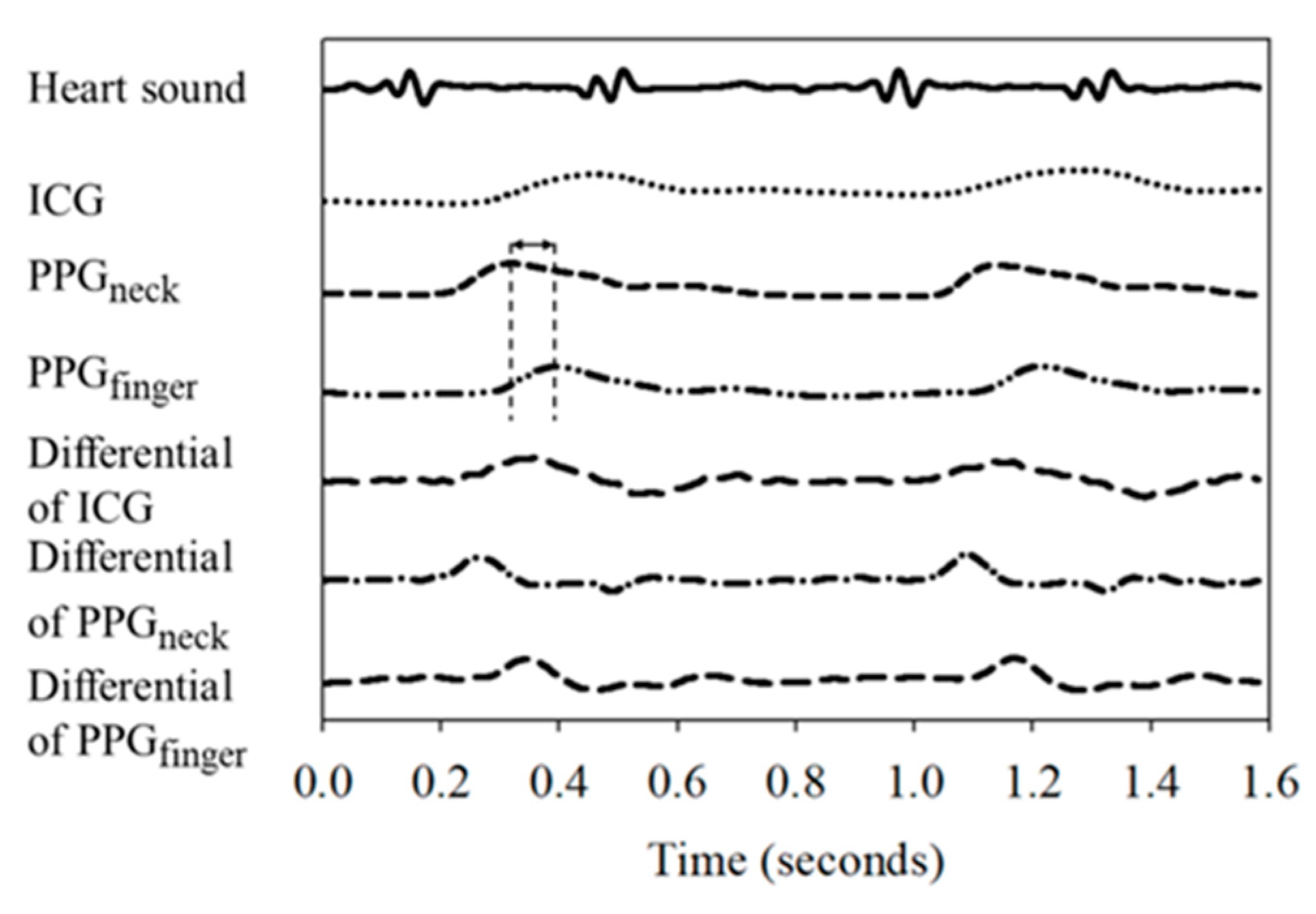
2.2. Arrangement of Placement of Sensing Elements
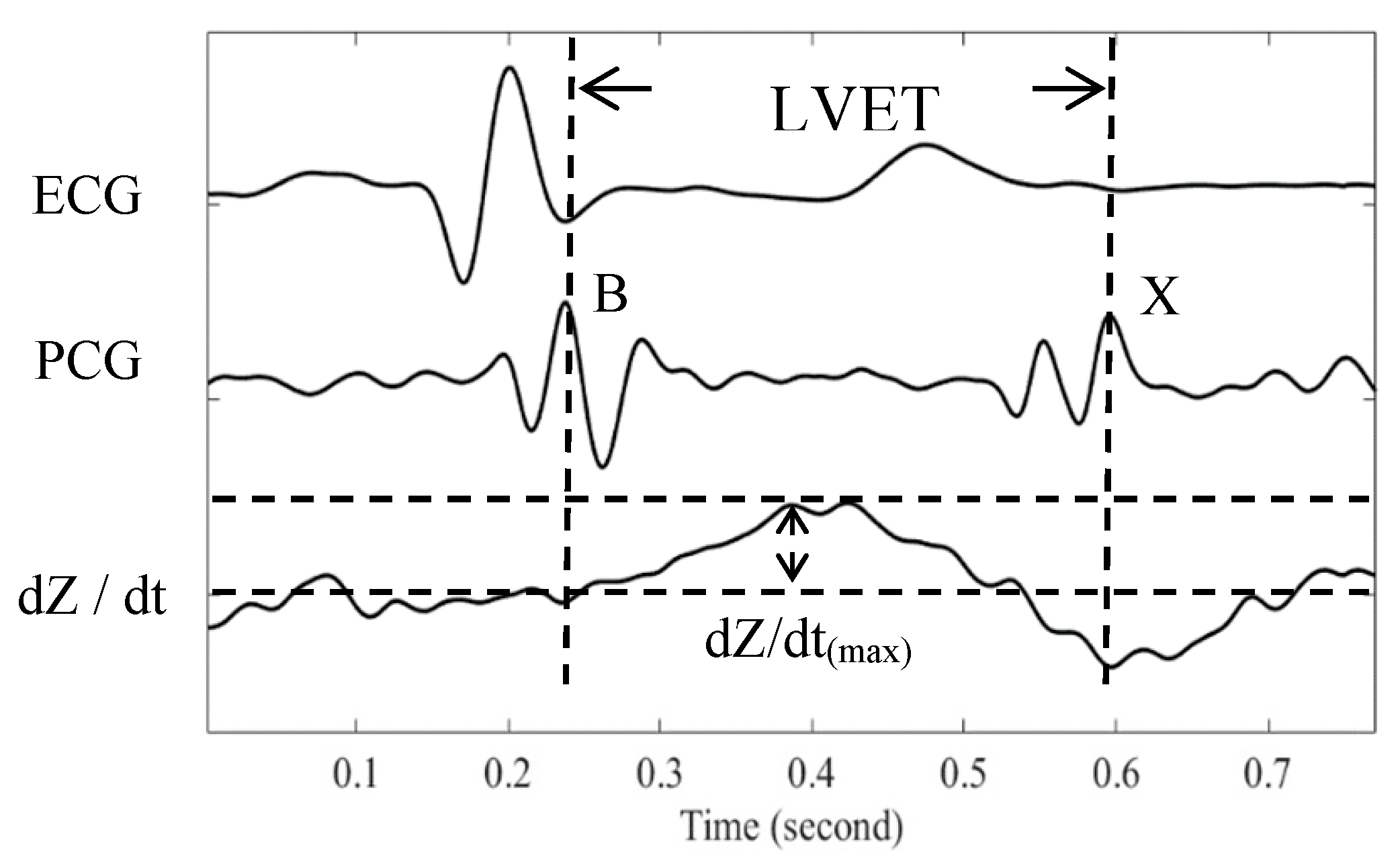
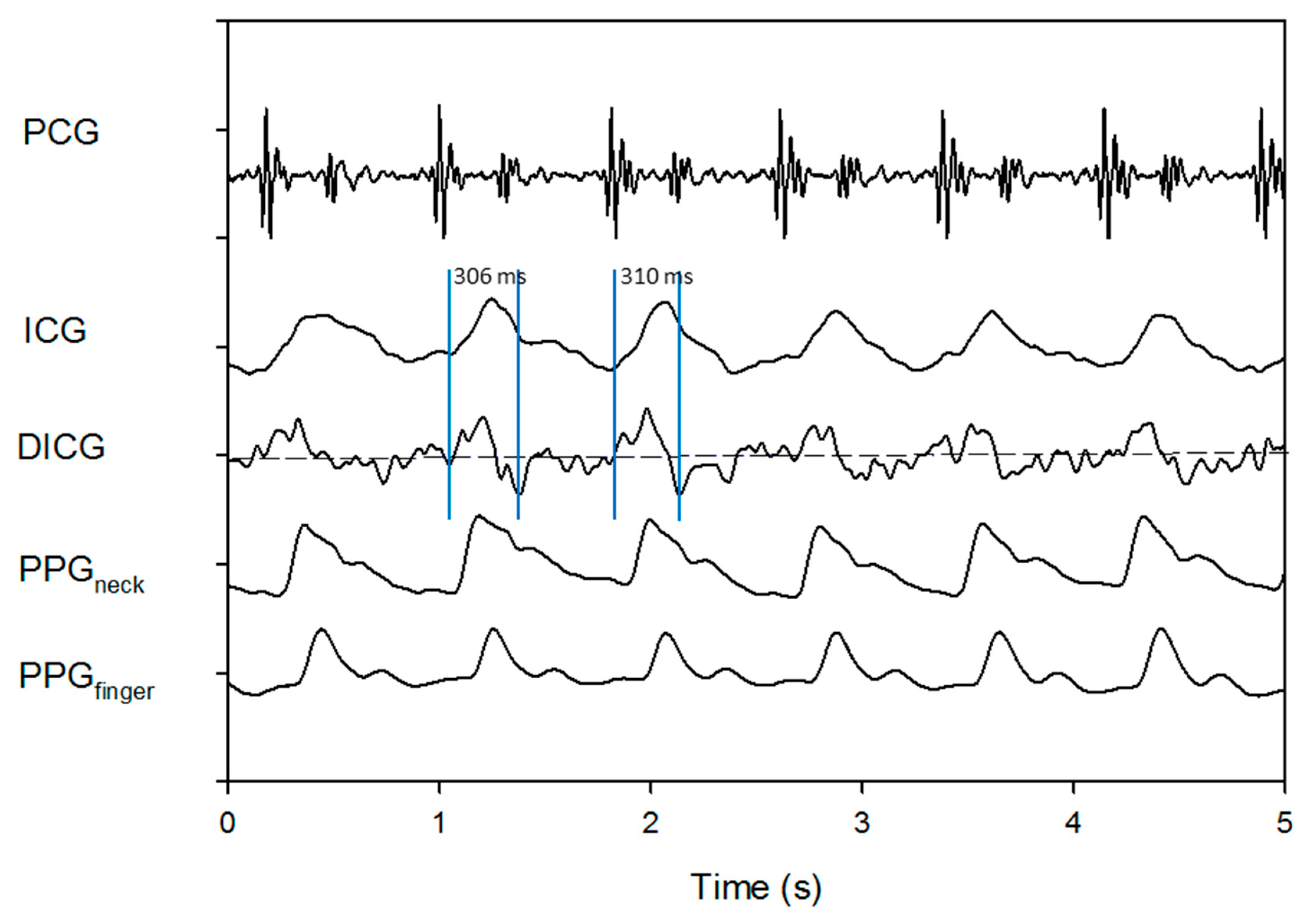
2.3. Stroke Volume Measurement
2.4. Experimental Protocol
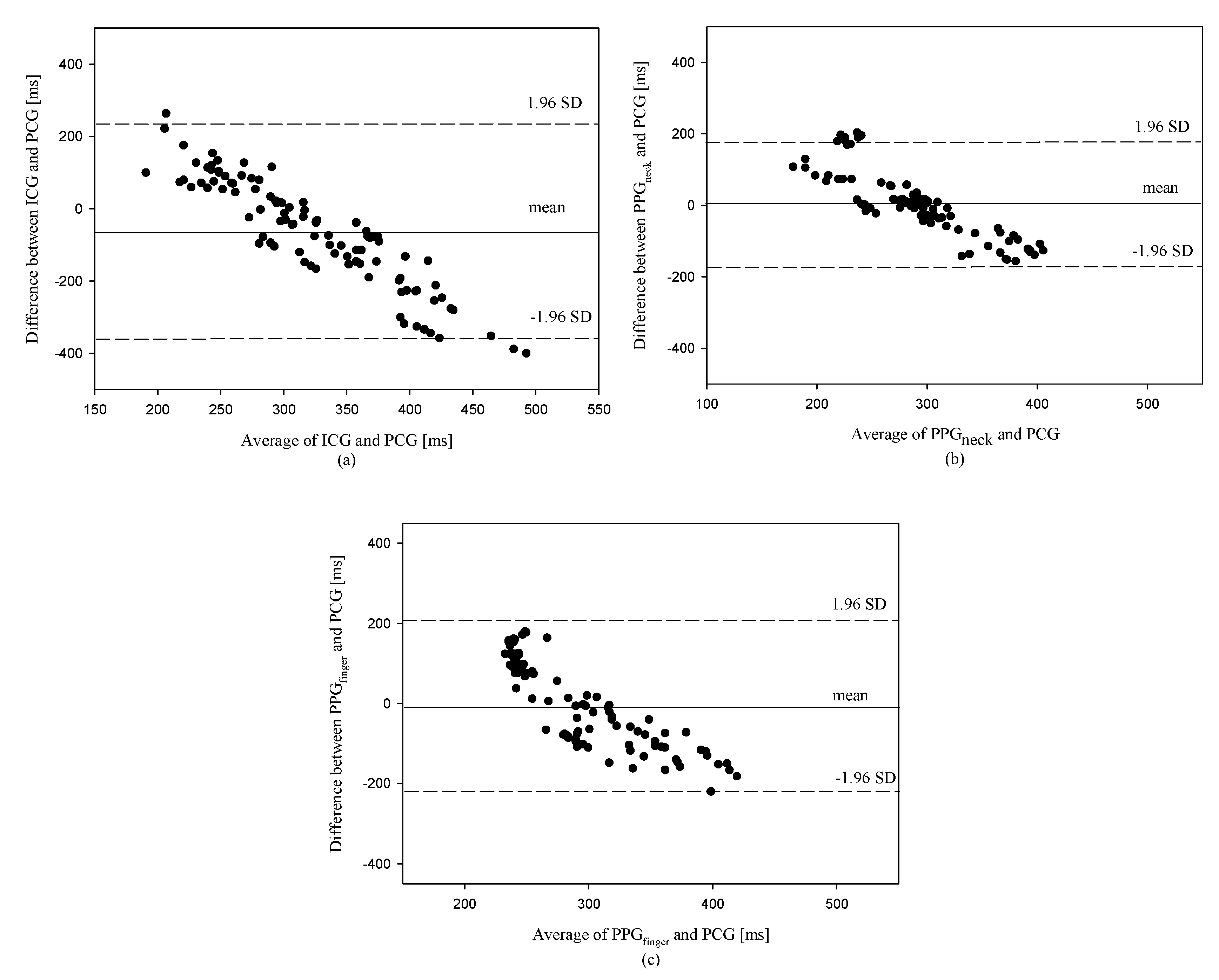
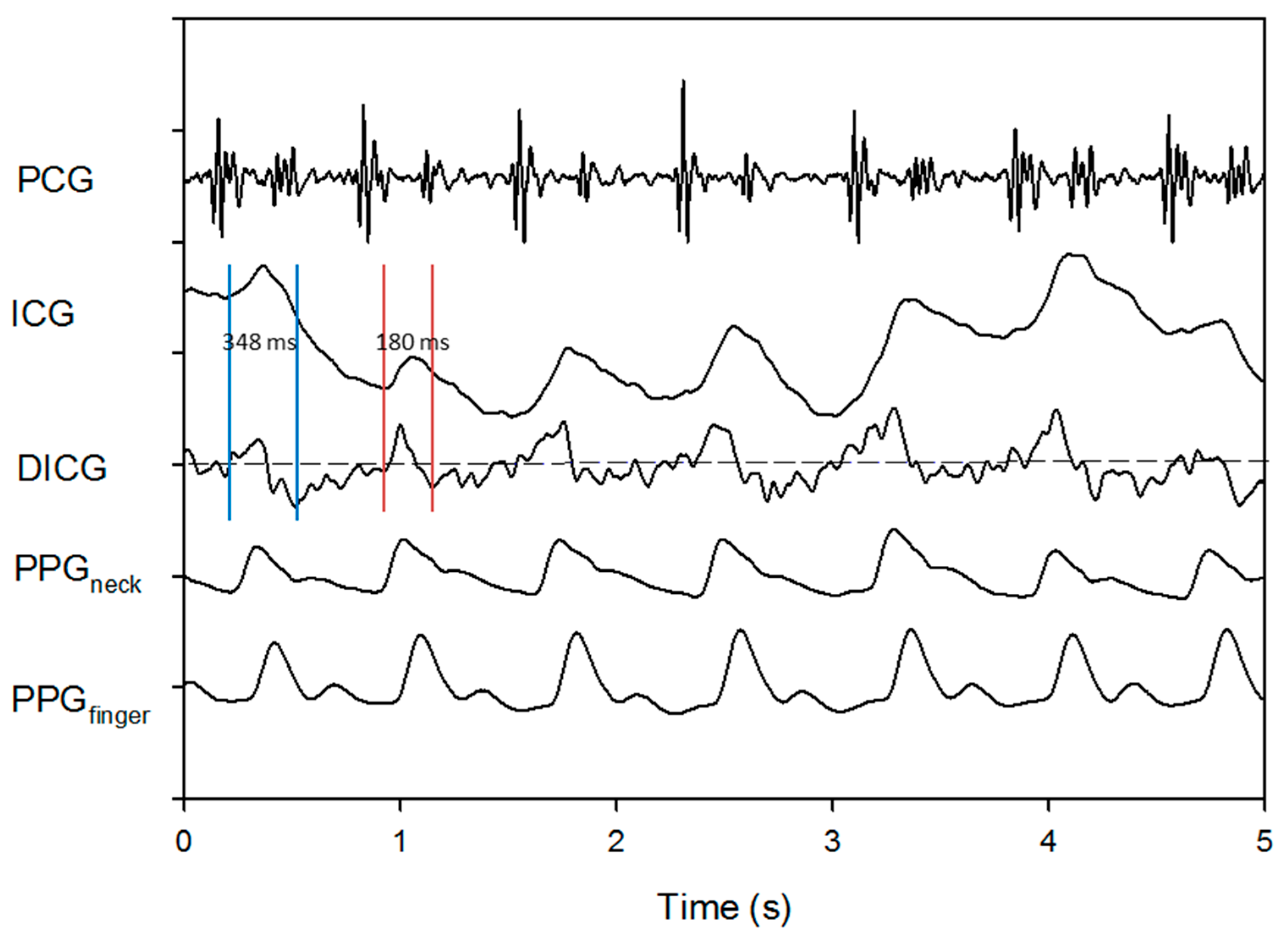
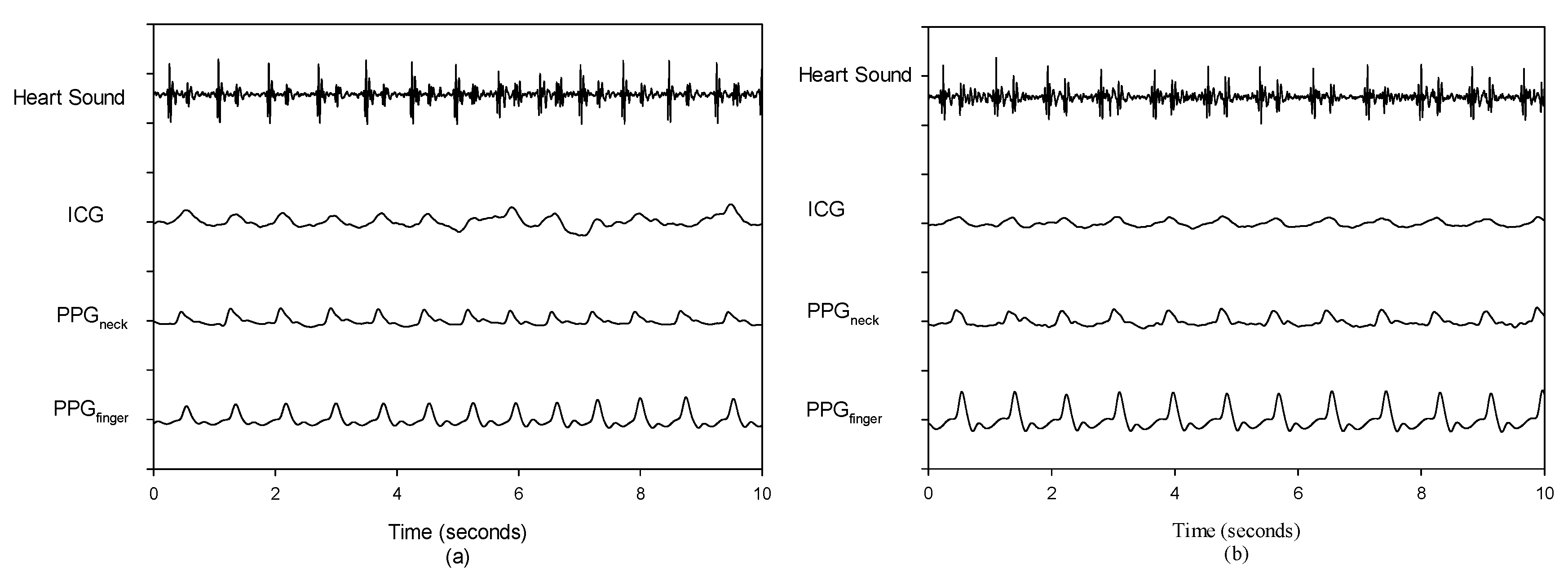
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Kern, J.W.; Shoemaker, W.C. Meta-analysis of hemodynamic optimization in high-risk patients. Crit. Care Med. 2002, 30, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Michard, F.; Teboul, J.L. Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest 2002, 121, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.J.; Brown, J.M. Introduction to Biomedical Equipment Technology; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Kubicek, W.G.; Karnegis, J.N.; Patterson, R.P.; Witsoe, D.A.; Mattson, R.H. Development and evaluation of an impedance cardiograph system. Aerosp. Med. 1966, 37, 1208–1212. [Google Scholar] [PubMed]
