Non-Linear Cellular Dielectrophoretic Behavior Characterization Using Dielectrophoretic Tweezers-Based Force Spectroscopy inside a Microfluidic Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chip Design and Fabrication
2.2. Cell Culture and Viability
2.3. Modeling and Simulations of DEP Force Acting on MCF-7 Cells
2.4. Experimental Configuration
2.5. Method for Observing Cellular Behavior Using DEP Tweezers in a Microfluidic Device
3. Results and Discussions
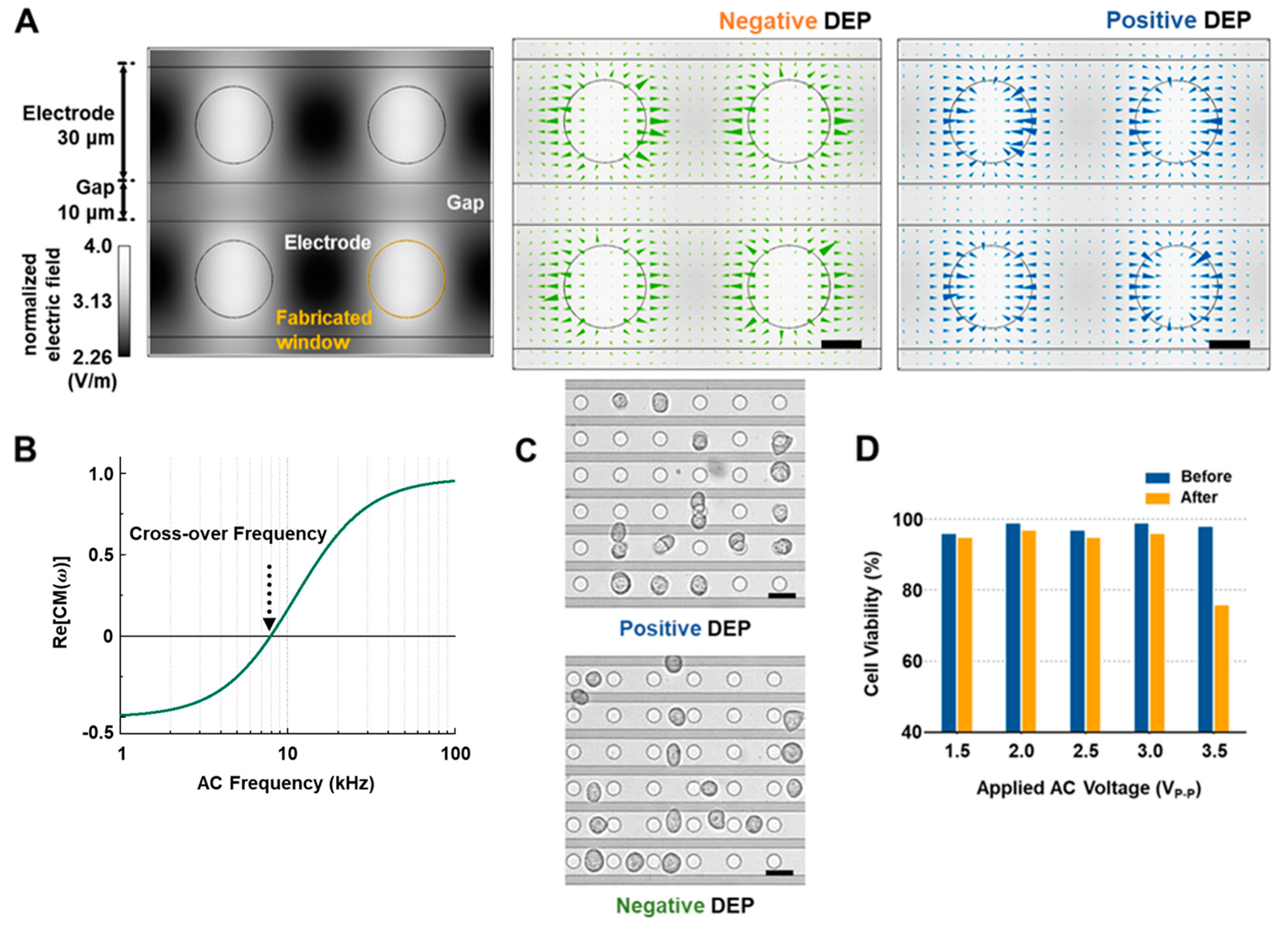
3.1. Numerical Simulation, Cell Alignment and Viability Results
3.2. Cellular Behaviors Characterization near Windows
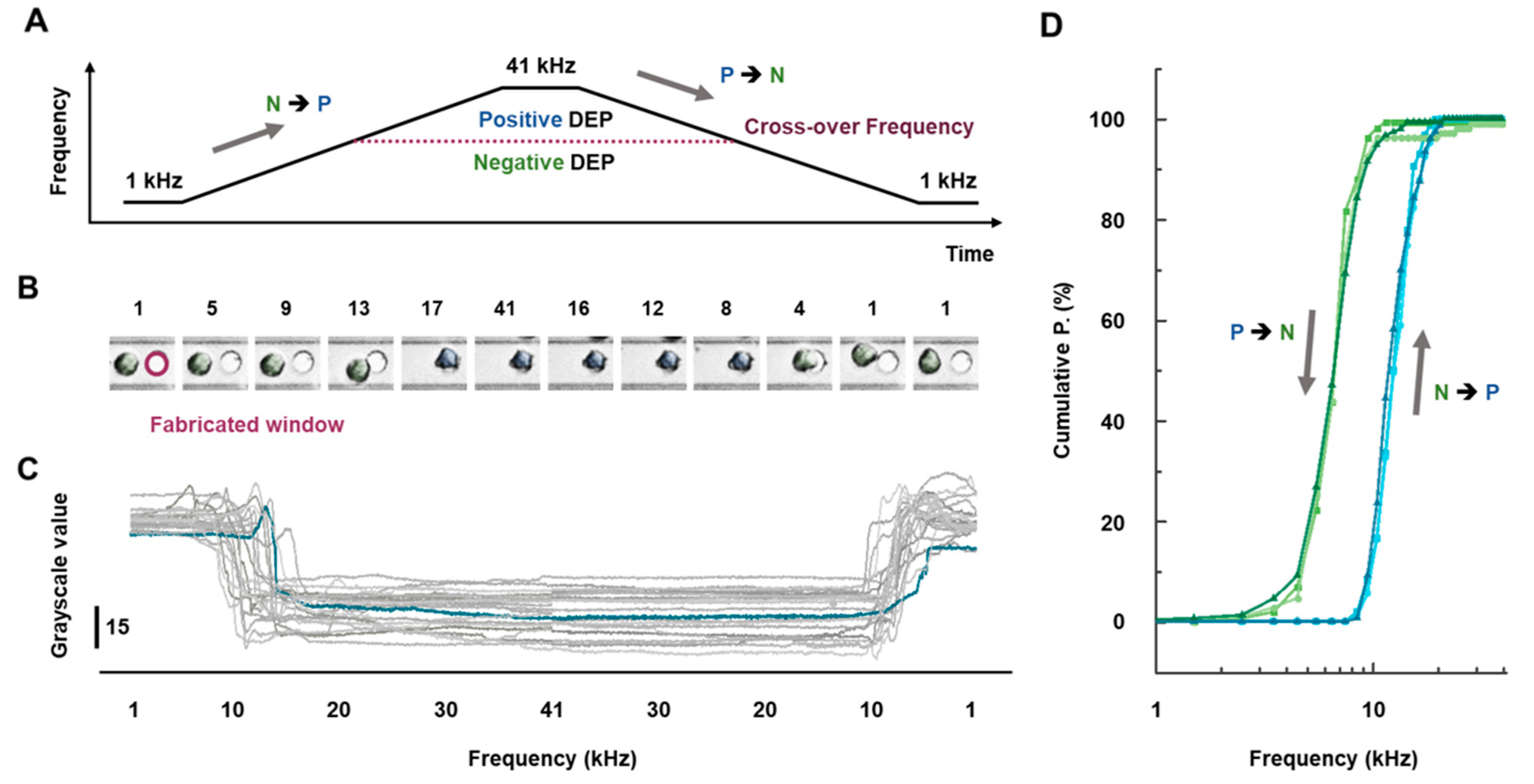
3.3. Cellular Cross-Over Frequency Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pethig, R. Dielectrophoresis: Theory, Methodology and Biological Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-118-67145-0. [Google Scholar]
- Coley, H.M.; Labeed, F.H.; Thomas, H.; Hughes, M.P. Biophysical characterization of MDR breast cancer cell lines reveals the cytoplasm is critical in determining drug sensitivity. Biochim. Biophys. Acta-Gen. Subj. 2007, 1770, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henslee, E.A.; Sano, M.B.; Rojas, A.D.; Schmelz, E.M.; Davalos, R.V. Selective concentration of human cancer cells using contactless dielectrophoresis. Electrophoresis 2011, 32, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.-C.; Huang, K.-W.; Fan, Y.-J.; Chiou, P.-Y. Fabrication of 3D high aspect ratio PDMS microfluidic networks with a hybrid stamp. Lab Chip 2015, 15, 1861–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, Y.C.; Huang, K.W.; Chong, W.; Chiou, P.Y. Tunnel Dielectrophoresis for Tunable, Single-Stream Cell Focusing in Physiological Buffers in High-Speed Microfluidic Flows. Small 2016, 12, 4343–4348. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bessette, P.H.; Qian, J.; Meinhart, C.D.; Daugherty, P.S.; Soh, H.T. Marker-specific sorting of rare cells using dielectrophoresis. Proc. Natl. Acad. Sci. USA 2005, 102, 15757–15761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clow, A.L.; Gaynor, P.T.; Oback, B.J. A novel micropit device integrates automated cell positioning by dielectrophoresis and nuclear transfer by electrofusion. Biomed. Microdevices 2010, 12, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-S.; Kwon, K.; Kim, S.-I.; Han, H.; Sohn, J.; Lee, S.; Jung, H.