Detecting Sulfuric and Nitric Acid Rain Stresses on Quercus glauca through Hyperspectral Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Spectral Measurement
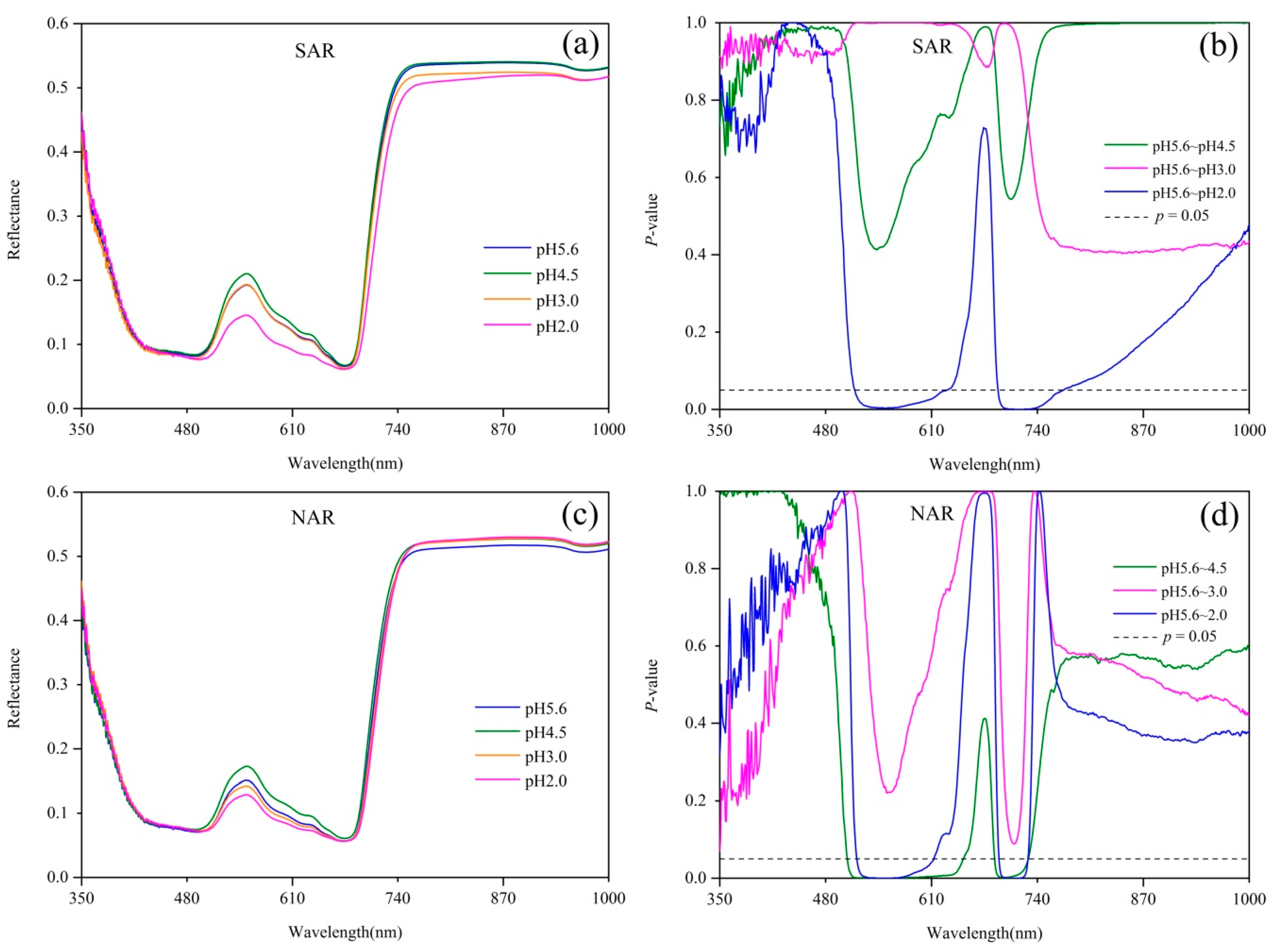
2.3. Sensitive Bands Detection
2.4. Hyperspectral Index Construction
3. Results
3.1. Sensitive Hyperspectral Bands Detection and Hyperspectral Index Construction
3.2. Indicator of GPAI on the Responses of Q. glauca under SAR Treatment
3.3. Indicator of GPAI on the Responses of Q. glauca under NAR Treatment
4. Discussion
4.1. The Influence of Acid Rain on Spectral Reflectance and the Indicator of GPAI
4.2. Responses of GPAI to SAR and NAR Stresses
4.3. GPAI as an Indicator for Assessing the Stress of SAR and NAR on Q. glauca at pH 4.5 Level
4.4. GPAI as an Indicator for Assessing the Stress of SAR and NAR on Q. glauca at pH 3.0 Level
4.5. GPAI as an Indicator for Assessing the Stress of SAR and NAR on Q. glauca at pH 2.0 Level
4.6. Limitations and Recommendations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Menz, F.C.; Seip, H.M. Acid rain in Europe and the United States: An update. Environ. Sci. Policy 2004, 7, 253–265. [Google Scholar] [CrossRef]
- Larssen, T.; Seip, H.M.; Semb, A.; Mulder, J.; Muniz, I.P.; Vogt, R.D.; Lydersen, E.; Angell, V.; Tang, D.; Eilertsen, O. Acid deposition and its effects in China: An overview. Environ. Sci. Policy 1999, 2, 9–24. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.H.; Liu, T.W.; Wu, F.H.; Zheng, H.L. Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol. Biochem. 2013, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.K.; Ji, G.L. Effects of H2SO4 and HNO3 on soil acidification and aluminum speciation in variable and constant charge soils. Water Air Soil Pollut. 2001, 129, 33–43. [Google Scholar] [CrossRef]
- Meng, H.; Dong, D.M.; Wang, J.; Yang, K.N.; Tian, L.; Sun, W.; Fang, C.S. Effects of simulated acid rain on main nutritional indicators of three leafy vegetables. Chem. Res. Chin. Univ. 2011, 27, 397–401. [Google Scholar]
- Zhang, X.M.; Chai, F.H.; Wang, S.L.; Sun, X.Z.; Han, M. Research progress of acid precipitation in China. Res. Environ. Sci. 2010, 23, 527–532. [Google Scholar]
- Larssen, T.; Lydersen, E.; Tang, D.; He, Y.; Gao, J.; Liu, H.; Duan, L.; Seip, H.M.; Vogt, R.D.; Mulder, J. Acid rain in China. Environ. Sci. Technol. 2006, 40, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Belesky, D.P.; Fedders, J.M. Tall fescue development in response to acremonium coenophialum and soil acidity. Crop Sci. 1995, 35, 529–533. [Google Scholar] [CrossRef]
- Shukla, J.B.; Sundar, S.; Naresh, R. Modeling and analysis of the acid rain formation due to precipitation and its effect on plant species. Nat. Resour. Model. 2013, 26, 53–65. [Google Scholar] [CrossRef]
- Larssen, T.; Carmichael, G.R. Acid rain and acidification in China: The importance of base cation deposition. Environ. Pollut. 2000, 110, 89–102. [Google Scholar] [CrossRef]