- Bernstein, D.P. A new stroke volume equation for thoracic electrical bioimpedance: Theory and rationale. Crit. Care Med. 1986, 14, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Coté, C.J.; Sui, J.; Anderson, T.A.; Bhattacharya, S.T.; Shank, E.S.; Tuason, P.M.; August, D.A.; Zibaitis, A.; Firth, P.G.; Fuzaylov, G.; et al. Continuous noninvasive cardiac output in children: Is this the next generation of operating room monitors? Initial experience in 402 pediatric patients. Pediatr. Anesth. 2015, 25, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pian-Smith, M.M.; Leffert, L.R.; Minehart, R.D.; Torri, A.; Coté, C.; Kacmarek, R.M.; Jiang, Y. Continuous measurement of cardiac output with the electrical velocimetry method in patients under spinal anesthesia for cesarean delivery. J. Clin. Monit. Comput. 2015, 29, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Hasija, S.; Chauhan, S.; Makhija, N. Comparison of the hemodynamic effects of the induction agents ketamine, etomidate and sevoflurane using the model of electrical velocimetry based cardiac output monitoring in pediatric cardiac surgical patients. World J. Cardiovasc. Surg. 2014, 4, 167–175. [Google Scholar] [CrossRef]
- DeMarzo, A.P. Using impedance cardiography with postural change to stratify patients with hypertension. Ther. Adv. Cardiovasc. Dis. 2011, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Charioux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Branson, R.D.; Mannheimer, P.D. Forehead oximetry in critically ill patients: The case for a new monitoring sit. Respir. Care Clin. N. Am. 2004, 10, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Cybulski1, G.; Strasz, A.; Niewiadomski, W.; Gąsiorowska, A. Impedance cardiography: Recent advancements. Cardiol. J. 2012, 19, 550–556. [Google Scholar] [CrossRef]