-I. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 2011, 11, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Faraghat, S.A.; Hoettges, K.F.; Steinbach, M.K.; van der Veen, D.R.; Brackenbury, W.J.; Henslee, E.A.; Labeed, F.H.; Hughes, M.P. High-throughput, low-loss, low-cost, and label-free cell separation using electrophysiology-activated cell enrichment. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-J.; Lee, T.; Choi, S.; Lee, S.-Y.; Key, J.; Yoo, Y.-M.; Chang, W.-J.; Kim, H.-S.; Lee, S.W. Selective position of individual cells without lysis on a circular window array using dielectrophoresis in a microfluidic device. Microfluid. Nanofluid. 2017, 21, 150. [Google Scholar] [CrossRef]
- Vaillire, C.; Honegger, T.; Kermarrec, F.; Gidrol, X.; Peyrade, D. Label-Free Electric Monitoring of Human Cancer Cells as a Potential Diagnostic Tool. Anal. Chem. 2016, 88, 9022–9028. [Google Scholar] [CrossRef] [PubMed]
- Velugotla, S.; Pells, S.; Mjoseng, H.K.; Duffy, C.R.E.; Smith, S.; De Sousa, P.; Pethig, R. Dielectrophoresis based discrimination of human embryonic stem cells from differentiating derivatives. Biomicrofluidics 2012, 6, 044113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elitas, M.; Dhar, N.; Schneider, K.; Valero, A.; Braschler, T.; McKinney, J.D.; Renaud, P. Dielectrophoresis as a single cell characterization method for bacteria. Biomed. Phys. Eng. Express 2017, 3, 015005. [Google Scholar] [CrossRef]
- Mulhall, H.J.; Cardnell, A.; Hoettges, K.F.; Labeed, F.H.; Hughes, M.P. Apoptosis progression studied using parallel dielectrophoresis electrophysiological analysis and flow cytometry. Integr. Biol. 2015, 7, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Henslee, E.A.; Torcal Serrano, R.M.; Labeed, F.H.; Jabr, R.I.; Fry, C.H.; Hughes, M.P.; Hoettges, K.F. Accurate quantification of apoptosis progression and toxicity using a dielectrophoretic approach. Analyst 2016, 141, 6408–6415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.S.; Lee, J.; Lee, G.; Nam, K.; Lee, T.; Chang, W.-J.; Kim, H.; Lee, S.-Y.; Seo, J.; Yoon, D.S.; et al. Real-Time Analysis of Cellular Response to Small-Molecule Drugs within a Microfluidic Dielectrophoresis Device. Anal. Chem. 2015, 87, 5914–5920. [Google Scholar] [CrossRef] [PubMed]