- Bellani, L.M.; Rinallo, C.; Muccifora, S.; Gori, P. Effects of simulated acid rain on pollen physiology and ultrastructure in the apple. Environ. Pollut. 1997, 95, 357–362. [Google Scholar] [CrossRef]
- Huhn, G.; Schulz, H. Contents of free amino acids in Scots pine needles from field sites with different levels of nitrogen deposition. New Phytol. 1996, 134, 95–101. [Google Scholar] [CrossRef]
- Liu, T.W.; Fu, B.; Niu, L.; Chen, J.; Wang, W.H.; He, J.X.; Pei, Z.M.; Zheng, H.L. Comparative proteomic analysis of proteins in response to simulated acid rain in arabidopsis. J. Proteom. Res. 2011, 10, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Thelin, G.; Rosengrenbrinck, U.; Nihlgård, B.; Barkman, A. Trends in needle and soil chemistry of Norway spruce and Scots pine stands in South Sweden 1985–1994. Environ. Pollut. 1998, 99, 149–158. [Google Scholar] [CrossRef]
- Fan, H.B.; Wang, Y.H. Effects of simulated acid rain on germination, foliar damage, chlorophyll contents and seedling growth of five hardwood species growing in China. For. Ecol. Manag. 2000, 126, 321–329. [Google Scholar] [CrossRef]
- Percy, K.E.; Baker, E.A. Effects of simulated acid rain on production, morphology and composition of epicuticular wax and on cuticular membrane development. New Phytol. 1987, 107, 577–589. [Google Scholar] [CrossRef]
- Liu, E.U.; Liu, C.P. Effects of simulated acid rain on the antioxidative system in cinnamomum philippinense seedlings. Water Air Soil Pollut. 2011, 215, 127–135. [Google Scholar] [CrossRef]
- Xie, X.Z.; Jiang, H.; Shu-Quan, Y.U.; Liu, Y.Y.; Yuan, H.Y. Effect of simulated acid rain on soil respiration of Pinus massoniana and Cunninghamia lanceolata. Acta Ecol. Sin. 2009, 29, 5713–5720. [Google Scholar]
- Cotrozzi, L.; Townsend, P.A.; Pellegrini, E.; Nali, C.; Couture, J.J. Reflectance spectroscopy: A novel approach to better understand and monitor the impact of air pollution on Mediterranean plants. Environ. Sci. Pollut. Res. 2017, 25, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Delalieux, S.; Auwerkerken, A.; Verstraeten, W.W.; Somers, B.; Valcke, R.; Lhermitte, S.; Keulemans, J.; Coppin, P. Hyperspectral reflectance and fluorescence imaging to detect scab induced stress in apple leaves. Remote Sens. 2009, 1, 858–874. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and caroteniods at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Rock, B.N.; Hoshizaki, T.; Miller, J.R. Comparison of in situ and airborne spectral measurements of the blue shift associated with forest decline. Remote Sens. Environ. 1988, 24, 109–127. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, T.; Qiu, Z.; Peng, J.; Zhang, C.; He, Y. Fast detection of striped stem-borer (chilo suppressaliswalker) infested rice seedling based on visible/near-infrared hyperspectral imaging system. Sensors 2017, 17, 2470. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Mirik, M.; Michels, G.J., Jr.; Kassymzhanova-Mirik, S.; Elliott, N.C. Reflectance characteristics of Russian wheat aphid (Hemiptera: Aphididae) stress and abundance in winter wheat. Comput. Electronics Agric. 