Rapid and Sensitive Determination of Vanillin Based on a Glassy Carbon Electrode Modified with Cu2O-Electrochemically Reduced Graphene Oxide Nanocomposite Film
Abstract
:1. Introduction
2. Experiment
2.1. Chemical and Solutions
2.2. Apparatus
2.3. Synthesis of Cuprous Oxide
2.4. Preparation of Cu2O–GO Nanocomposite
2.5. Fabrication of Electrochemical Sensor
2.6. Electrochemical Measurements
3. Results and Discussion
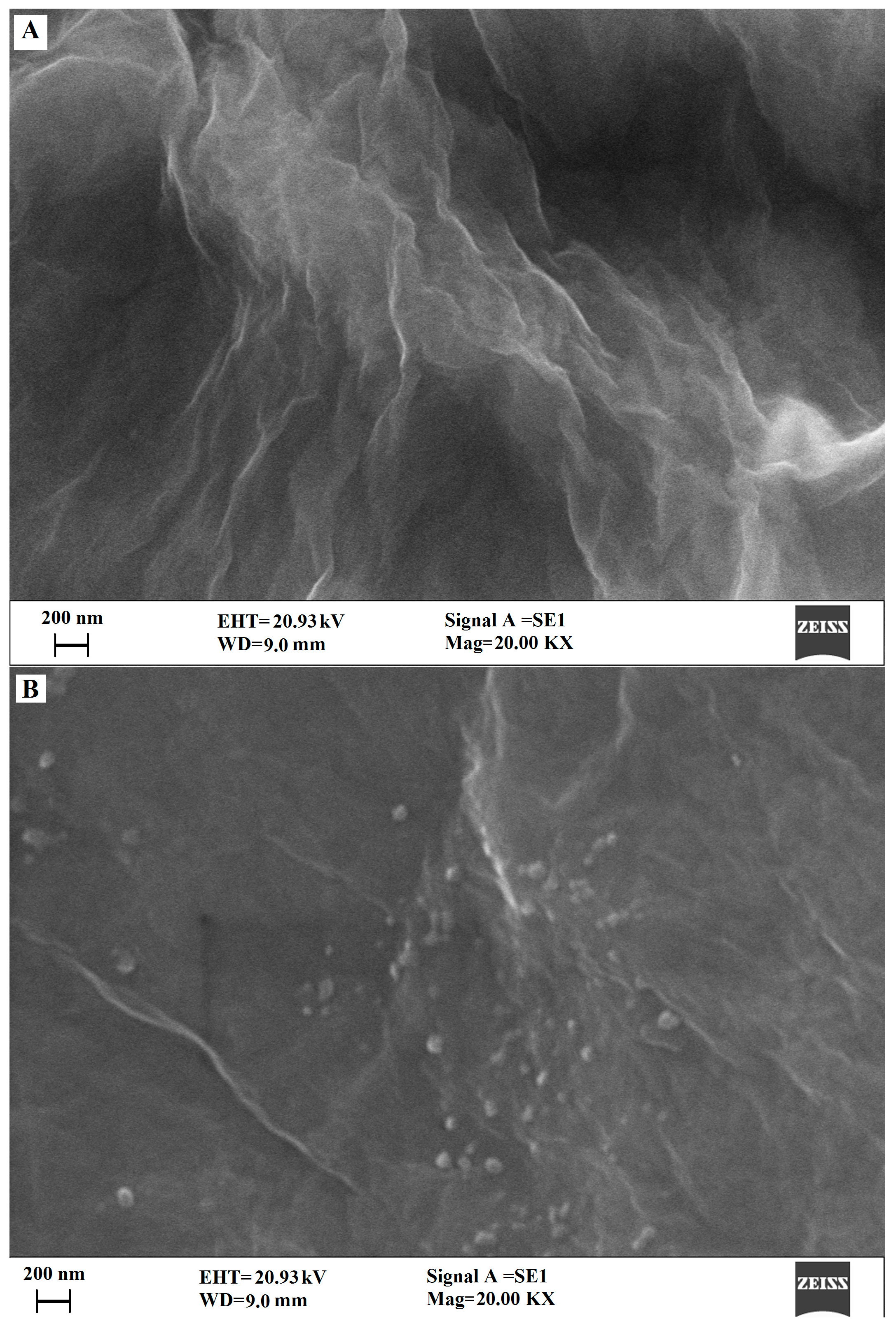
3.1. Characterization of the GO and Cu2O-ERGO Nanocomposites
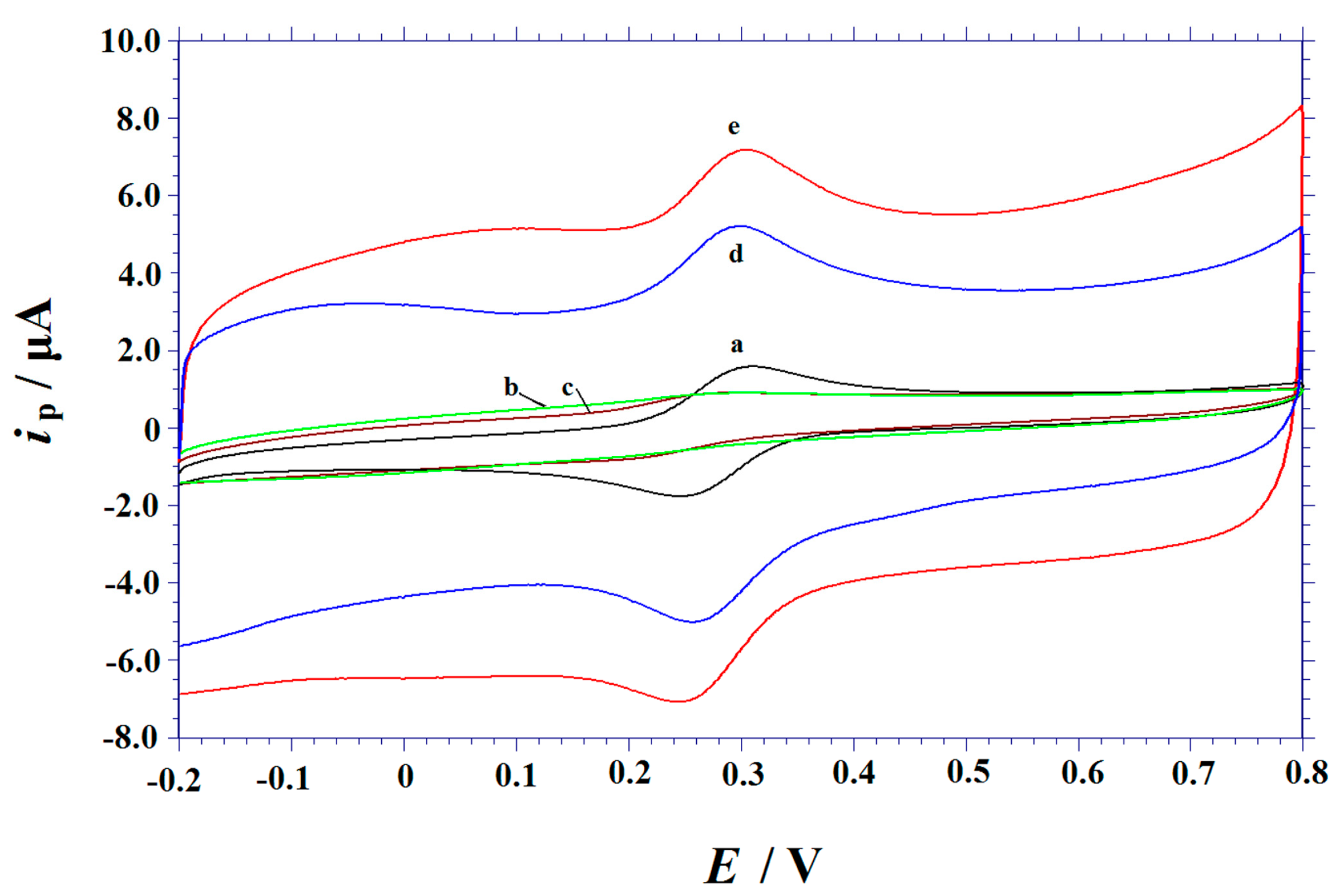
3.2. Electrochemical Characterization of Modified Electrodes
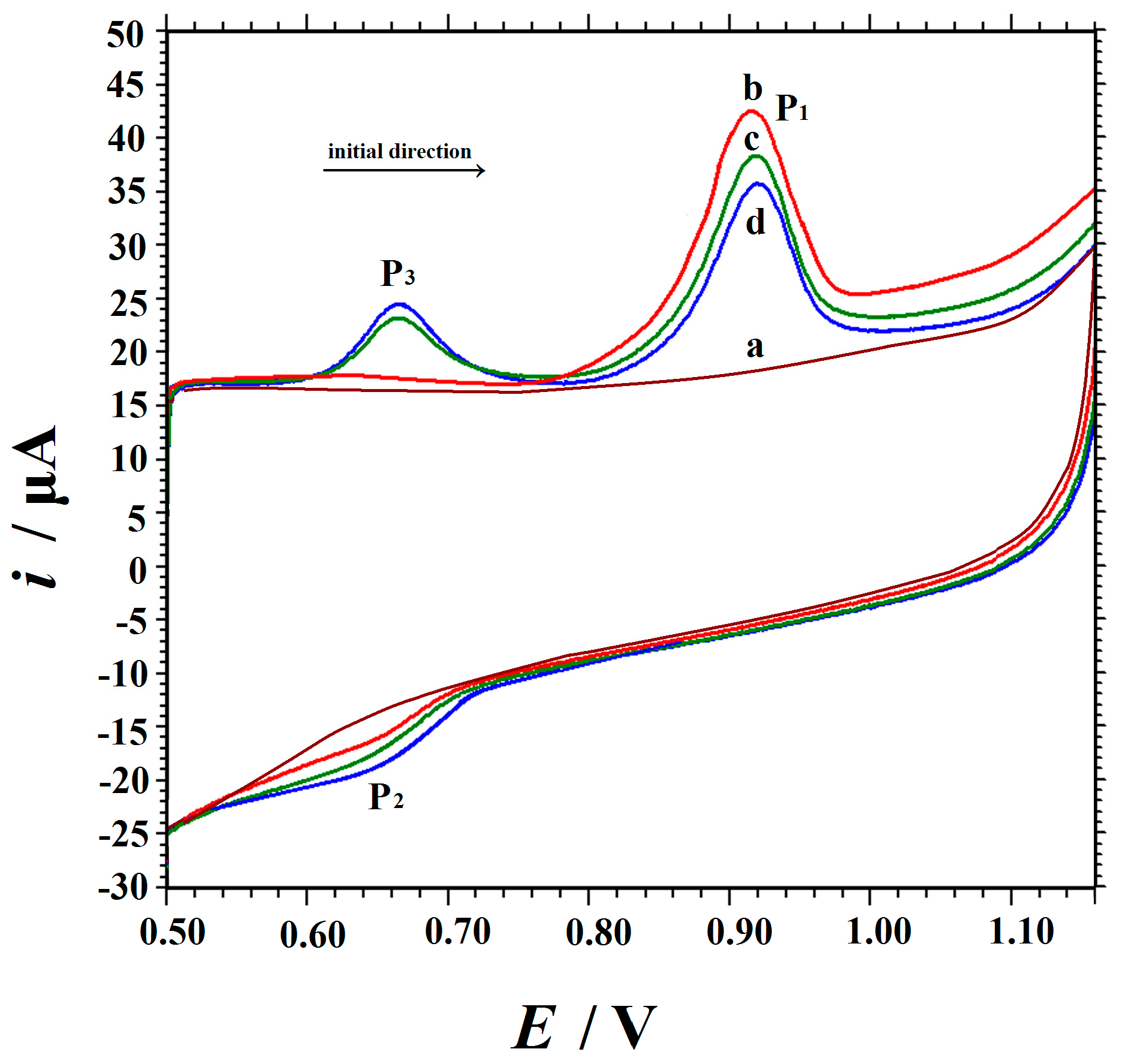
3.3. Electrocatalytic Activity of Cu2O NPs-ERGO/GCE Towards Vanillin
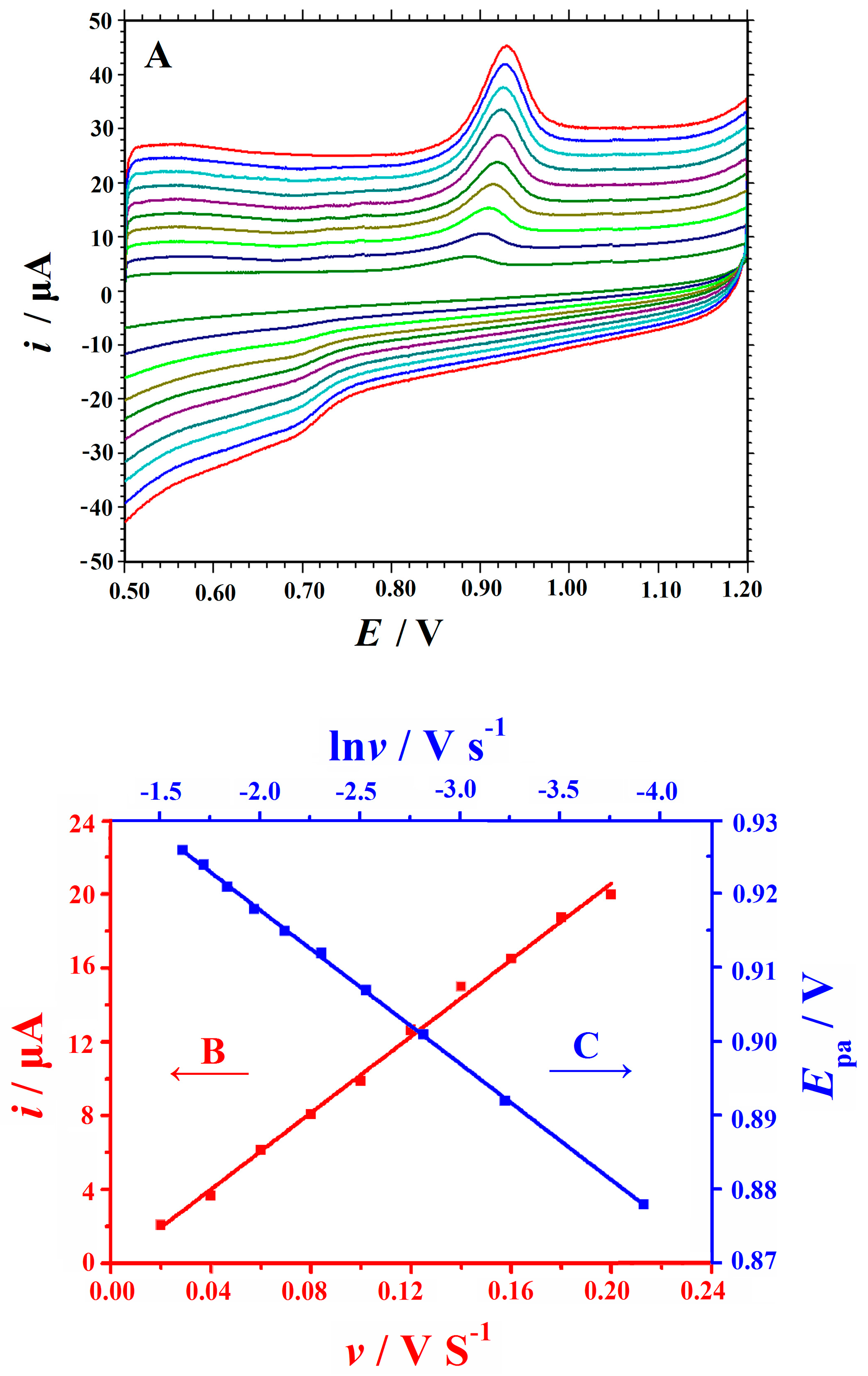
3.4. Cyclic Voltammetric Behaviors
3.5. Chronocoulometry Study
3.6. Optimization of Analytical Parameters
3.6.1. Effect of Supporting Electrolytes
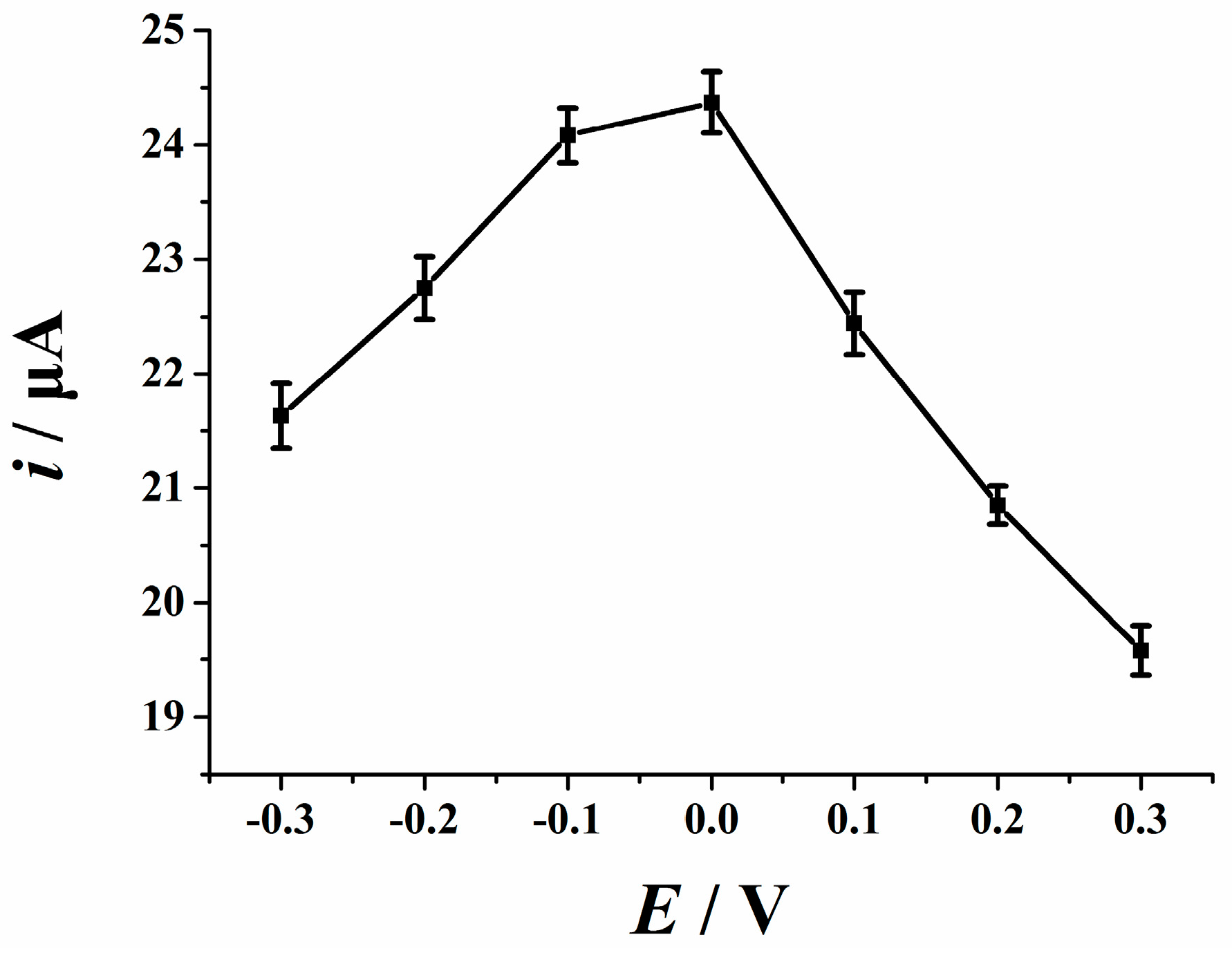
3.6.2. Accumulation Potential and Time
3.7. Analytic Properties
3.7.1. Repeatability, Reproducibility, and Stability
3.7.2. Interference Studies
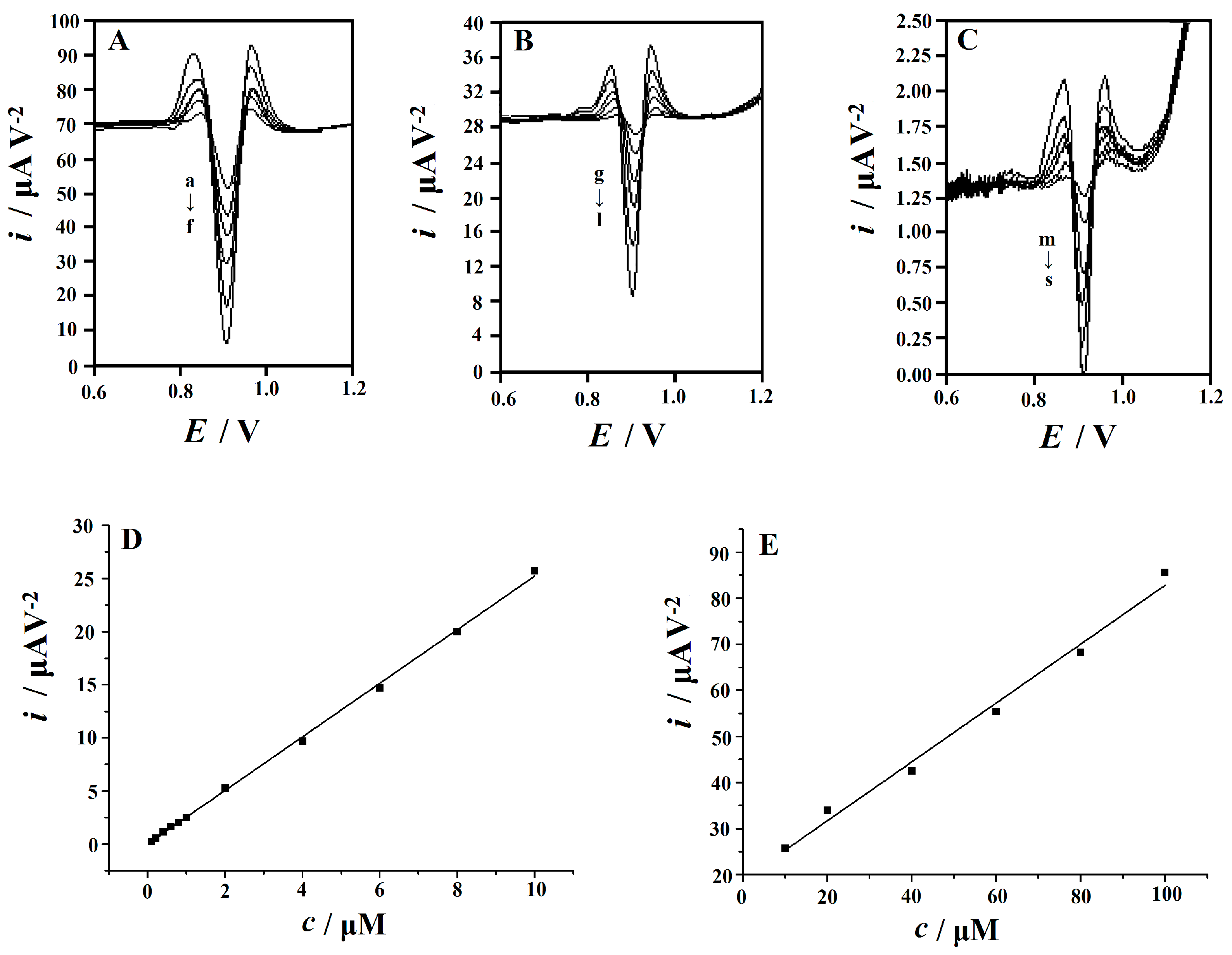
3.7.3. Calibration and Limit of Detection
3.7.4. Analytical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yardım, Y.; Gülcan, M.; Sentürk, Z. Determination of vanillin in commercial food product by adsorptive stripping voltammetry using a boron-doped diamond electrode. Food