A Room-Temperature CNT/Fe3O4 Based Passive Wireless Gas Sensor
Abstract
:1. Introduction
2. Sensor Testing Principle
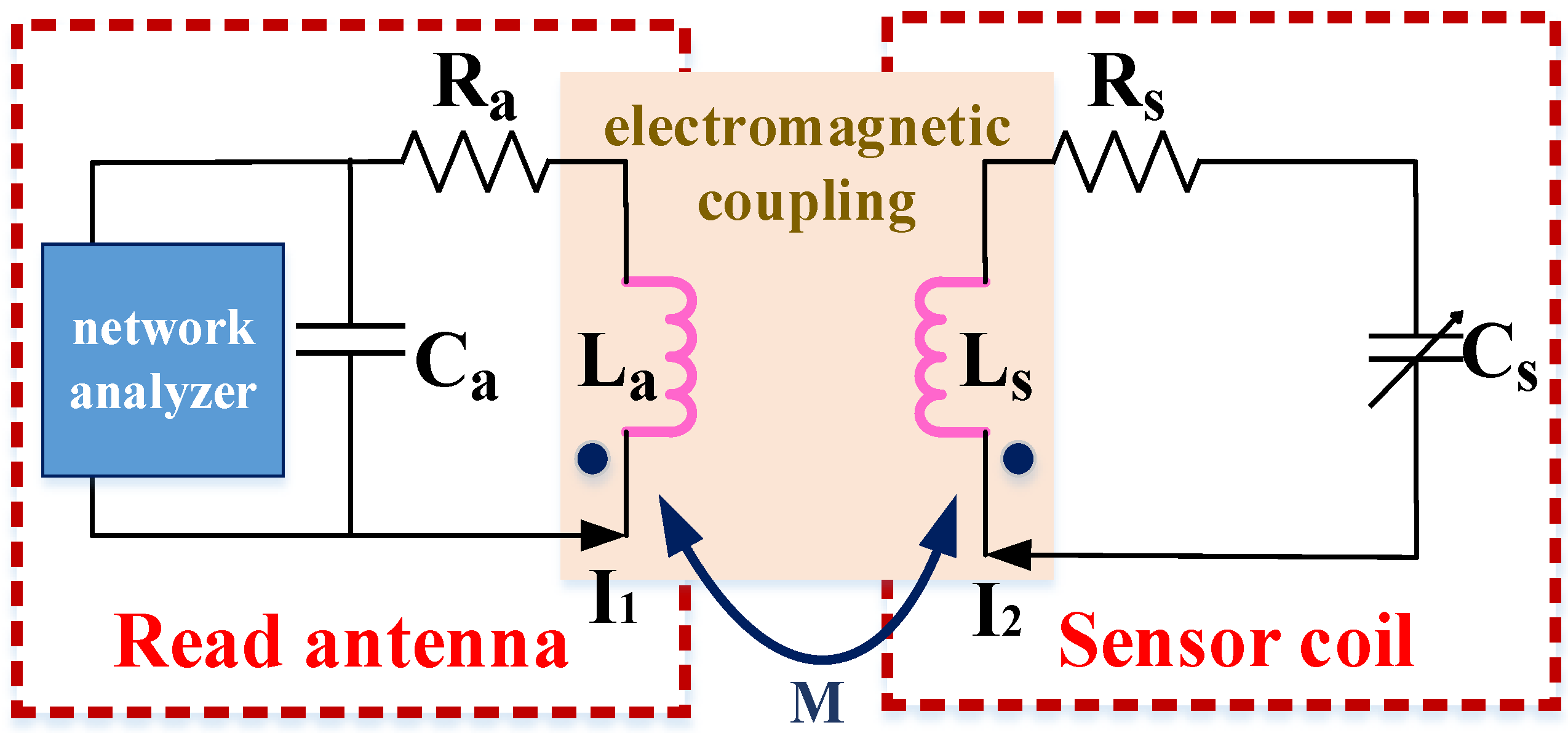
2.1. Wireless Coupling Principle
2.2. Gas Sensing Mechanism
3. Experimental Section
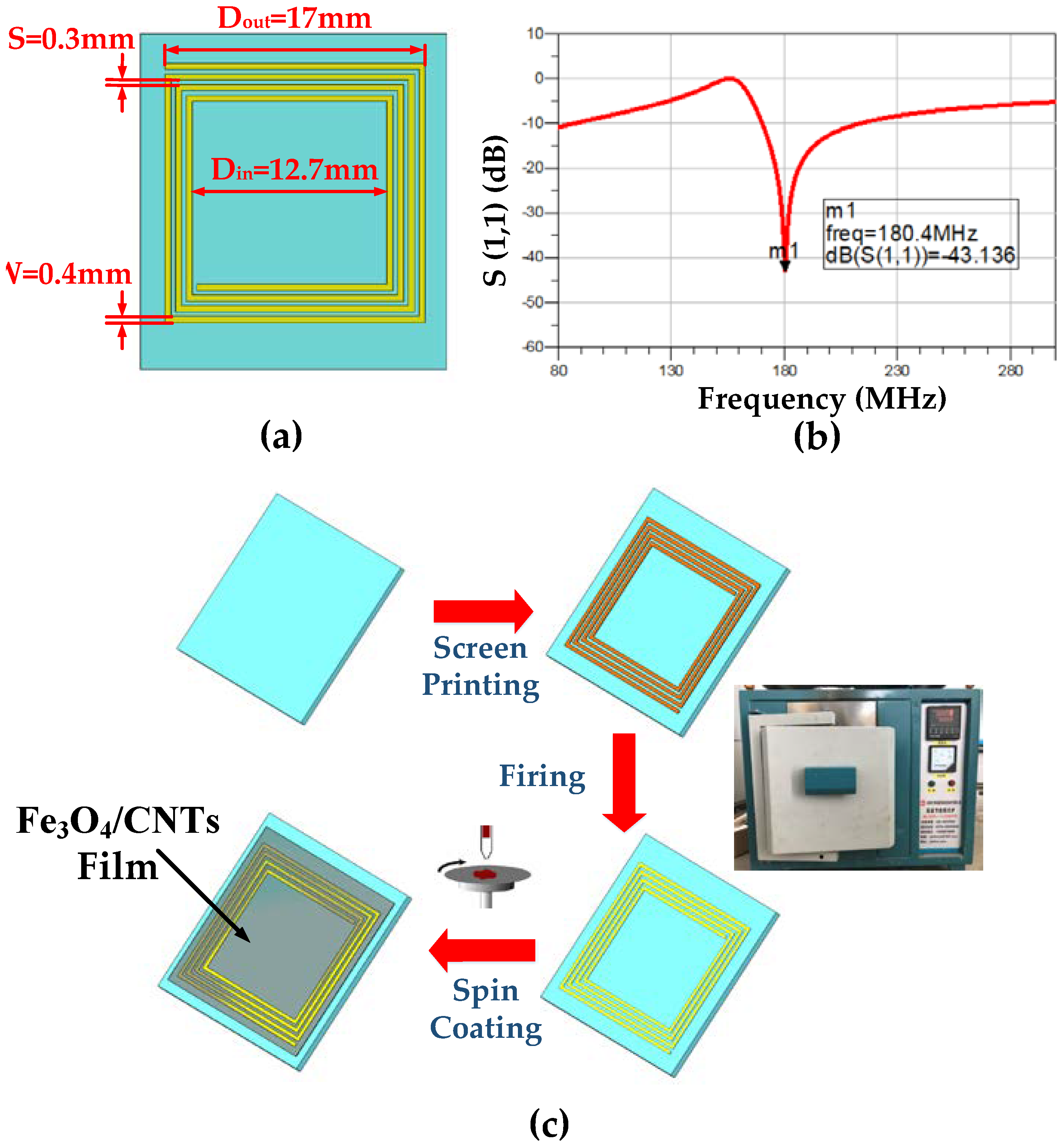
3.1. Design and Manufacturing of the Sensor
3.2. Sensor Test
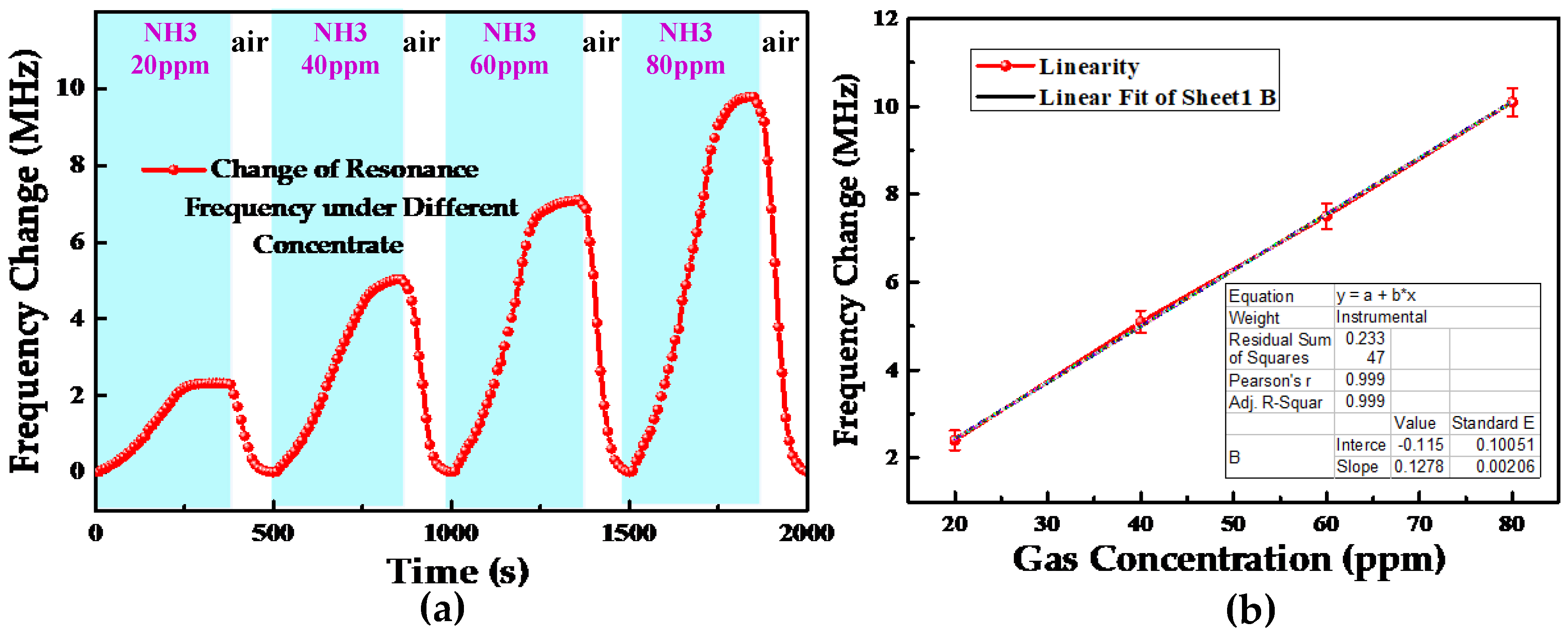
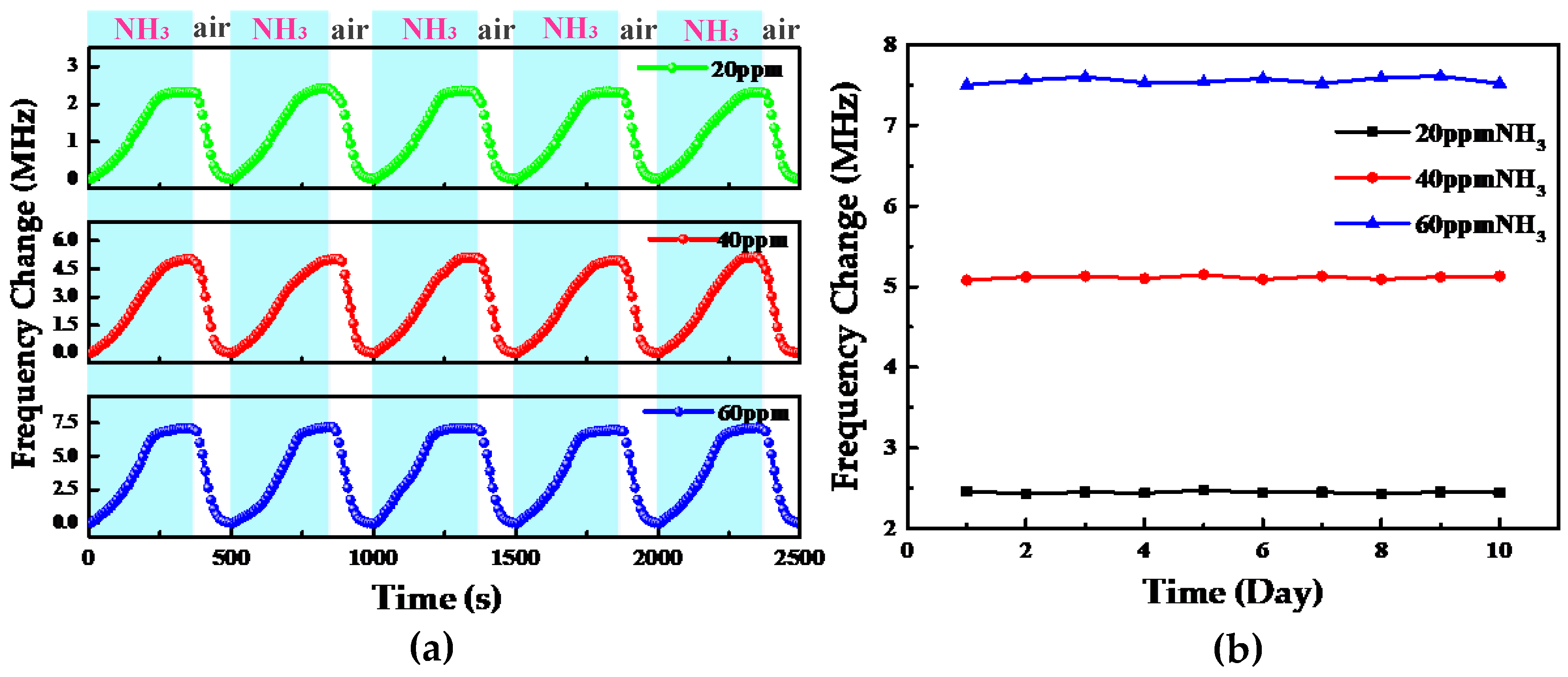
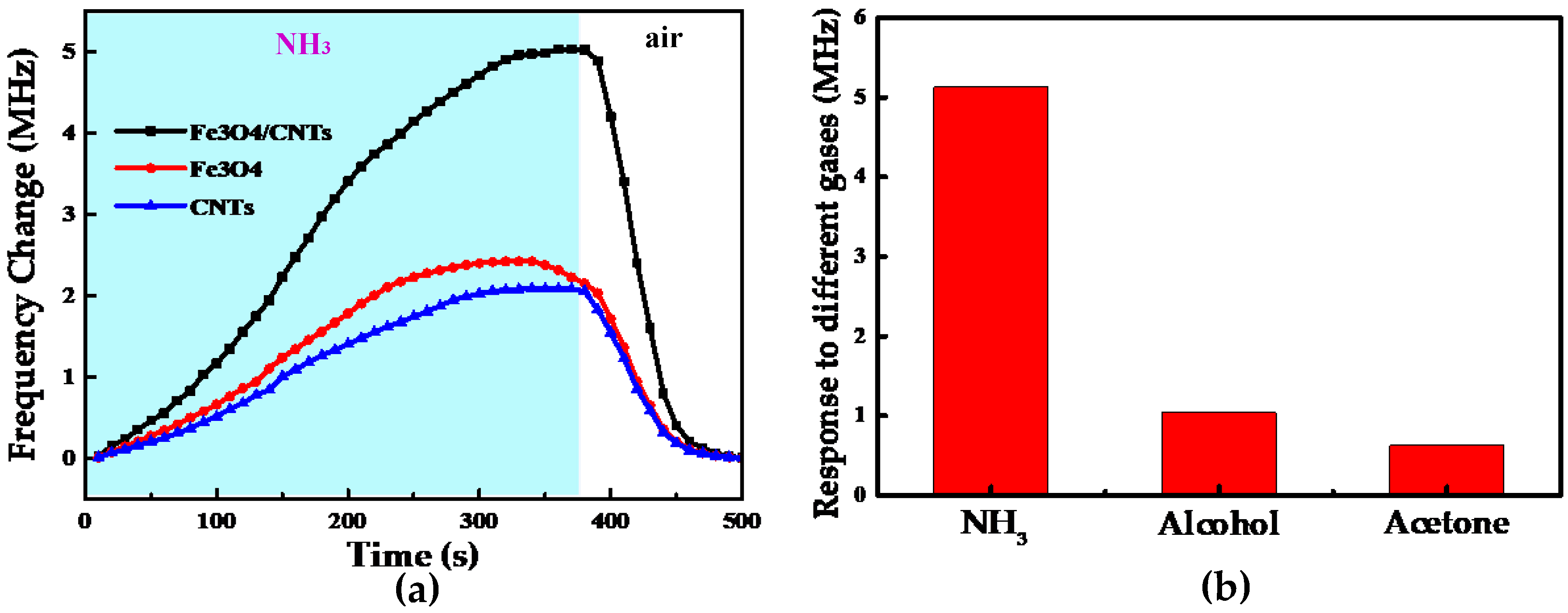
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Wolfe, D.W. Out of Thin Air—Nitrogen Fixers. Natural History, September 2001. [Google Scholar]
- Yoo, K.P.; Kwon, K.H.; Min, N.K.; Lee, M.J.; Lee, C.J. Effects of O2 plasma treatment on NH3 sensing characteristics of multiwall carbon nanotube/polyanilinecomposite films. Sens. Actuators B Chem. 2009, 143, 333–340. [Google Scholar] [CrossRef]
- Pushkarsky, M.; Webber, M.; Patel, C.K.N. Ultra-sensitive ambient ammonia detection using CO2-laser-based photoacoustic spectroscopy. Appl. Phys. B 2003, 77, 381–385. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, L.; Zhang, A.; Jiang, G.; Tang, H. A Peculiar Cyclic Voltammetric Behavior of Polyaniline in Acetonitrile and Its Application in Ammonia Vapor Sensor. J. Electroanal. Chem. 2007, 608, 141–147. [Google Scholar] [CrossRef]
- Dickey, E.C.; Varghese, O.K.; Ong, K.G.; Gong, D.; Paulose, M.; Grimes, C.A. Room Temperature Ammonia and Humidity Sensing Using Highly Ordered Nanoporous Alumina Films. Sensors 2002, 2, 91–110. [Google Scholar] [CrossRef] [Green Version]