- Limper, U.; Gauger, P.; Beck, L.E.J. Upright cardiac output measurements in the transition to weightlessness during parabolic flights. Aviat. Space Environ. Med. 2011, 82, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Gielerak, G.; Piotrowicz, E.; Krzesiński, P.; Kowal, J.; Grzęda, M.; Piotrowicz, R. The effects of cardiac rehabilitation on hemodynamic parameters measured by impedance cardiography in patients with heart failure. Kardiol. Pol. 2011, 69, 309–317. [Google Scholar] [PubMed]
- Khan, F.Z.; Virdee, M.S.; Hutchinson, J.; Smith, B.; Pugh, P.J.; Read, P.A.; Fynn, S.P.; Dutka, D.P. Cardiac resynchronization therapy optimization using noninvasive cardiac output measurement. Pacing Clin. Electrophysiol. 2011, 34, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- De Zambotti, M.; Covassin, N.; De Min Tona, G.; Sarlo, M.; Stegagno, L. Sleep onset and cardiovascular activity in primary insomnia. J. Sleep Res. 2011, 20, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, J.S.; Bakker, J.P.; Rahangdale, S.; Yim-Yeh, S.; Mietus, J.E.; Goldberger, A.L.; Malhotra, A. Effect of mild, asymptomatic obstructive sleep apnea on daytime heart rate variability and impedance cardiography measurements. Am. J. Cardiol. 