- Vaillier, C.; Honegger, T.; Kermarrec, F.; Gidrol, X.; Peyrade, D. Involvement of membrane proteins and ion channels on the self-rotation of human cells in a non-rotating AC electric field. Electrophoresis 2015, 36, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Hoettges, K.F.; Hübner, Y.; Broche, L.M.; Ogin, S.L.; Kass, G.E.N.; Hughes, M.P. Dielectrophoresis-Activated Multiwell Plate for Label-Free High-Throughput Drug Assessment. Anal. Chem. 2008, 80, 2063–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.; Pethig, R.; Smith, S.; Waterfall, M. Intracellular potassium under osmotic stress determines the dielectrophoresis cross-over frequency of murine myeloma cells in the MHz range. Electrophoresis 2017, 39, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Henslee, E.A.; Crosby, P.; Kitcatt, S.J.; Parry, J.S.W.; Bernardini, A.; Abdallat, R.G.; Braun, G.; Fatoyinbo, H.O.; Harrison, E.J.; Edgar, R.S.; et al. Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat. Commun. 2017, 8, 1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broche, L.M.; Bhadal, N.; Lewis, M.P.; Porter, S.; Hughes, M.P.; Labeed, F.H. Early detection of oral cancer—Is dielectrophoresis the answer? Oral Oncol. 2007, 43, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Labeed, F.H.; Coley, H.M.; Thomas, H.; Hughes, M.P. Assessment of Multidrug Resistance Reversal Using Dielectrophoresis and Flow Cytometry. Biophys. J. 2003, 85, 2028–2034. [Google Scholar] [CrossRef] [Green Version]
- Zellner, P.; Shake, T.; Sahari, A.; Behkam, B.; Agah, M. Off-chip passivated-electrode, insulator-based dielectrophoresis (OπDEP). Anal. Bioanal. Chem. 2013, 405, 6657–6666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shake, T.; Zellner, P.; Sahari, A.; Breazeal, M.V.R.; Behkam, B.; Pruden, A.; Agah, M. Embedded passivated-electrode insulator-based dielectrophoresis (EπDEP). Anal. Bioanal. Chem. 2013, 405, 9825–9833. [Google Scholar] [CrossRef] [PubMed]
- Nakidde, D.; Zellner, P.; Alemi, M.M.; Shake, T.; Hosseini, Y.; Riquelme, M.V.; Pruden, A.; Agah, M. Three dimensional passivated-electrode insulator-based dielectrophoresis. Biomicrofluidics 2015, 9, 014125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.J.; Lin, Y.Y.; Lei, U.; Wu, M.S.; Yang, P.C. Measurement of the Clausius-Mossotti factor of generalized dielectrophoresis. Appl. Phys. Lett. 2014, 104, 083701. [Google Scholar] [CrossRef]