2007, 57, 123–134. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Detecting sugarcane ‘orange rust’ disease using EO-1 Hyperion hyperspectral imagery. Int. J. Remote Sens. 2004, 25, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Dobrowski, S.Z.; Pushnik, J.C.; Zarco-Tejada, P.J.; Ustin, S.L. Simple reflectance indices track heat and water stress-induced changes in steady-state chlorophyll fluorescence at the canopy scale. Remote Sens. Environ. 2005, 97, 403–414. [Google Scholar] [CrossRef]
- Ranjan, R.; Chopra, U.K.; Singh, A.K.; Singh, A.K.; Pradhan, S. Assessment of plant nitrogen stress in wheat (Triticum aestivum L.) through hyperspectral indices. Int. J. Remote Sens. 2012, 33, 6342–6360. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, H.; Qin, Q.; Ersoy, O.K. A new narrow band vegetation index for characterizing the degree of vegetation stress due to copper: The copper stress vegetation index (CSVI). Remote Sens. Lett. 2017, 8, 576–585. [Google Scholar] [CrossRef]
- Delalieux, S.; Aardt, J.V.; Keulemans, W.; Schrevens, E.; Coppin, P. Detection of biotic stress (Venturia inaequalis) in apple trees using hyperspectral data: Non-parametric statistical approaches and physiological implications. Eur. J. Agron. 2007, 27, 130–143. [Google Scholar] [CrossRef]
- Vittorio, A.V.D.; Biging, G.S. Spectral identification of ozone-damaged pine needles. Int. J. Remote Sens. 2009, 30, 3041–3073. [Google Scholar] [CrossRef]
- Xie, X.Z.; Jiang, H.; Song, X.D.; Zhuo, G.M.; Shu-Quan, Y.U.; Wang, B. Hyperspectral characteristics of elaeocarpus glabripetalus merr and carya cathayensis at different levels of simulated acid rain. Remote Sens. Inf. 2010, 31, 32–38. [Google Scholar]
- Cheng, M.M.; Hong, J.; Jian, C.; Shu-Quan, Y.U.; Song, X.D.; Wang, B.; Xie, X.Z.; Zheng, G. Evaluation of stress in the seedlings of succession typical species induced by acid rain using hyperspectral remote sensing. Acta Ecol. Sin. 2009, 29, 5953–5962. [Google Scholar]
- Song, X.D.; Jiang, H.; Yu, S.Q.; Zhou, G.M.; Jiang, Z.S. Relationship between simulated acid rain stress and leaf reflectance. Spectrosc. Spectr. Anal. 2010, 30, 165–169. [Google Scholar]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Penuelas, J.; Valentini, R. Relationships between ndvi, canopy structure, and photosynthesis in three californian vegetation types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Gitelson, A.; Lang, M. Non-destructive determination of chlorophyll content of leaves of a green and an aurea mutant of tobacco by reflectance measurements. J. Plant Physiol. 1996, 148, 483–493. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with erts. Nasa Spec. Publ. 1974, 351, 309–317. [Google Scholar]
- Suárez, L.; Zarcotejada, P.J.; Gonzálezdugo, V.; Berni, J.A.J.; Sagardoy, R.; Morales, F.; Fereres, E. Detecting water stress effects on fruit quality in orchards with time-series PRI airborne imagery. Remote Sens. Environ. 2010, 114, 286–298. [Google Scholar] [CrossRef]
