A Multifunctional Molecular Probe for Detecting Hg2+ and Ag+ Based on Ion-Mediated Base Mismatch
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fluorescent Signal Detection
3. Principles
4. Results and Discussion
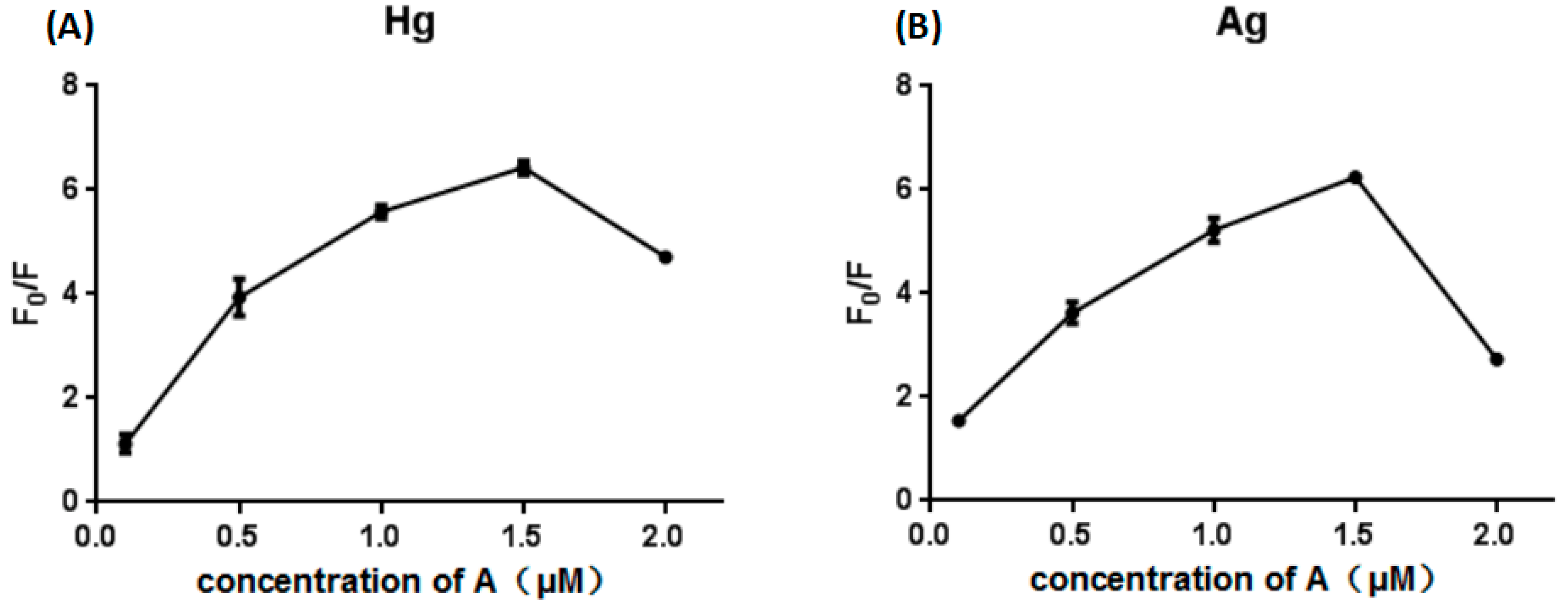
4.1. Orthonormal Preliminary Optimization
4.2. Optimization of Reaction Conditions
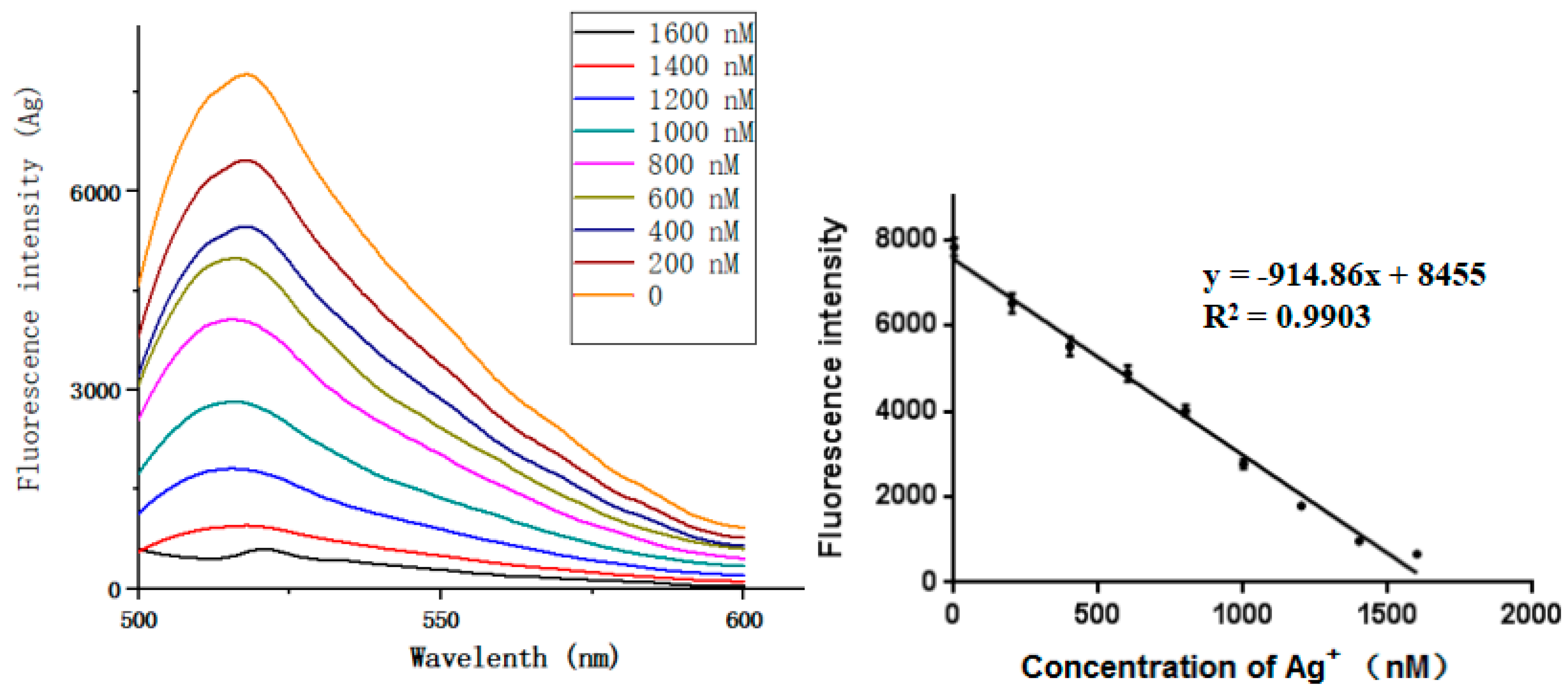
4.3. Sensitivity
5. Conclusions
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Xu, J. The era of bio-computers is coming. Chin. Acad. Sci. 2014, 29, 42–54. [Google Scholar]
- Wan, F.; Dong, C.; Yang, J.; Dong, Y.; Zhang, C. The development and application of DNA computing technology. Bull. Chin. Acad. Sci. 2014, 29, 94–105. [Google Scholar]
- Li, C.H.; Xiao, X.; Tao, J.; Wang, D.M.; Huang, C.Z.; Zhen, S.J. A graphene oxide-based strand displacement amplification platform for ricin detection using aptamer as recognition element. Biosens. Bioelectron. 2017, 91, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, Z.; Deng, C.; Song, T.; Pan, L.; Chen, Z. A novel bio-sensor based on DNA strand displacement. PLoS ONE 2014, 9, e108856. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jiang, S.; Liu, X.; Pan, L.; Zhang, C. Aptamer-binding directed DNA origami pattern for logic gates. ACS Appl. Mater. Interfaces 1944, 8, 34054–34060. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, Z.; Li, Y.; Xu, F.; Zhang, Q.; Zhang, C. Nicking enzyme-controlled toehold regulation for DNA logic circuits. Nanoscale 2017, 9, 18223–18228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Desilva, A.P.; Mcclenaghan, N.D. Molecular-scale logic gates. Chem. Eur. J. 2004, 10, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.W. Value of DNA evidence in detecting crime. J. Forensic Sci. 2007, 52, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zeng, X.; Dandapat, A.; Chi, Y.; Kim, D. Installing logic gates in permeability controllable polyelectrolyte-carbon nitride films for detecting proteases and nucleases. Anal. Chem. 2015, 87, 8851–8857. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, L.; Mao, X.; Lu, M.; Zhao, J.; Yin, Y. Electrochemical detection of glutathione based on Hg2+-mediated strand displacement reaction strategy. Biosens. Bioelectron. 2016, 85, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Adleman, L.M. Molecular Computation of solutions to combinatorial problems. Science 1994, 266, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D. (Bio)sensors for measurement of analytes implicated in food safety: A review. Trends Anal. Chem. 2002, 21, 96–115. [Google Scholar] [CrossRef]
- Bedelean, H.; Măicăneanu, A.; Burcă, S.; Stanca, M. Removal of heavy metal ions from wastewaters using natural clays. Clay Miner. 2009, 44, 487–495. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; And, R.C.J.; Hupp, J.T. Gold Nanoparticle-based sensing of “spectroscopically silent” heavy metal ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Mo, J.; Han, Y. Research progress of Hg2+ detection technology in water environment. Mod. Instrum. Med. Treat. 2010, 3, 14–17. [Google Scholar]
- Huang, Y.; Wang, Z.; Xue, Y. Research progress on the detection of Hg2+ based on DNA. Public Health Cap. 2016, 10, 263–266. [Google Scholar]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Cheminform 2008, 39, 3443–3480. [Google Scholar] [CrossRef]
- Wang, S. Metal Poisoning; People’s Health Press: Metairie, LA, USA, 1988. [Google Scholar]
- Zhang, Y.; Jiang, H.; Wang, X. Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag+ and Hg2+. Anal. Chim. Acta 2015, 870, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, PRC. GB5749-2006 Hygienic Standard for Drinking Water; China Standard Press: Beijing, China, 2007.
- Zhou, Y.; Yoon, J. Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem. Soc. Rev. 2011, 41, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorber, K.E. Monitoring of heavy metals by energy dispersive X-ray fluorescence spectrometry. Waste Manag. Res. 1986, 4, 3–13. [Google Scholar] [CrossRef]