- Luis, R.G.; Loredana, L.; Pilar, B.; Ignacio, R.J. A Review of Wireless Sensor Technologies and Applications in Agriculture and Food Industry: State of the Art and Current Trends. Sensors 2009, 9, 4728–4750. [Google Scholar] [Green Version]
- Mitchell, D.; Kulkarni, A.; Lostetter, A.; Schupbach, M.; Fraley, J.; Waits, R. Development and Testing of Harsh Environment, Wireless Sensor Systems for Industrial Gas Turbines. In Proceedings of the ASME Turbo Expo 2009: Power for Land, Sea, and Air, Orlando, FL, USA, 8–12 June 2009; pp. 139–149. [Google Scholar]
- Abu-Mahfouz, A.M.; Isaac, S.J.; Kruger, C.P.; Aakvaag, N.; Fismen, B. Wireless gas sensing in South African underground platinum mines. In Proceedings of the Wireless Communications and NETWORKING Conference, Istanbul, Turkey, 6–9 April 2014; pp. 3432–3437. [Google Scholar]
- Huang, J.; Wang, J.; Gu, C.; Yu, K.; Meng, F.; Liu, J. A novel highly sensitive gas ionization sensor for ammonia detection. Sens. Actuators A Phys. 2009, 150, 218–223. [Google Scholar] [CrossRef]
- Koul, S.; Chandra, R.; Dhawan, S. Conducting polyaniline composite: A reusable sensor material for aqueous ammonia. Sens. Actuators B Chem. 2001, 75, 151–159. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X. Fabrication and gas sensitivity of polyaniline–titanium dioxide nanocomposite thin film. Sens. Actuators B Chem. 2007, 125, 644–650. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Bin, L. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Wathes, C.M.; Demmers, T.G.M.; Xin, H. Ammonia Concentrations and Emissions in Livestock Production Facilities: Guidelines and Limits in the USA and UK. In Proceedings of the 2003 ASAE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003. [Google Scholar]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors forgas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Choi, S.J.; Jang, B.H.; Lee, S.J.; Min, B.K.; Rothschild, A.; Kim, I.D. Selective detectionof acetone and hydrogen sulfide for the diagnosis of diabetes and halitosisusing SnO2nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2587–2596. [Google Scholar]
- Yang, Y.; Sun, L.; Dong, X.; Yu, H.; Wang, T.; Wang, J.; Wang, R.; Yu, W.; Liu, G. Fe3O4/rGO nanocomposite: Synthesis and enhanced NOx gas-sensing properties at room temperature. RSC Adv. 2016, 6, 37085–37092. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. ChemInform Abstract: Carbon Nanomaterials for Electronics, Optoelectronics, Photovoltaics, and Sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Baggett, D.W.; Kim, J.W.; Siochi, E.J.; Connell, J.W. Instantaneous formation of metal and metal oxide nanoparticles on carbon nanotubes and graphene via solvent-free microwave heating. ACS Appl. Mater. Interfaces 2011, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Flujiwara, A.; Ishii, K.; Suematsu, H.; Kataura, K.; Maniwa, Y.; Suzuki, S.; Achiba, Y. Gas adsorption in the inside and outside of single-walled carbon nanotubes. Chem. Phys. Lett. 2001, 336, 205–211. [Google Scholar] [CrossRef]