Chem. 2013, 141, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Waliszewski, K.N.; Pardio, V.T.; Ovando, S.L. A simple and rapid HPLC technique for vanillin determination in alcohol extract. Food Chem. 2006, 101, 1059–1062. [Google Scholar] [CrossRef]
- Goodner, K.L.; Jella, P.; Rouseff, R.L. Determination of vanillin in orange, grapefruit, tangerine, lemon, and lime juices using GC–Olfactometry and GC–MS/MS. J. Agric. Food Chem. 2000, 48, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, A.V.; Gornova, N.V.; Rudometova, N.V. Determination of vanillin and ethylvanillin in vanilla flavorings by planar (thin-layer) chromatography. J. Anal. Chem. 2003, 58, 677–684. [Google Scholar] [CrossRef]
- Ohashi, M.; Omae, H.; Hashida, M.; Sowa, Y.; Imai, S. Determination of vanillin and related flavor compounds in cocoa drink by capillary electrophoresis. J. Chromatogr. A 2007, 1138, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhang, G.; Kokot, S. Simultaneous spectrophotometric determination of maltol, ethyl maltol, vanillin and ethyl vanillin in foods by multivariate calibration and artificial neural networks. Food Chem. 2005, 89, 465–473. [Google Scholar] [CrossRef]
- Timotheou-Potamia, M.; Calokerinos, A.C. Chemiluminometric determination of vanillin in commercial vanillin products. Talanta 2007, 71, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhao, F.; Zeng, B. Sensitive voltammetric