- Boluda, R.; Andreu, V.; Gilabert, M.A.; Sobrino, P. Relation between reflectance of rice crop and indices of pollution by heavy metals in soils of albufera natural park (Valencia, Spain). Soil Technol. 1994, 6, 351–363. [Google Scholar] [CrossRef]
- Garty, J.; Tamir, O.; Hassid, I.; Eshel, A.; Cohen, Y.; Karnieli, A.; Orlovsky, L. Photosynthesis, chlorophyll integrity, and spectral reflectance in lichens exposed to air pollution. J. Environ. Qual. 2001, 30, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Deventer, H.V.; Cho, M.A. Assessing leaf spectral properties of Phragmites australis impacted by acid mine drainage. South Afr. J. Sci. 2014, 110, 1–12. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2006, 58, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Ping, W.; Liu, X.N.; Huang, F. Retrieval model for subtle variation of contamination stressed maize chlorophyll using hyperspectral data. Spectrosc. Spectr. Anal. 2010, 30, 197–201. [Google Scholar]
- Jiang, D.; Wang, A.B.; Yang, X.H.; Lu, H.H. Ecological connotation and application of the vegetation index-surface temperature feature space. Prog. Geogr. 2001, 20, 146–152. [Google Scholar]
- Adams, C.M.; Hutchinson, T.C. Comparative abilities of leaf surfaces to neutralize acidic raindrops. II. The influence of leaf wettability, leaf age and rain duration on changes in droplet ph and chemistry on leaf surfaces. New Phytol. 2010, 106, 437–456. [Google Scholar] [CrossRef]
- Xiao-Peng, Y.U.; Li-Ta, Y.I.; Shu-Quan, Y.U.; Shen, L. Effects of acid rainfall intensities and treatment ways on chlorophyll fluorescence parameters of Myrica rubra seedlings. Chin. J. Ecol. 2015, 34, 1246–1252. [Google Scholar]
- Cheng, M.; Jiang, H.; Guo, Z.; Zhang, X. Assessing nitrogen treatment efficiency in schima superba seedlings detected using hyperspectral reflectance. Terr. Atmos. Ocean. Sci. 2014, 25, 369–380. [Google Scholar] [CrossRef]
- Newete, S.W.; Erasmus, B.F.N.; Weiersbye, I.M.; Cho, M.A.; Byrne, M.J. Hyperspectral reflectance features of water hyacinth growing under feeding stresses of Neochetina spp. and different heavy metal pollutants. Int. J. Remote Sens. 2014, 35, 799–817. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Jiang, H.; Jin, J. Hyperspectral response of rice under acid rain stress. Remote Sens. Inf. 2014, 29, 94–99. [Google Scholar]
- Rayapatia, N.; Eileenm, P.; Francisj, P.; Tefera, M. The potential of spectral reflectance technique for the detection of Grapevine leafroll-associated virus-3 in two red-berried wine grape cultivars. Comput. Electron. Agric. 2009, 66, 38–45. [Google Scholar]
- Du, H.Q.; Jin, W.; Ge, H.L.; Fan, W.Y.; Xu, X.J. Using fractal dimensions of hyperspectral curves to analyze the healthy status of vegetation. Spectrosc. Spectr. Anal. 2009, 29, 2136–2140. [Google Scholar]