- Su, H.-W.; Prieto, J.L.; Voldman, J. Rapid dielectrophoretic characterization of single cells using the dielectrophoretic spring. Lab Chip 2013, 13, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, J.; Clausen, C.; Rodriguez-Trujillo, R.; Svendsen, W. Study of Paclitaxel-Treated HeLa Cells by Differential Electrical Impedance Flow Cytometry. Biosensors 2014, 4, 257–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnaninchi, P.O.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitinger, S.; Wissenwasser, J.; Kapferer, W.; Heer, R.; Lepperdinger, G. Electric impedance sensing in cell-substrates for rapid and selective multipotential differentiation capacity monitoring of human mesenchymal stem cells. Biosens. Bioelectron. 2012, 34, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Asami, K.; Gheorghiu, E.; Yonezawa, T. Real-Time Monitoring of Yeast Cell Division by Dielectric Spectroscopy. Biophys. J. 1999, 76, 3345–3348. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, L.; Yin, H.; Xing, W.; Yu, Z.; Guo, M.; Cheng, J. Real-time, label-free monitoring of the cell cycle with a cellular impedance sensing chip. Biosens. Bioelectron. 2010, 25, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Sabuncu, A.C.; Liu, J.A.; Beebe, S.J.; Beskok, A. Dielectrophoretic separation of mouse melanoma clones. Biomicrofluidics 2010, 4, 021101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, H.A. Dielectrophoresis—The Behavior of Neutral Matter in Nonuniform Electric Fields; Cambridge Monographs on Physics; Cambridge University Press: Cambridge, UK, 1978; Chapter 1–3. [Google Scholar]
- Kim, M.H.; Lee, J.; Nam, K.; Park, I.S.; Son, M.; Ko, H.; Lee, S.; Yoon, D.S.; Chang, W.-J.; Lee, S.Y.; et al. Automated Dielectrophoretic Tweezers-Based Force Spectroscopy System in a Microfluidic Device. Sensors 2017, 17, 2272. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R. Rapid, accurate particle tracking by calculation of radial symmetry centers. Nat. Methods 2012, 9, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Eom, K.; Son, J.; Chang, W.J.; Park, K.; Kwon, T.; Yoon, D.S.; Bashir, R.; Lee, S.W. Microfluidic multifunctional probe array dielectrophoretic force spectroscopy with wide loading rates. ACS Nano 2012, 6, 8665–8673. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Choi, S.; Ko, K.H.; Kim, M.H.; Lee, S.-Y.; Key, J.; Yoon, Y.-R.; Park, I.S.; Lee, S.W. Characterization of the Stiffness of Multiple Particles Trapped by Dielectrophoretic Tweezers in a Microfluidic Device. Langmuir 2016, 32, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Where is dielectrophoresis (DEP) going? J. Electrochem. Soc. 2017, 164, B3049–B3055. [Google Scholar] [CrossRef]
