Fecal Volatile Organic Compounds in Preterm Infants Are Influenced by Enteral Feeding Composition
Abstract
:1. Introduction
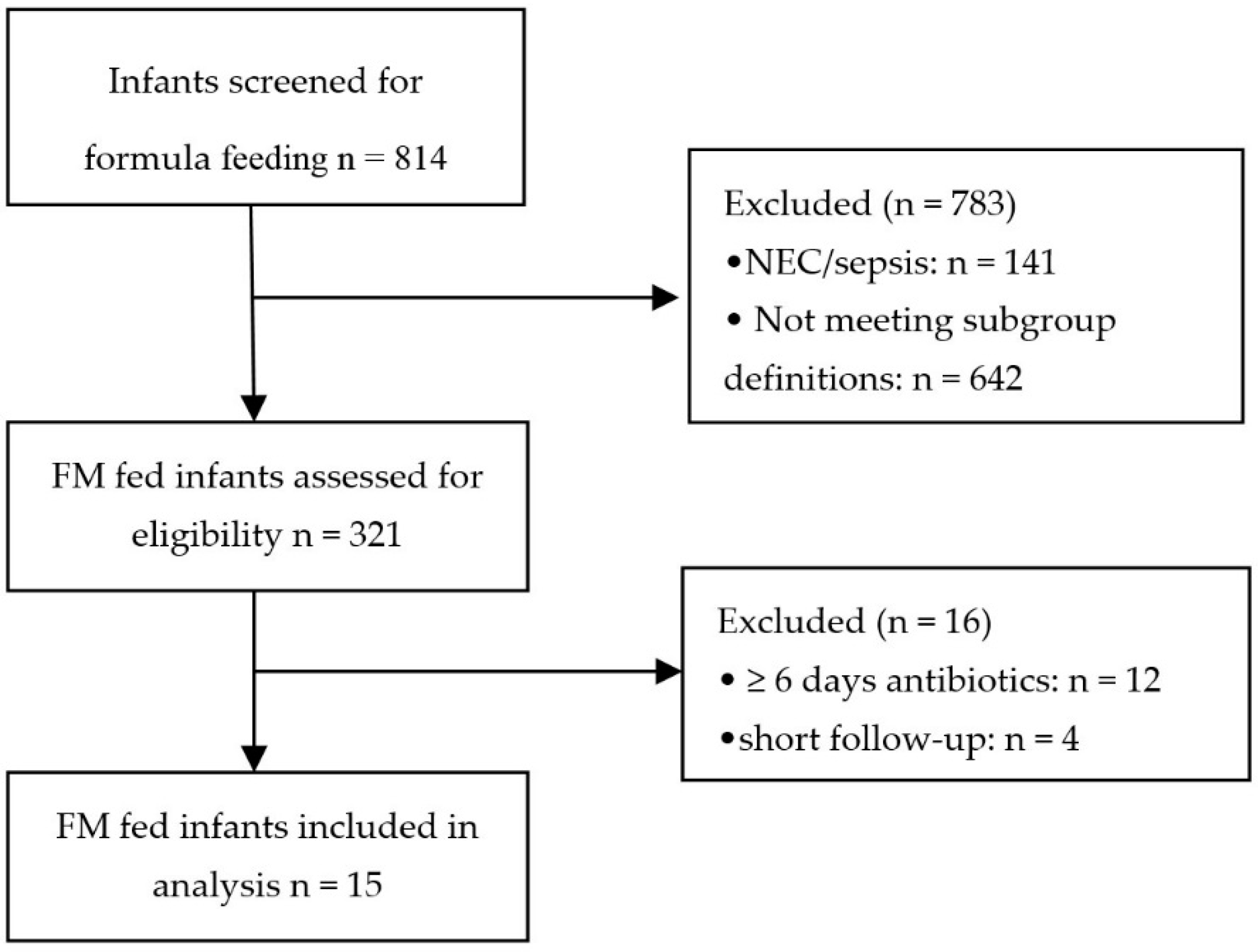
2. Materials and Methods
2.1. Subjects
2.2. Study Groups
2.3. Sample Collection
2.4. VOC Analysis by eNose
2.5. Statistical Analysis
2.5.1. Demographic and Clinical Data
2.5.2. eNose Data
3. Results
3.1. Patient Population
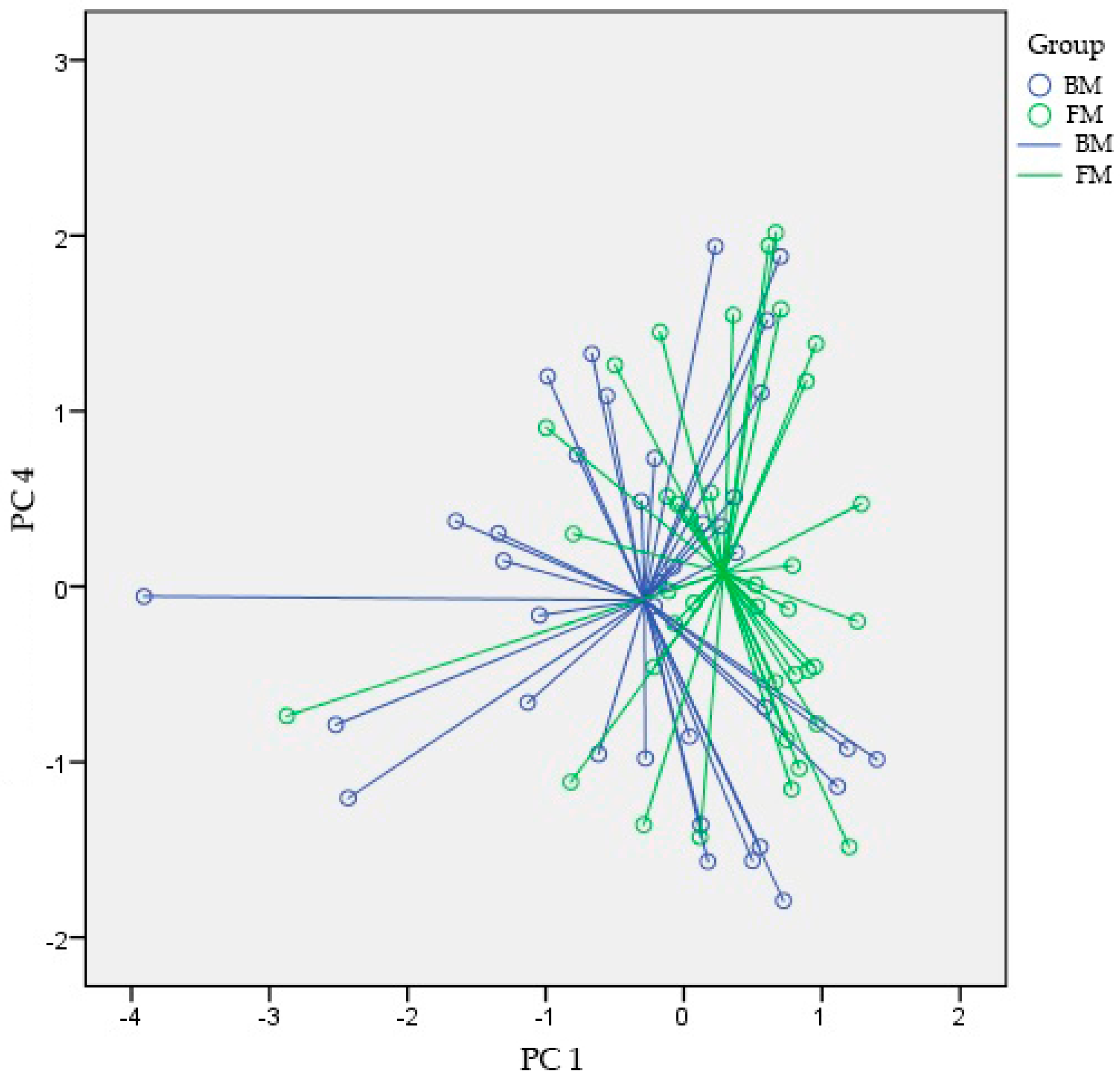
3.2. VOC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lough, F.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Detection of exogenous VOCs as a novel in vitro diagnostic technique for the detection of pathogenic bacteria. TrAC Trends Anal. Chem. 2017, 87, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Probert, C.S.; Khalid, T.; Ahmed, I.; Johnson, E.; Smith, S.; Ratcliffe, N.M. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J. Gastrointest. Liver Dis. 2009, 18, 337–343. [Google Scholar]
- Walton, C.; Fowler, D.P.; Turner, C.; Jia, W.; Whitehead, R.N.; Griffiths, L.; Dawson, C.; Waring, R.H.; Ramsden, D.B.; Cole, J.A.; et al. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflamm. Bowel Dis. 2013, 19, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Buijck, M.; Berkhout, D.J.; de Groot, E.F.; Benninga, M.A.; van der Schee, M.P.; Kneepkens, C.M.F.; de Boer, N.K.; de Meij, T.G. Sniffing out Paediatric Gastrointestinal Diseases: The Potential of Volatile Organic Compounds as Biomarkers for Disease. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 585–591. [Google Scholar] [CrossRef] [PubMed]
- De Meij, T.G.; van der Schee, M.P.; Berkhout, D.J.; van de Velde, M.E.; Jansen, A.E.; Kramer, B.W.; van Weissenbruch, M.M.; van Kaam, A.H.; Andriessen, P.; van Goudoever, J.B.; et al. Early Detection of Necrotizing Enterocolitis by Fecal Volatile Organic Compounds Analysis. J. Pediatr. 2015, 167, 562–567. [Google Scholar] [CrossRef] [PubMed]