- Kunkel, R.; Manahan, S.E. Atomic absorption analysis of strong heavy metal chelating agents in water and waste water. Anal. Chem. 1973, 45, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Bings, N.H.; Bogaerts, A.; Broekaert, J.A.C. Atomic spectroscopy. Anal. Chem. 2013, 85, 670–704. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Oda, S.; Yamaguchi, H.; Kondo, Y.; Kojima, C.; Ono, A. 15N-15N J-coupling across Hg(II): Direct observation of Hg(II)-mediated T-T base pairs in a DNA duplex. J. Am. Chem. Soc. 2007, 129, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Cao, S.; Togashi, H.; Tashiro, M.; Fujimoto, T.; Machinami, T.; Oda, S.; Miyake, Y.; Okamoto, I.; Tanaka, Y. Specific interactions between silver(I) ions and cytosine-cytosine pairs in DNA duplexes. Chem. Commun. 2008, 4825–4827. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xue, X.; Liu, X. One-step, room-temperature, colorimetric detection of Hg+(Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245. [Google Scholar] [CrossRef]
- Jing, X.U.; Kong, D.M. Specific Hg2+ quantitation using intramolecular split G-quadruplex DNAzyme. Chin. J. Anal. Chem. 2012, 40, 347–353. [Google Scholar]
- Ge, J.; Li, X.P.; Jiang, J.H.; Yu, R.Q. A highly sensitive label-free sensor for mercury ion (Hg2+) by inhibiting thioflavin T as DNA G-quadruplexes fluorescent inducer. Talanta 2014, 122, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wong, J.X.; Li, X.; Li, Y.; Yu, H.Z. Detection and quantitation of heavy metal ions on bona fide DVDs using DNA molecular beacon probes. Anal. Chem. 2015, 87, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, X.; Wu, T.; Dong, Y. An ions-medicated single molecular multi-functional DNA cascade logic circuit and signal amplifier model. In Bio-Inspired Computing: Theories and Applications, Proceedings of the 12th International Conference, BIC-TA 2017, Harbin, China, 1–3 December 2017; Springer: Singapore, 2017. [Google Scholar]
- Cui, C.; Feng, S.; Li, Y.; Wang, S. Orthogonal analysis for perovskite structure microwave dielectric ceramic thin films fabricated by the RF magnetron-sputtering method. J. Mater. Sci. Mater. Electron. 2010, 21, 349–354. [Google Scholar]