- Zhao, M.; Heinsch, F.A.; Nemani, R.R.; Running, S.W. Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens. Environ. 2005, 95, 164–176. [Google Scholar] [CrossRef]
- Asner, G.P.; Carlson, K.M.; Martin, R.E. Substrate age and precipitation effects on Hawaiian forest canopies from spaceborne imaging spectroscopy. Remote Sens. Environ. 2005, 98, 457–467. [Google Scholar] [CrossRef]
- Dong, L.; Yu, F.; Liu, M.; Yu, S.; Wang, S.; Yi, L. Effects of three kinds of acid rain treatments on chlorophyll fluorescence and photosynthetic characteristics of Camellia sinensis under different acid rain gradients. Acta Sci. Circumst. 2016, 36, 3495–3504. [Google Scholar]
- Liu, J. Current and future study about effects of acid deposition on forest ecosystems. Chin. J. Ecol. 2003, 22, 113–117. [Google Scholar]
- Parmar, S.; Buchner, P.; Hawkesford, M.J. Leaf developmental stage affects sulfate depletion and specific sulfate transporter expression during sulfur deprivation in Brassica napus L. Plant Biology 2007, 9, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, L.; Li, N.; Fu, B. Long-term effects of simulated acid rain stress on a staple forest plant, Pinus massoniana Lamb: A proteomic analysis. Trees 2013, 27, 297–309. [Google Scholar] [CrossRef]
- Qin, H.; Yi, H. Genetic damage of Vicia faba root tip cells caused by combination of simulated acid rain and aluminum. Chin. J. Appl. Environ. Biol. 2007, 13, 799–802. [Google Scholar]
- Chen, J.S.; Tang, S.H.; Zhang, F.B.; Pei-Zhi, X.U.; Zhang, Z.X. Effects of N and S fertilizer combination on yield and quality of flowering chinese cabbage. Chin. J. Soil Sci. 2003, 34, 36–39. [Google Scholar]
- Byers, M.; Bolton, J. Effects of nitrogen and sulphur fertilisers on the yield, N and S content, and amino acid composition of the grain of spring wheat. J. Sci. Food Agric. 1979, 30, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Meng, C.; Ma, J.; Wu, Z.; Hu, J. Interaction of nitrogen and sulfur fertilizers on the yield of rapeseed and rice. Acta Agric. Zhejiangensis 2000, 12, 61–65. [Google Scholar]
- Xin-hui, L.; Xiu-ying, Z.; Yuan-dan, M.; Jia-xin, J.; Min, C.; Xiao-min, Z.; Hong, J. The hyperspectral diagnosis model for acid stress of cinnamomum camphora. Spectrosc. Spectr. Anal. 2017, 37, 1872–1878. [Google Scholar]
- XiaoDong, S.; Hong, J.; ShuQuan, Y.; GuoMo, Z.; Jie, C.; ZiShan, J.; Bo, J. Relationship between chlorophyll concentrations and spectral reflectance feature of the typical evergreen hardwood species in subtropical region of China. Acta Ecol. Sin. 2008, 28, 1959–1963. [Google Scholar]
- Shui, D.J.; Wang, X.Y.; Shao, Q.; Li-Yong, Y.E.; Sun, J.; Wang, B.L. Effects of simulated acid rain stress on physiological characteristics of Brassica chinensis L. J. South. Agric. 2016, 47, 1155–1158. [Google Scholar]
- Yan, M.; Li, Z.; Cong, Z.; Yin, Y.; Li, L.; Xin, Q.; Sun, X.; Jiang, H. Effect of water stress on drought-resistance of root in Yannong 21. Chin. Agric. Sci. Bull. 2010, 26, 113–117. [Google Scholar]