- Marliere, C.; Poncharal, P.; Vaccarini, L.; Zahab, A. Effect of gas adsorption on the electrical properties of single-walled carbon nanotube mats. Proc. Mater. Res. Soc. Symp. 2000, 593, 173–179. [Google Scholar] [CrossRef]
- Sharma, D.K.; Rajput, M.; Akbar, S.A.; Kumar, A. Development of embedded system for carbon nano tube (CNT) based Ammonia (NH3) gas sensor. In Proceedings of the IEEE India Conference, New Delhi, India, 17–20 December 2015; pp. 1–4. [Google Scholar]
- Kumar, N.; Sahatiya, P.; Dubey, P. Fabrication of CNT based Gas Sensor Using Interdigitated Gold Electrodes. In Proceedings of the International Conference on Materials Processing and Characterisation, Hyderabad, India, 8–9 March 2014. [Google Scholar]
- Isa, S.S.M.; Ramli, M.M.; Jamlos, M.F.; Hambali, N.A.M.A.; Isa, M.M.; Kasjoo, S.R.; Ahmad, N.; Nor, N.I.M.; Khalid, N. Multi-walled carbon nanotubes plastic NH3 gas sensor. In Proceedings of the Asian Conference on Chemical Sensor, Penang, Malaysia, 16–18 November 2015. [Google Scholar]
- Salpavaara, T.; Verho, J.; Kumpulainen, P.; Lekkala, J. Wireless interrogation techniques for sensors utilizing inductively coupled resonance circuits. Procedia Eng. 2010, 5, 216–219. [Google Scholar] [CrossRef]
- Maksimovic, M.; Stojanovic, G.M.; Radovanovic, M.; Malešev, M.; Radonjanin, V.; Radosavljević, G.; Smetana, W. Application of a LTCC sensor for measuring moisture content of building materials. Constr. Build. Mater. 2011, 26, 327–333. [Google Scholar] [CrossRef]