determination of vanillin with an AuPd nanoparticlesgraphene composite modified electrode. Food Chem. 2014, 151, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Hu, C.; Gan, T.; Dang, X.; Hu, S. Preparation and application of a novel vanillin sensor based on biosynthesis of Au–Ag alloy nanoparticles. Sens. Actuators B 2010, 148, 247–252. [Google Scholar] [CrossRef]
- Bettazzi, F.; Palchetti, I.; Sisalli, S.; Mascini, M. A disposable electrochemical sensor for vanillin detection. Anal. Chim. Acta 2006, 555, 134–138. [Google Scholar] [CrossRef]
- Peng, J.; Hou, C.; Hu, X. A graphene-based electrochemical sensor for sensitive detection of vanillin. Int. J. Electrochem. Sci. 2012, 7, 1724–1733. [Google Scholar]
- Kong, D.; Shen, S.; Yu, H.; Wang, J.; Chen, N. Chemical modification of multi-walled carbon nanotubes by tetraaminophthalocyaninatocobalt for the electrocatalytic oxidation of vanillin. Chin. J. Inorg. Chem. 2010, 26, 817–821. [Google Scholar]
- Li, J.; Feng, H.; Li, J.; Jiang, J.; Feng, Y.; He, L.; Qian, D. Bimetallic Ag-Pd nanoparticles-decorated graphene oxide: A fascinating three-dimensional nanohybrid as an efficient electrochemical sensing platform for vanillin determination. Electrochim. Acta 2015, 176, 827–835. [Google Scholar] [CrossRef]