- Yizong, H.; Li, Z.; Li, X.; Wu, K.; Jianzhong, S. Impact of simulated acid rain on growth and nutrient elements uptake by Eucalyptus urophylla and Pinus massoniana. Ecol. Environ. 2006, 15, 331–336. [Google Scholar]
- Wang, S.; Yi, L.T.; Yu, S.Q.; Zhang, C.; Shi, J.J. Effects of simulating acid rain on photosynthesis and chlorophyll fluorescence parameters of Quercus glauca. Chin. J. Appl. Ecol. 2014, 25, 2183–2192. [Google Scholar]
- Kohno, Y. Leaf surface observations of radish and bush bean plants exposed to sulfuric acid by cryo-scanning electron microscope. J. Jpn. Soc. Atmos. Environ. 2011, 29, 71–79. [Google Scholar]
- Boying, M.; Ligen, X.; De’an, J. Effects of simulated acid rain on chlorophyll fluorescence characteristics in Eremochloa ophiuroides. Sci. Silvae Sin. 2006, 42, 8–11. [Google Scholar]
- Tian, D. Effect of simulated acid rain on photosynthetic characteristics in cinnamomum camphora seedlings. Sci. Silvae Sin. 2007, 43, 29–35. [Google Scholar]
- Shi, Q.; Jiang, H.; Chen, J.; Zhang, Q. Hyperspectral characteristics of typical subtopical trees at different levels of simulated acid rain. Acta Ecol. Sin. 2012, 32, 5621–5629. [Google Scholar]
- Singh, A.; Agrawal, M. Acid rain and its ecological consequences. J. Environ. Biol. 2008, 29, 15–24. [Google Scholar] [PubMed]
- Devi, S.R.; Prasad, M.N.V. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Guo, L.Z.; Ling, W.M.; Wu, J.; Bing, J.W. Effects of simulated acid rain on some physiological indices of Parakmeria lotungensis seedlings. Chin. J. Ecol. 2007, 26, 31–34. [Google Scholar]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A.J. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarcotejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L.; Ustin, S.L.; Schaepman, M.E. PROSPECT+SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]



| Winter | Spring | Summer | Autumn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | 12 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Rainfall | 51 | 72 | 85 | 125 | 127 | 157 | 211 | 147 | 148 | 150 | 78 | 61 |
| Max T | 11 | 8 | 10 | 14 | 21 | 25 | 29 | 33 | 33 | 28 | 23 | 17 |
| Min T | 3 | 1 | 3 | 6 | 12 | 17 | 21 | 25 | 25 | 21 | 15 | 9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, X.; Ma, Y.; Li, X.; Cheng, M.; Zhang, X.; Liu, L. Detecting Sulfuric and Nitric Acid Rain Stresses on Quercus glauca through Hyperspectral Responses. Sensors 2018, 18, 830. https://doi.org/10.3390/s18030830
Wang S, Zhang X, Ma Y, Li X, Cheng M, Zhang X, Liu L. Detecting Sulfuric and Nitric Acid Rain Stresses on Quercus glauca through Hyperspectral Responses. Sensors. 2018; 18(3):830. https://doi.org/10.3390/s18030830
Chicago/Turabian StyleWang, Shanqian, Xiuying Zhang, Yuandan Ma, Xinhui Li, Min Cheng, Xiaomin Zhang, and Lei Liu. 2018. "Detecting Sulfuric and Nitric Acid Rain Stresses on Quercus glauca through Hyperspectral Responses" Sensors 18, no. 3: 830. https://doi.org/10.3390/s18030830