- Wu, X.; Leung, D.Y.C. Optimization of biodiesel production from camelina oil using orthogonal experiment. Appl. Energy 2011, 88, 3615–3624. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, K.; Wu, L.; Wang, D.; Ge, S. Synthesis and characterization of PVA-HA-Silk composite hydrogel by orthogonal experiment. J. Bionic Eng. 2012, 9, 234–242. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Zhou, S.; Weng, J. Investigation on process parameters of electrospinning system through orthogonal experimental design. J. Appl. Polym. Sci. 2010, 103, 3105–3112. [Google Scholar] [CrossRef]
- Xuan, F.; Luo, X.; Hsing, I.M. Conformation-dependent exonuclease III activity mediated by metal ions reshuffling on thymine-rich DNA duplexes for an ultrasensitive electrochemical method for Hg2+ detection. Anal. Chem. 2013, 85, 4586–4593. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Wen, W.; Zhang, X.; Xia, Q.; Wang, S. An exonuclease-assisted amplification electrochemical aptasensor for Hg2+ detection based on hybridization chain reaction. Biosens. Bioelectron. 2015, 70, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; She, Z.; Sharma, R.; Kraatz, H.B. A study of the interactions of Hg(II) with T-T mispair containing hairpin loops. Electrochim. Acta 2017, 243, 44–52. [Google Scholar] [CrossRef]
- Lee, J.S.; Mirkin, C.A. Chip-based scanometric detection of mercuric ion using DNA-functionalized gold nanoparticles. Anal. Chem. 2008, 80, 6805–6808. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Balcioglu, M.; Robertson, N.M.; Hizir, M.S.; Yumak, S.; Yigit, M.V. Low picomolar, instrument-free visual detection of mercury and silver ions using low-cost programmable nanoprobes. Chem. Sci. 2017, 8, 1200–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyur, D.B.; Disha, J.V.; Bharat, A.M.; Savan, M.D.; Vinod, K.J.; Hemangini, S. Turn-on fluorescence probe for selective detection of Hg(II) by calixpyrrole hydrazide reduced silver nanoparticle: Application to real water sample. Chin. Chem. Lett. 2016, 27, 731–737. [Google Scholar]
- Jiang, X.D.; Yu, H.F.; Zhao, J.L.; Sun, C.L.; Xie, Y.; Xiao, L.J. A colorimetric chemosensor based on new water-soluble PODIPY dye for Hg2+ detection. Chin. Chem. Lett. 2015, 26, 1241–1245. [Google Scholar] [CrossRef]