- Salpavaara, T.; Verho, J.; Kumpulainen, P.; Lekkala, J. Readout methods for an inductively coupled resonance sensor used in pressure garment application. Sens. Actuators A Phys. 2010, 107, 109–116. [Google Scholar] [CrossRef]
- Huang, Z.; Yin, X.; Cui, T.; Hong, W. Equivalent Circuit Model and Parameter Extraction of Silicon-Based Planar Spiral Inductors. Chin. J. Radio Sci. 2005, 20, 777–783. [Google Scholar]
- Boyd, A.; Dube, I.; Fedorov, G.; Paranjape, M.; Barbara, P. Gas sensing mechanism of carbon nanotubes: From single tubes to high-density networks. Carbon 2014, 69, 417–423. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Kim, D.H.; Perello, D.; Park, Y.J.; Hong, S.H.; Yun, M.; Kim, S. Integrating Metal-Oxide-Decorated CNT Networks with a CMOS Readout in a Gas Sensor. Sensors 2012, 12, 2582–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, M.A. Polymer/Ceramic Wireless MEMS Pressure Sensors for Harsh Environments: High Temperature and Biomedical Applications; Georgia Institute of Technology: Atlanta, GA, USA, 2007. [Google Scholar]
- Liu, Q.; Zhang, X.; Zhang, B.; Luo, Y.; Cui, G.; Xie, F.; Sun, X. Ambient N2 fixation to NH3 electrocatalyzed by a spinel Fe3O4 nanorod. Nanoscale 2018, 10, 14386–14389. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J. The Effect on the Gas Selectivity of CNT-Based Gas Sensors by Binder in SWNT/Silane Sol Solution. IEEE Sens. J. 2009, 10, 173–177. [Google Scholar] [CrossRef]