2012, 109, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Zhang, Y.; Webster, J.G.; Tompkins, W.J. Motion artifact from spot and band electrodes during impedance cardiography. IEEE Trans. Biomed. Eng. 1986, 33, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- ADPD174GGI Datasheet and Product Info | Analog Devices. Available online: http://www.analog.com/en/products/adpd174ggi.html (accessed on 10 September 2018).
- MAX30101 Datasheet and Product Info | Maxim Integrated. Available online: https://www.maximintegrated.com/en/products/sensors/MAX30101.html (accessed on 10 September 2018).
- Newlin, D.B.; Levenson, R.W. Pre-ejection period: Beta-adrenergic influences upon the heart. Psychophysiology 1979, 16, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Levison, G.E.; Schwartz, C.J.; Ettinger, P.D. Systolic time intervals as measures of contractile state of the left ventricular myocardium in man. Circulation 1972, 46, 559–571. [Google Scholar] [CrossRef] [PubMed]
| Subject No. | PCG (ms) | ICG (ms) | PPGneck (ms) | PPGfinger (ms) |
|---|---|---|---|---|
| 1 | 337.8 ± 96.1 | 286.8 ± 12.4 | 376.2 ± 81.4 | 293.8 ± 10.8 |
| 2 | 413 ± 46.7 | 439.4 ± 26 | 453.4 ± 45.6 | 331.2 ± 5.1 |
| 3 | 506.8 ± 109.9 | 246.8 ± 9.9 | 339.6 ± 13 | 242.2 ± 2.4 |
| 4 | 351.4 ± 92.3 | 275.8 ± 6.8 | 204.2 ± 7 | 283.2 ± 2.5 |
| 5 | 338 ± 90.6 | 286.4 ± 7.7 | 300.2 ± 97.6 | 300 ± 7 |
| 6 | 193.8 ± 86 | 137.8 ± 7.4 | 161.4 ± 2.3 | 321.4 ± 10.8 |
| 7 | 199.2 ± 25.4 | 422.4 ± 32.9 | 193.6 ± 29.2 | 296.8 ± 23.1 |
| 8 | 506.8 ± 132.6 | 322.2 ± 14.4 | 305.2 ± 119.1 | 285.8 ± 6.9 |
| 9 | 452 ± 117.3 | 278.2 ± 37.9 | 309 ± 18.3 | 302.4 ± 9.3 |
| 10 | 288.2 ± 98.2 | 162 ± 25.3 | 332.6 ± 45.9 | 248.6 ± 11.1 |
| Subjects No. | ICG | PPGneck | PPGfinger |
|---|---|---|---|
| 1 | 0.001 | 0.435 | 0.070 |
| 2 | 0.493 | 0.028 | 0.001 |
| 3 | 0.004 | 0.131 | 0.017 |