- Lee, T.W.; Nam, K.; Baek, S.H.; Chang, W.J.; Kim, S.K.; Kim, H.S.; Yoon, D.S.; Lee, S.W. Numerical and Experimental Study on Dielectrophoretic and Electrohydrodynamic Traps using Micro-Particles on An Interdigitated Electrode Array System. Int. J. Nonlinear Sci. Numer. Simul. 2010, 11, 777–784. [Google Scholar] [CrossRef]
- Valero, A.; Braschler, T.; Renaud, P. A unified approach to dielectric single cell analysis: Impedance and dielectrophoretic force spectroscopy. Lab Chip 2010, 10, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Raicu, V.; Feldman, Y. Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Applications; Oxford University Press: Oxford, MS, USA, 2015. [Google Scholar]
- Pethig, R.; Menachery, A.; Pells, S.; De Sousa, P. Dielectrophoresis: A Review of Applications for Stem Cell Research. J. Biomed. Biotechnol. 2010, 2010, 182581. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.N.G.; Leonard, K.M.; Minerick, A.R. Frequency sweep rate dependence on the dielectrophoretic response of polystyrene beads and red blood cells. Biomicrofluidics 2013, 7, 064114. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Ko, K.; Lim, J.; Kim, S.H.; Woo, S.-H.; Kim, Y.S.; Key, J.; Lee, S.Y.; Park, I.S.; Lee, S.W. Non-Linear Cellular Dielectrophoretic Behavior Characterization Using Dielectrophoretic Tweezers-Based Force Spectroscopy inside a Microfluidic Device. Sensors 2018, 18, 3543. https://doi.org/10.3390/s18103543
Choi S, Ko K, Lim J, Kim SH, Woo S-H, Kim YS, Key J, Lee SY, Park IS, Lee SW. Non-Linear Cellular Dielectrophoretic Behavior Characterization Using Dielectrophoretic Tweezers-Based Force Spectroscopy inside a Microfluidic Device. Sensors. 2018; 18(10):3543. https://doi.org/10.3390/s18103543
Chicago/Turabian StyleChoi, Seungyeop, Kwanhwi Ko, Jongwon Lim, Sung Hoon Kim, Sung-Hun Woo, Yoon Suk Kim, Jaehong Key, Sei Young Lee, In Su Park, and Sang Woo Lee. 2018. "Non-Linear Cellular Dielectrophoretic Behavior Characterization Using Dielectrophoretic Tweezers-Based Force Spectroscopy inside a Microfluidic Device" Sensors 18, no. 10: 3543. https://doi.org/10.3390/s18103543