- Alshammari, A.S.; Alenezi, M.R.; Lai, K.T.; Silva, S.R.P. Inkjet printing of polymer functionalized CNT gas sensor with enhanced sensing properties. Mater. Lett. 2016, 189, 299–302. [Google Scholar] [CrossRef]
- Ke, S.C.; Shen, C.H. Fe3O4 magnetic enhanced CMOS MEMS compatible gas sensor. In Proceedings of the IEEE International Conference of IEEE Region 10 (TENCON 2013), Xi’an, China, 22–25 October 2013; pp. 1–4. [Google Scholar]
- Ma, M.; Khan, H.; Shan, W.; Wang, Y.; Ou, J.Z.; Liu, Z.; Kalantar-Zadeh, K.; Li, Y. A novel wireless gas sensor based on LTCC technology. Sens. Actuators B Chem. 2017, 239, 711–717. [Google Scholar] [CrossRef]
- Bhadra, J.; Al-Thani, N.J.; Madi, N.K.; Al-Maadeed, M.A. High performance sulfonic acid doped polyaniline–polystyrene blend ammonia gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 8206–8216. [Google Scholar] [CrossRef]
- Tran, Q.T.; Hoa, H.T.M.; Yoo, D.H.; Cuong, T.V.; Hur, S.H.; Jin, S.C.; Kim, E.J.; Kohl, P.A. Reduced graphene oxide as an over-coating layer on silver nanostructures for detecting NH 3 gas at room temperature. Sens. Actuators B Chem. 2014, 194, 45–50. [Google Scholar] [CrossRef]
- Schütt, F.; Postica, V.; Adelung, R.; Lupan, O. Single and Networked ZnO-CNT Hybrid Tetrapods for Selective Room-Temperature High-Performance Ammonia Sensors. ACS Appl. Mater. Interfaces 2017, 9, 23107–23118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S.S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B Chem. 