- Park, C.; Jang, K.; Lee, S.; You, J.; Lee, S.; Ha, H.; Yun, K.; Kim, J.; Lee, H.; Prak, J.; et al. A highly sensitive, direct and label-free technique for Hg2+ detection using Kelvin probe force microscopy. Nanotechnology 2015, 26, 305501. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X.; Willner, I. DNA switches: From principles to applications. Angew. Chem. Int. Ed. 2015, 54, 1098–1129. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.D.; Xia, L.; Xu, D.D.; Xing, X.J.; Pang, D.W.; Tang, H.W. DNA-stabilized silver nanoclusters and carbon nanoparticles oxide: A sensitive platform for label-free fluorescence turn-on detection of HIV-DNA sequences. Biosens. Bioelectron. 2016, 85, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, N.; Lan, J.; Ji, X.; He, Z. A label-free colorimetric platform for DNA via target-catalyzed hairpin assembly and the peroxidase-like catalytic of graphene/Au-NPs hybrids. Anal. Chim. Acta 2016, 902, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, Y.; Xu, Y.; Liu, Y.; Li, D.; Fan, C. A highly sensitive chemiluminescence sensor for detecting mercury (II) ions: A combination of Exonuclease III-aided signal amplification and graphene oxide-assisted background reduction. Sci. China Chem. 2015, 58, 514–518. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, L.; Ge, S.; Yan, M.; Yu, J.; Huang, J.; Liu, S. Three-dimensional paper-based electrochemiluminescence device for simultaneous detection of Pb2+ and Hg2+ based on potential-control technique. Biosens. Bioelectron. 2013, 41, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, X.; Kraatz, H.B. Impedimetric immobilized DNA-based sensor for simultaneous detection of Pb2+, Ag+, and Hg2+. Anal. Chem. 2011, 83, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Hui, B.T.; Wu, H.; Zuo, X.; Li, S.F.Y. Detection of Hg2+ using molecular beacon-based fluorescent sensor with high sensitivity and tunable dynamic range. Sens. Actuators B Chem. 2014, 195, 623–629. [Google Scholar]