- Huang, L.; Hou, K.; Jia, X.; Pan, H.; Du, M. Preparation of novel silver nanoplates/graphene composite and their application in vanillin electrochemical detection. Mater. Sci. Eng. C 2014, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Shi, Z.; Deng, Y.; Sun, J.; Wang, H. Morphology-dependent electrochemical sensing properties of manganese dioxide–graphene oxide hybrid for guaiacol and vanillin. Electrochim. Acta 2014, 147, 157–166. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Zeng, R.; Ding, C. Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 2015, 180, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ratinac, K.R.; Yang, W.; Gooding, J.J.; Thordarson, P.; Braet, F. Graphene and related materials in electrochemical sensing. Electroanalysis 2011, 23, 803–826. [Google Scholar] [CrossRef]
- Yang, J.; Strickler, J.R.; Gunasekaran, S. Indium tin oxide-coated glass modified with reduced graphene oxide sheets and gold nanoparticles as disposable working electrodes for dopamine sensing in meat samples. Nanoscale 2012, 4, 4594–4602. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Qu, Z.-W.; Zhu, H.; Kroes, G.-J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Xu, F.; Deng, M.; Li, G.; Chen, S.; Wang, L. Electrochemical behavior of cuprous oxide–reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta 2013, 88, 59–65. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, Y.; Zhang, Y.; Weng, W.; Li, S. Carbon quantum dots/octahedral Cu2O nanocomposites for non-enzymatic glucose and hydrogen peroxide amperometric sensor. Sens. Actuators B 2015, 206, 735–743. [Google Scholar] [CrossRef]