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Ghule, B.; Shaikh, S.F.; Ekar, S.; Nakate, U.T.; Gunturu, K.C.; Shinde, N.; Naushad, M.; Kim, K.H.; O’Dwyer, C.; Mane, R. Natural Carbonized Sugar as a Low-temperature Ammonia Sensor Material: Experimental, Theoretical and Computational Studies. ACS Appl. Mater. Interfaces 2017, 9, 43051–43060. [Google Scholar] [CrossRef] [PubMed]




| Materials | NH3 Concentration | Response Time | Recovery Time | Reference |
|---|---|---|---|---|
| Fe3O4/CNTs | 20 ppm | 290 s | 100 s | This work |
| PANI/HCSA | 100 ppm | 20 s | 80 s | [39] |
| RGO-A (aniline reducing) | 20 ppm | 1200 s | 300 s | [40] |
| single ZnO-T−CNT | 100 ppm | 20 s | 420 s | [41] |
| MWCNT/PEDOT:PSS | 30 ppm | 1200 s | 300 s | [42] |
| Natural Carbonized Sugar | 100 ppm | 50 s | 42 s | [43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; Zhou, T.; Tan, Q.; Guo, Q.; Lu, F.; Xiong, J. A Room-Temperature CNT/Fe3O4 Based Passive Wireless Gas Sensor. Sensors 2018, 18, 3542. https://doi.org/10.3390/s18103542
Guo T, Zhou T, Tan Q, Guo Q, Lu F, Xiong J. A Room-Temperature CNT/Fe3O4 Based Passive Wireless Gas Sensor. Sensors. 2018; 18(10):3542. https://doi.org/10.3390/s18103542
Chicago/Turabian StyleGuo, Tao, Tianhao Zhou, Qiulin Tan, Qianqian Guo, Fengxiang Lu, and Jijun Xiong. 2018. "A Room-Temperature CNT/Fe3O4 Based Passive Wireless Gas Sensor" Sensors 18, no. 10: 3542. https://doi.org/10.3390/s18103542