- De Meij, T.G.; De Boer, N.K.; Benninga, M.A.; Lentferink, Y.E.; de Groot, E.F.; van de Velde, M.E.; van Bodegraven, A.A.; van der Schee, M.P. Faecal gas analysis by electronic nose as novel, non-invasive method for assessment of active and quiescent paediatric inflammatory bowel disease: Proof of principle study. J. Crohns Colitis 2014. [Google Scholar] [CrossRef] [PubMed]
- De Meij, T.G.; Larbi, I.B.; van der Schee, M.P.; Lentferink, Y.E.; Paff, T.; Terhaar sive Droste, J.S.; Mulder, C.J.; van Bodegraven, A.A.; de Boer, N.K. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: Proof of principle study. Int. J. Cancer 2014, 134, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.E.; Ewer, A.K.; Elasouad, K.; Power, F.; Greenwood, R.; Ratcliffe, N.M.; de Lacy Costello, B.; Probert, C.S. Analysis of faecal volatile organic compounds in preterm infants who develop necrotising enterocolitis: A pilot study. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.; Niemarkt, H.J.; Buijck, M.; Van Weissenbruch, M.M.; Brinkman, P.; Benninga, M.A.; van Kaam, A.H.; Kramer, B.W.; Andriessen, P.; de Boer, N.K.; et al. Detection of Sepsis in Preterm Infants by Fecal Volatile Organic Compounds Analysis: A Proof of Principle Study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e47–e52. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.; Niemarkt, H.J.; Benninga, M.A.; Budding, A.E.; van Kaam, A.H.; Kramer, B.W.; Pantophlet, C.M.; van Weissenbruch, M.M.; de Boer, N.K.; de Meij, T.G. Development of severe bronchopulmonary dysplasia is associated with alterations in fecal volatile organic compounds. Pediatr. Res. 2018, 83, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.C.; van Keulen, B.J.; Niemarkt, H.J.; Bessem, J.R.; de Boode, W.P.; Cossey, V.; Hoogenes, N.; Hulzebos, C.V.; Klaver, E.; Andriessen, P.; et al. Late-onset sepsis in preterm infants can be detected preclinically by fecal volatile organic compound analysis: A prospective, multicenter cohort study. Clin. Infect. Dis. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Covington, J.A.; Harmston, C.; Nwokolo, C.U. Review article: Next generation diagnostic modalities in gastroenterology—Gas phase volatile compound biomarker detection. Aliment. Pharmacol. Ther. 2014, 39, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases (dagger). Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Covington, J.A.; Wedlake, L.; Andreyev, J.; Ouaret, N.; Thomas, M.G.; Nwokolo, C.U.; Bardhan, K.D.; Arasaradnam, R.P. The detection of patients at risk of gastrointestinal toxicity during pelvic radiotherapy by electronic nose and FAIMS: A pilot study. Sensors 2012, 12, 13002–13018. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N.; Bailey, C.; Nwokolo, C.; Bardhan, K.D.; Covington, J.A. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Ouaret, N.; Thomas, M.G.; Gold, P.; Quraishi, M.N.; Nwokolo, C.U.; Bardhan, K.D.; Covington, J.A. Evaluation of gut bacterial populations using an electronic e-nose and field asymmetric ion mobility spectrometry: Further insights into ‘fermentonomics’. J. Med. Eng. Technol. 2012, 36, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.; Benninga, M.A.; van Stein, R.M.; Brinkman, P.; Niemarkt, H.J.; de Boer, N.K.; de Meij, T.G. Effects of Sampling Conditions and Environmental Factors on Fecal Volatile Organic Compound Analysis by an Electronic Nose Device. Sensors 2016, 16, 1967. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; el Manouni el Hassani, S.; Covington, J.A.; Wicaksono, A.N.; Bomers, M.K.; Benninga, M.A.; Mulder, C.J.; de Boer, N.K.; de Meij, T.G. Optimized Sampling Conditions for Fecal Volatile Organic Compound Analysis by Means of Field Asymmetric Ion Mobility Spectrometry. Anal. Chem. 2018, 90, 7972–7981. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.H.; Tsai, M.H.; Lai, M.Y.; Hsu, L.F.; Chiang, M.C.; Lien, R.; Fu, R.H.; Huang, H.R.; Chu, S.M.; Hsu, J.F. Incidence, clinical features, and implications on outcomes of neonatal late-onset sepsis with concurrent infectious focus. BMC Infect. Dis. 2017, 17, 465. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.; Klaassen, P.; Niemarkt, H.J.; de Boode, W.P.; Cossey, V.; van Goudoever, J.B.; Hulzebos, C.V.; Andriessen, P.; van Kaam, A.H.; Kramer, B.W.; et al. Risk Factors for Necrotizing Enterocolitis: A Prospective Multicenter Case-Control Study. Neonatology 2018, 114, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Corvaglia, L.; Fantini, M.P.; Aceti, A.; Gibertoni, D.; Rucci, P.; Baronciani, D.; Faldella, G. Predictors of full enteral feeding achievement in very low birth weight infants. PLoS ONE 2014, 9, e92235. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Judge, M.; Xu, W.; Diallo, A.; Janton, S.; Brownell, E.A.; Maas, K.; Graf, J. Influence of Feeding Type on Gut Microbiome Development in Hospitalized Preterm Infants. Nurs. Res. 2017, 66, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, X.; Xu, W.; Janton, S.; Henderson, W.A.; Matson, A.; McGrath, J.M.; Maas, K.; Graf, J. Gut Microbiome Developmental Patterns in Early Life of Preterm Infants: Impacts of Feeding and Gender. PLoS ONE 2016, 11, e0152751. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.E.; Samuel, B.S.; Houghteling, P.; Shan, G.; Ausubel, F.M.; Sadreyev, R.I.; Walker, W.A. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 2016, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Hausner, H.; Philipsen, M.; Skov, T.H.; Petersen, M.A.; Bredie, W.L. Characterization of the Volatile Composition and Variations between Infant Formulas and Mother’s Milk. Chemosens. Percept. 2009, 2, 79–93. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [PubMed]
- Mshvildadze, M.; Neu, J.; Shuster, J.; Theriaque, D.; Li, N.; Mai, V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J. Pediatr. 2010, 156, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.; Sterk, P.J.; Schultz, M.J. Volatile metabolites of pathogens: A systematic review. PLoS Pathog. 2013, 9, e1003311. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E.; Shaikh, N.; Linneman, L.A.; et al. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, C.; Stigum, H.; Valeur, J.; Iszatt, N.; Lenters, V.; Peddada, S.; Bjørnholt, J.V.; Midtvedt, T.; Mandal, S.; Eggesbø, M. Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int. J. Epidemiol. 2018, dyy064. [Google Scholar] [CrossRef] [PubMed]