- Cao, H.; Yang, A.; Li, H.; Wang, L.; Li, S.; Kong, J.; Bao, X.; Yang, R. A non-enzymatic glucose sensing based on hollow cuprous oxidenanospheres in a Nafion matrix. Sens. Actuators B 2015, 214, 169–173. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, C.; Liu, L.; Zhang, Q.; Ji, S.; Pi, L.; Zhang, D.; Huo, Q. Cu2O/NiOx/graphene oxide modified glassy carbon electrode for the enhanced electrochemical oxidation of reduced glutathione and nonenzyme glucose sensor. Electrochim. Acta 2013, 104, 78–83. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Gu, Y.; Wang, Z.; Wang, C. One-pot solvothermal synthesis of a Cu2O/Graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim. Acta 2011, 173, 103–109. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Gu, Y.; Wang, C. Fabrication of the Cu2O/polyvinyl pyrrolidone-graphene modified glassy carbon-rotating disk electrode and its application for sensitive detection of herbicide paraquat. Sens. Actuators B 2012, 173, 530–539. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gu, A.; Wu, H.; Fang, B. Preparation of porous Cu2O octahedron and its application as L-Tyrosine sensors. Solid State Commun. 2008, 148, 525–528. [Google Scholar] [CrossRef]
- Shishiyanu, S.T.; Shishiyanu, T.S.; Lupan, O.I. Novel NO2 gas sensor based on cuprous oxide thin films. Sens. Actuators B 2006, 113, 468–476. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Feng, Y. Acetylene black paste electrode modified with graphene as the voltammetric sensor for selective determination of tryptophan in the presence of high concentrations of tyrosine. Mater. Sci. Eng. C 2014, 35, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, H.; Zhou, F. Determination of trace tin by catalytic adsorptive cathodic stripping voltammetry. Anal. Chim. Acta 2005, 554, 86–91. [Google Scholar] [CrossRef]
- Li, Y.; Xie, H.; Zhou, F. Alizarin violet modified carbon paste electrode for the determination of trace silver(I) by adsorptive voltammetry. Talanta 2005, 67, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Yin, H.; Cui, L.; Chen, Q.; Shi, W.; Ai, S.; Zhu, L.; Lu, L. Amperometric determination of bisphenol A in milk using PAMAM–Fe3O4 modified glassy carbon electrode. Food Chem. 2011, 125, 1097–1103. [Google Scholar] [CrossRef]
- Anson, F. Application of potentiostatic current integration to the study of the adsorption of cobalt(III)-(ethylenedinitrilo tetraacetate) on mercury electrodes. Anal. Chem. 1964, 36, 932–934. [Google Scholar] [CrossRef]
- Adams, R. Electrochemistry at Solid Electrodes; Marcel Dekker: New York, NY, USA, 1969. [Google Scholar]




| Electrochemical Sensors | Technique | Supporting Electrolyte | Linear Range/μM | Correlation Coefficient | Sensitivity (μA/μM) | Detection Limit/μM | References |
|---|---|---|---|---|---|---|---|