| 4 | 2.31 × 10−6 | 0.309 | 0.002 |
| 5 | 0.153 | 0.680 | 0.342 |
| 6 | 0.065 | 0.042 | 0.025 |
| 7 | 0.281 | 0.187 | 0.350 |
| 8 | 0.240 | 0.101 | 0.359 |
| 9 | 0.384 | 0.347 | 0.124 |
| 10 | 0.161 | 0.441 | 0.001 |
| All | 0.178 ± 0.162 | 0.270 ± 0.199 | 0.129 ± 0.149 |
| Subjects No. | ICG (ms) | PPGneck (ms) | PPGfinger (ms) |
|---|---|---|---|
| 1 | −44.0 ± 97.0 | 7.0 ± 9.7 | −82.4 ± 79.2 |
| 2 | −81.8 ± 43.3 | −108.2 ± 25.7 | −122.2 ± 45.7 |
| 3 | −264.6 ± 110.0 | −4.6 ± 11.0 | −97.4 ± 12.9 |
| 4 | −68.2 ± 92.4 | 7.4 ± 5.8 | 79.0 ± 7.5 |
| 5 | −38.0 ± 93.5 | 13.6 ± 4.4 | −0.2 ± 93.7 |
| 6 | 127.6 ± 83.9 | 183.6 ± 11.8 | 160.0 ± 11.4 |
| 7 | 97.6 ± 23.6 | −125.6 ± 31.0 | 103.2 ± 46.7 |
| 8 | −221.0 ± 129.4 | −36.4 ± 13.9 | −19.4 ± 123.4 |
| 9 | −149.6 ± 123.3 | 24.2 ± 33.3 | −6.6 ± 17.3 |
| 10 | −39.6 ± 103.2 | 86.6 ± 19.8 | −84.0 ± 47.6 |