| Formula Fed Infants (n = 15) | Breastmilk Fed Infants (n = 15) | p-Value | |
|---|---|---|---|
| Gestational age (median [IQR], weeks + days) | 29 + 0 [28 + 1–29 + 6] | 29 + 1 [28 + 2–29 + 4] | 0.723 |
| Birth weight (median [IQR], g) | 1200 [1030–1280] | 1220 [1005–1285] | 0.740 |
| Gender Male (n[%]) | 10 [66.7] | 8 [53.3] | 0.464 |
| Delivery mode Vaginal delivery (n[%]) | 5 [33.3] | 7 [46.7] | 0.464 |
| Age at full enteral feeding (median [IQR] days) | 10 [8–12] | 9 [8–15] | 0.964 |
| Parental feeding (median [IQR], days) | 8 [7–10] | 8 [6–13] | 0.893 |
| t1 | 9 | 9 | |
| t2 | 0 | 2 | |
| t3 | 0 | 0 | |
| Type of parental feeding (n[%]) | |||
| Lipids | 3 [20] | 3 [20] | 0.878 |
| Combination Lipids and Proteins | 3 [20] | 3 [20] | |
| Intralipid 20% + vaminolact-glucose | 1 [6.7] | 2 [13.3] | |
| Smofolipid + vaminolact-glucose | 2 [13.3] | 1 [6.7] | |
| Enteral additives (n[%]) | |||
| None | 8 [53.3] | 2 [13.3] | 0.073 |
| MT | 1 [6.7] | 5 [33.3] | |
| PF | 1 [6.7] | 0 [0] | |
| MT and PF | 0 [0] | 1 [6.7] | |
| BMF | 4 [26.7] | 7 [46.7] | |
| Amino acid formula | 1 [6.7] | 0 [0] | |
| Received donor milk (n[%]) | 2 [13.3] | 0 [0] | 0.150 |
| Sample masses (median [IQR] in g) | |||
| t1 (n = 14) | 0.150 [0.148–0.151] | 0.151 [0.150–0.153] | 0.211 |
| t2 (n = 14) | 0.150 [0.148–0.153] | 0.150 [0.148–0.152] | 0.780 |
| t3 (n = 10) | 0.151 [0.150–0.152] | 0.151 [0.150–0.153] | 0.538 |
| Sample age (median (IQR], in months) | |||
| t1 (n = 14) | 28 [16.5–38] | 26 [18–36] | 0.913 |
| t2 (n = 14) | 27.5 [16.5–38] | 25.5 [18–36] | 0.836 |
| t3 (n = 10) | 27.5 [15–38] | 24.5 [18–37] | 0.850 |
| Postpartum antibiotics (n[%]) | 0.513 | ||
| Not administered | 1 [6.7] | 0 | |
| 1–3 days administered | 13 [86.7] | 13 [86.7] | |
| >3 days administered | 1 [6.7] | 2 [13.3] | |
| Antibiotic exposure (n[%]) | 15 [100] | 15 [100] | 1.000 |
| Antibiotics days (median (IQR]) | 2 [1–2] | 2 [1–2] | 0.128 |
| Medication (n[%]) | |||