| a BDD electrode | i SWV | 0.1 M PBS, pH 2.5 | 3.3–98 | 0.999 | 0.5984 (μA mL μg−1) | 0.16 | [1] |
| b AuPd-graphene/GCE | j DPV | 0.1 M PBS, pH 7.0 | 0.1−7 and 10−40 | 0.996 and 0.999 | 0.113 and 0.0125 | 0.02 | [2] |
| c CDA/Au–AgNPs/GCE | Amperometry | 0.1 M PBS, pH 2.0 | 0.2−50 | - | - | 0.04 | [9] |
| disposable screen-printed electrode | SWV | 0.1 M PBS, pH 7.4 | 5−400 | 0.9994 | 0.0265 | 0.4 | [10] |
| graphene/GCE | DPV | Na2HPO4− C6H8O7 buffer, pH 5.0 | 0.6−48 | 0.9996 | 0.1523 | 0.056 | [11] |
| d MWCNTs-TAPcCo/GCE | SWV | 0.1 M PBS, pH 7.2 | 4.2−5000 | 0.9993 | 0.04725 | 0.44 | [12] |
| e Ag-Pd/GO/GCE | DPV | 0.1 M PBS, pH 6.0 | 0.02–45 | 0.9933 | 0.5733 | 0.005 | [13] |
| f Ag NPs/GN/GCE | SWV | 0.1 M PBS, pH 6.98 | 2−100 | 0.998 | 0.959 | 0.332 | [14] |
| g MFG/GCE | DPV | B–R buffer (pH 1.81) | 0.03−8 | 0.995 | 0.5733 | 0.0015 | [15] |
| h GR-PVP/ABPE | Derivative Voltammetry | 0.1 M H3PO4 | 0.02−2.0, 2.0−40, 40−100 | 0.9985, 0.9959, 0.9985 | 0.1714; 0.0805; 0.0269 | 0.01 | [16] |
| Cu2O-ERGO/GCE | Derivative Voltammetry | 0.1 M H2SO4 | 0.1−10; 10−100 | 0.9995 0.9953 | 2.5222; 0.6394 | 0.01 | This work |
| Sample a | Found b/μM | Added/μM | Total Found b/μM | Recovery/% | Content b/μg g−1 | Content Determined by HPLC b/μg g−1 |
|---|---|---|---|---|---|---|
| Biscuit | 0.84(±0.04) | 0.80 | 1.61(±0.03) | 96.3 | 25.56(±1.10) | 25.34 (±1.24) |
| Chocolate | 6.18(±0.24) | 6.00 | 12.25(±0.11) | 101.2 | 188.04(±7.17) | 187.81 (±6.86) |