| All | −68.2 ± 148.6 | 4.8 ± 86.5 | −7.0 ± 107.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Wang, J.-J.; Su, C.-H.; Cheng, D.-C. Improvement of Left Ventricular Ejection Time Measurement in the Impedance Cardiography Combined with the Reflection Photoplethysmography. Sensors 2018, 18, 3036. https://doi.org/10.3390/s18093036
Liu S-H, Wang J-J, Su C-H, Cheng D-C. Improvement of Left Ventricular Ejection Time Measurement in the Impedance Cardiography Combined with the Reflection Photoplethysmography. Sensors. 2018; 18(9):3036. https://doi.org/10.3390/s18093036
Chicago/Turabian StyleLiu, Shing-Hong, Jia-Jung Wang, Chun-Hung Su, and Da-Chuan Cheng. 2018. "Improvement of Left Ventricular Ejection Time Measurement in the Impedance Cardiography Combined with the Reflection Photoplethysmography" Sensors 18, no. 9: 3036. https://doi.org/10.3390/s18093036
APA StyleLiu, S.-H., Wang, J.-J., Su, C.-H., & Cheng, D.-C. (2018). Improvement of Left Ventricular Ejection Time Measurement in the Impedance Cardiography Combined with the Reflection Photoplethysmography. Sensors, 18(9), 3036. https://doi.org/10.3390/s18093036