| Antiviral medication | 2 [13.3] | 0 | 0.150 |
| Caffeine | 14 [93.3] | 15 [100] | 0.753 |
| Vitamin D | 9 [60] | 9 [60] | 1.000 |
| Vitamin E | 3 [20] | 2 [13.3] | 0.630 |
| Iron supplementation | 4 [26.7] | 6 [40] | 0.446 |
| Morphine | 3 [20] | 0 | 0.073 |
| Propofol | 2 [13.3] | 0 | 0.150 |
| Paracetamol | 1 [6.7] | 1 [6.7] | 1.000 |
| Ibuprofen | 1 [6.7] | 1 [6.7] | 1.000 |
| Indomethacin | 0 | 1 [6.7] | 0.317 |
| Chloral hydrate | 0 | 1 [6.7] | 0.317 |
| Nystatin | 0 | 2 [13.3] | 0.150 |
| Miconazole | 3 [20] | 1 [6.7] | 0.291 |
| Heparin | 1 [6.7] | 0 | 0.317 |
| Dopamine | 2 [13.3] | 0 | 0.150 |
| Dobutamine | 1 [6.7] | 0 | 0.317 |
| Hydrochlorothiazide | 2 [13.3] | 0 | 0.150 |
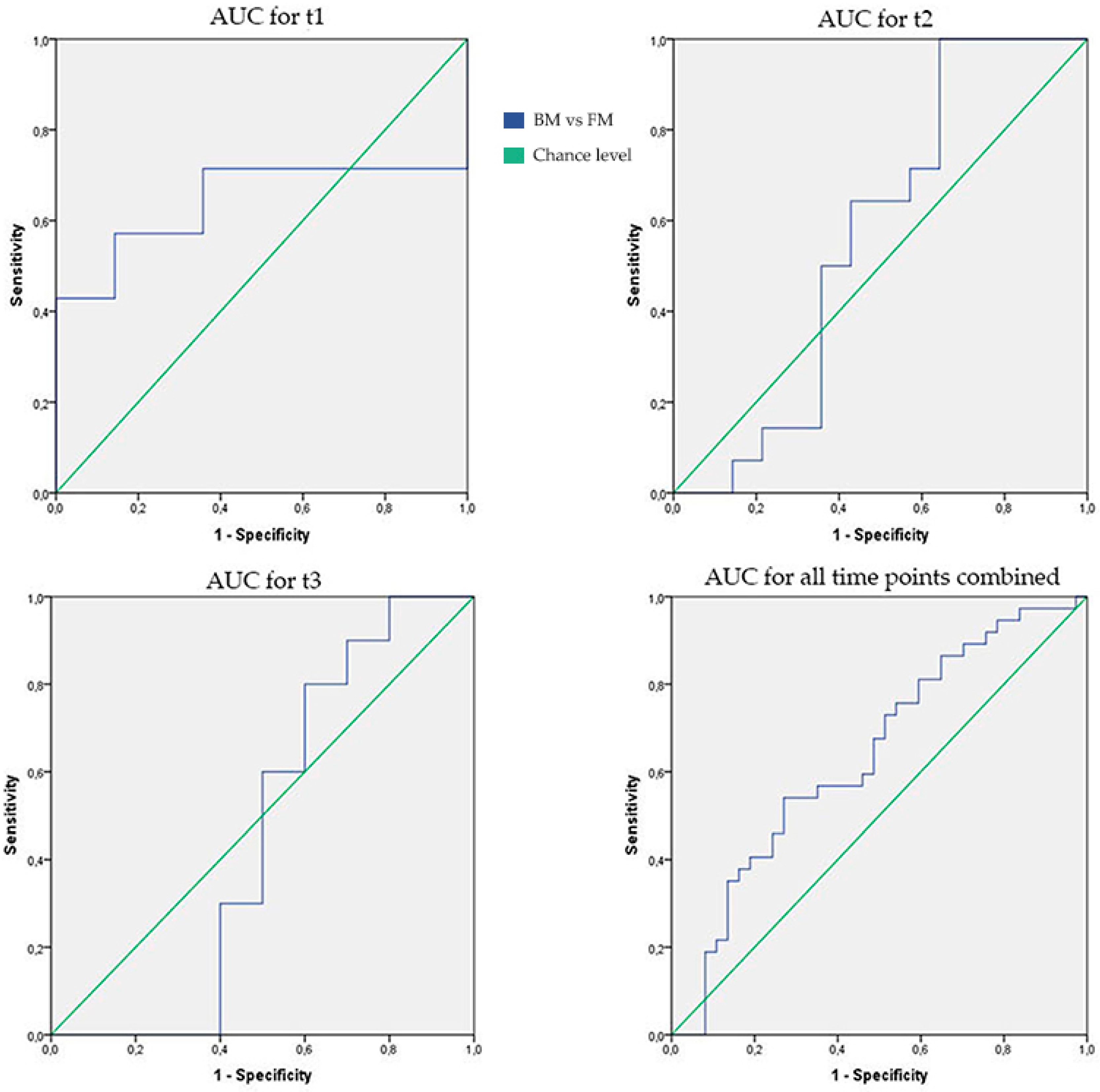
| AUC [95% CI] | p-Value | Sensitivity | Specificity | |
|---|---|---|---|---|
| t1 | 0.64 [0.41–0.87] | 0.20 | 71% | 64% |
| t2 | 0.56 [0.33–0.79] | 0.58 | 64% | 57% |
| t3 | 0.46 [0.18–0.74] | 0.76 | 60% | 50% |
| All time points combined | 0.64 [0.51–0.77] | 0.04 | 68% | 51% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Manouni el Hassani, S.; Niemarkt, H.J.; Said, H.; Berkhout, D.J.C.; Van Kaam, A.H.; Van Lingen, R.A.; Benninga, M.A.; De Boer, N.K.H.; De Meij, T.G.J. Fecal Volatile Organic Compounds in Preterm Infants Are Influenced by Enteral Feeding Composition. Sensors 2018, 18, 3037. https://doi.org/10.3390/s18093037
El Manouni el Hassani S, Niemarkt HJ, Said H, Berkhout DJC, Van Kaam AH, Van Lingen RA, Benninga MA, De Boer NKH, De Meij TGJ. Fecal Volatile Organic Compounds in Preterm Infants Are Influenced by Enteral Feeding Composition. Sensors. 2018; 18(9):3037. https://doi.org/10.3390/s18093037
Chicago/Turabian StyleEl Manouni el Hassani, Sofia, Hendrik J. Niemarkt, Hager Said, Daniel J. C. Berkhout, Anton H. Van Kaam, Richard A. Van Lingen, Marc A. Benninga, Nanne K. H. De Boer, and Tim G. J. De Meij. 2018. "Fecal Volatile Organic Compounds in Preterm Infants Are Influenced by Enteral Feeding Composition" Sensors 18, no. 9: 3037. https://doi.org/10.3390/s18093037