| Candy | 1.27(±0.06) | 1.00 | 2.25(±0.17) | 98.0 | 38.64(±1.72) | 38.95(±1.54) |
| custard | 5.32(±0.22) | 5.00 | 10.16(±0.47) | 96.8 | 161.87(±6.69) | 161.52 (±5.70) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Liu, J.; Li, G. Rapid and Sensitive Determination of Vanillin Based on a Glassy Carbon Electrode Modified with Cu2O-Electrochemically Reduced Graphene Oxide Nanocomposite Film. Sensors 2018, 18, 2762. https://doi.org/10.3390/s18092762
Ning J, He Q, Luo X, Wang M, Liu D, Wang J, Liu J, Li G. Rapid and Sensitive Determination of Vanillin Based on a Glassy Carbon Electrode Modified with Cu2O-Electrochemically Reduced Graphene Oxide Nanocomposite Film. Sensors. 2018; 18(9):2762. https://doi.org/10.3390/s18092762
Chicago/Turabian StyleNing, Jingheng, Quanguo He, Xin Luo, Min Wang, Donglin Liu, Jianhui Wang, Jun Liu, and Guangli Li. 2018. "Rapid and Sensitive Determination of Vanillin Based on a Glassy Carbon Electrode Modified with Cu2O-Electrochemically Reduced Graphene Oxide Nanocomposite Film" Sensors 18, no. 9: 2762. https://doi.org/10.3390/s18092762
APA StyleNing, J., He, Q., Luo, X., Wang, M., Liu, D., Wang, J., Liu, J., & Li, G. (2018). Rapid and Sensitive Determination of Vanillin Based on a Glassy Carbon Electrode Modified with Cu2O-Electrochemically Reduced Graphene Oxide Nanocomposite Film. Sensors, 18(9), 2762. https://doi.org/10.3390/s18092762