- Li, Y.; Liu, N.; Liu, H.; Wang, Y.; Hao, Y.; Ma, X.; Li, X.; Huo, Y.; Lu, J.; Tang, S.; et al. A novel label-free fluorescence assay for one-step sensitive detection of Hg2+ in environmental drinking water samples. Sci. Rep. 2017, 7, 45974. [Google Scholar] [CrossRef] [PubMed]
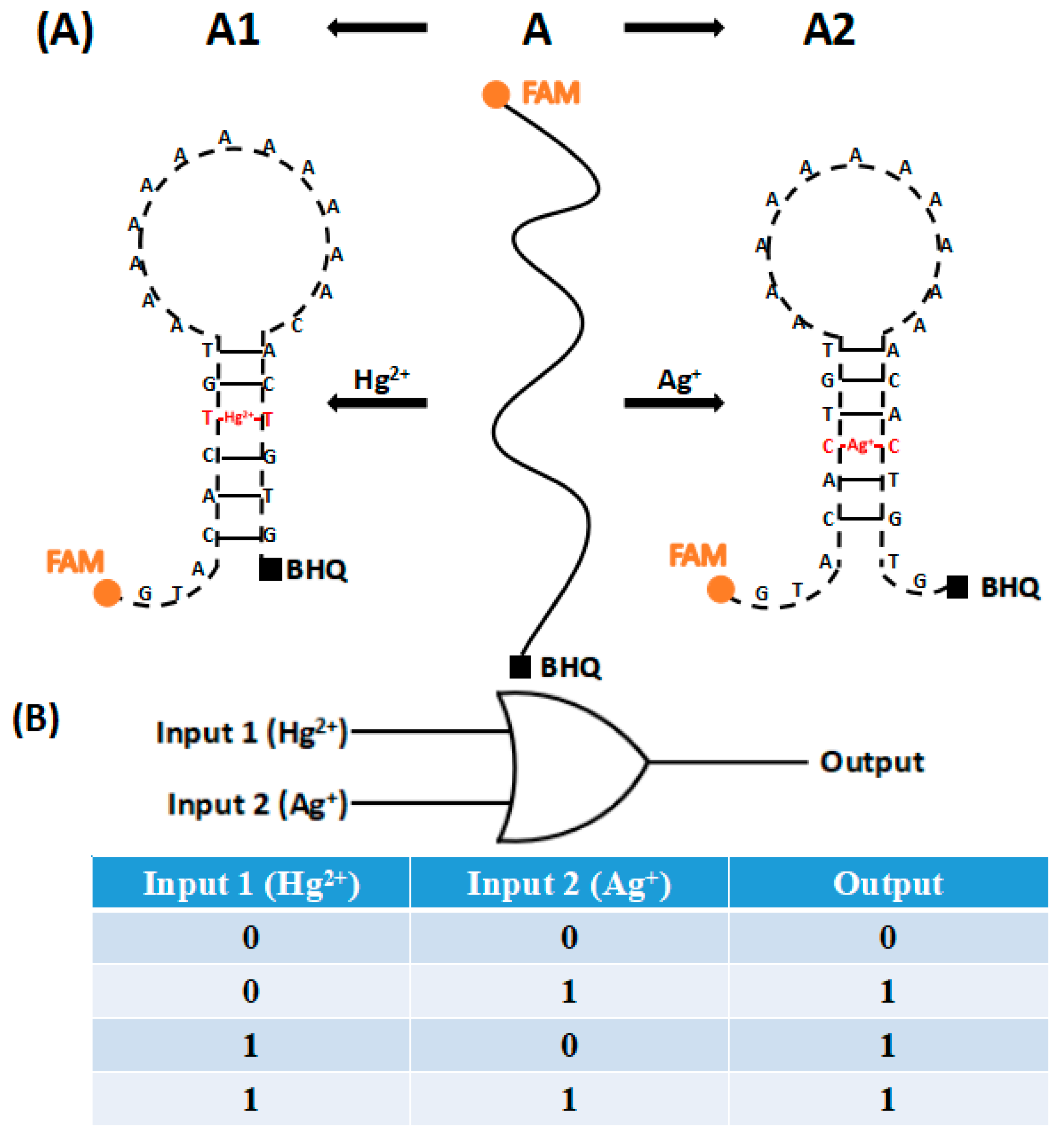





| Single Strand | Strand Sequence (5′–3′) |
|---|---|
| A | FAM–GTACACTGTAAAAAAAAAAAAAAACACTGTG–BHQ |
| Factor | Concentration of A Strand (A) | Concentration of Ions (B) | Reaction Time (C) |
|---|---|---|---|
| 1 | 0.5 μM | 100 nM | 30 min |
| 2 | 1 μM | 200 nM | 60 min |
| 3 | 1.5 μM | 500 nM | 90 min |
| Experiment Number | Concentration of A Strand (A) | Concentration of Ions (B) | Reaction Time (C) | F0–F | ||||
|---|---|---|---|---|---|---|---|---|
| Hg2+ | Ag+ | |||||||
| 1 | A1 | B1 | C1 | 1239.4 | 1042.8 | |||
| 2 | A1 | B2 | C3 | 1572.9 | 1806.4 | |||
| 3 | A1 | B3 | C2 | 1535.1 | 2010.4 | |||
| 4 | A2 | B1 | C3 | 3243.2 | 1090.8 | |||
| 5 | A2 | B2 | C2 | 6569.5 | 4773.2 | |||
| 6 | A2 | B3 | C1 | 7675.0 | 7103.0 | |||
| 7 | A3 | B1 | C2 | 3723.7 | 1201.6 | |||
| 8 | A3 | B2 | C1 | 8459.9 | 9090.4 | |||
| 9 | A3 | B3 | C3 | 11,151.3 | 10,298.2 | |||
| Hg2+ | Ag+ | Hg2+ | Ag+ | Hg2+ | Ag+ | |||
| K1 | 4347.4 | 4859.6 | 8206.3 | 3335.2 | 17,374.3 | 17,236.2 | ||
| K2 | 17,487.7 | 12,967.0 | 16,602.4 | 15,670.0 | 11,828.4 | 7985.2 | ||
| K3 | 23,334.9 | 20,590.2 | 20,361.4 | 19,411.6 | 15,967.5 | 13,195.4 | ||
| k1 | 1449.1 | 1619.9 | 2735.4 | 1111.7 | 5791.4 | 5745.4 | ||
| k2 | 5829.2 | 4322.3 | 5534.1 | 5223.3 | 3942.8 | 2661.7 | ||
| k3 | 7778.3 | 6863.4 | 6787.1 | 6470.5 | 5322.5 | 4398.5 | ||
| R | 6329.2 | 5243.5 | 4051.7 | 5358.8 | 1848.6 | 3083.7 | ||
| Added | Detected | Recovery (%) | SD | |
|---|---|---|---|---|
| Hg2+ | 3.9 pM | 10.2 pM | 262 | 4.1 |
| 5.0 pM | 13.6 pM | 272 | 3.6 | |
| 15.0 pM | 23.3 pM | 150 | 3.2 | |
| 25.0 pM | 30.3 pM | 121 | 4.7 | |
| 35.0 pM | 38.4 pM | 110 | 4.6 | |
| 45.0 pM | 47.7 pM | 106 | 5.0 | |
| Ag+ | 3.9 pM | 12.3 pM | 315 | 5.4 |
| 5.0 pM | 15.3 pM | 306 | 2.2 | |
| 15.0 pM | 21.3 pM | 142 | 3.7 | |
| 25.0 pM | 31.1 pM | 124 | 3.6 | |
| 35.0 pM | 39.0 pM | 111 | 5.4 | |
| 45.0 pM | 48.1 pM | 107 | 4.7 |
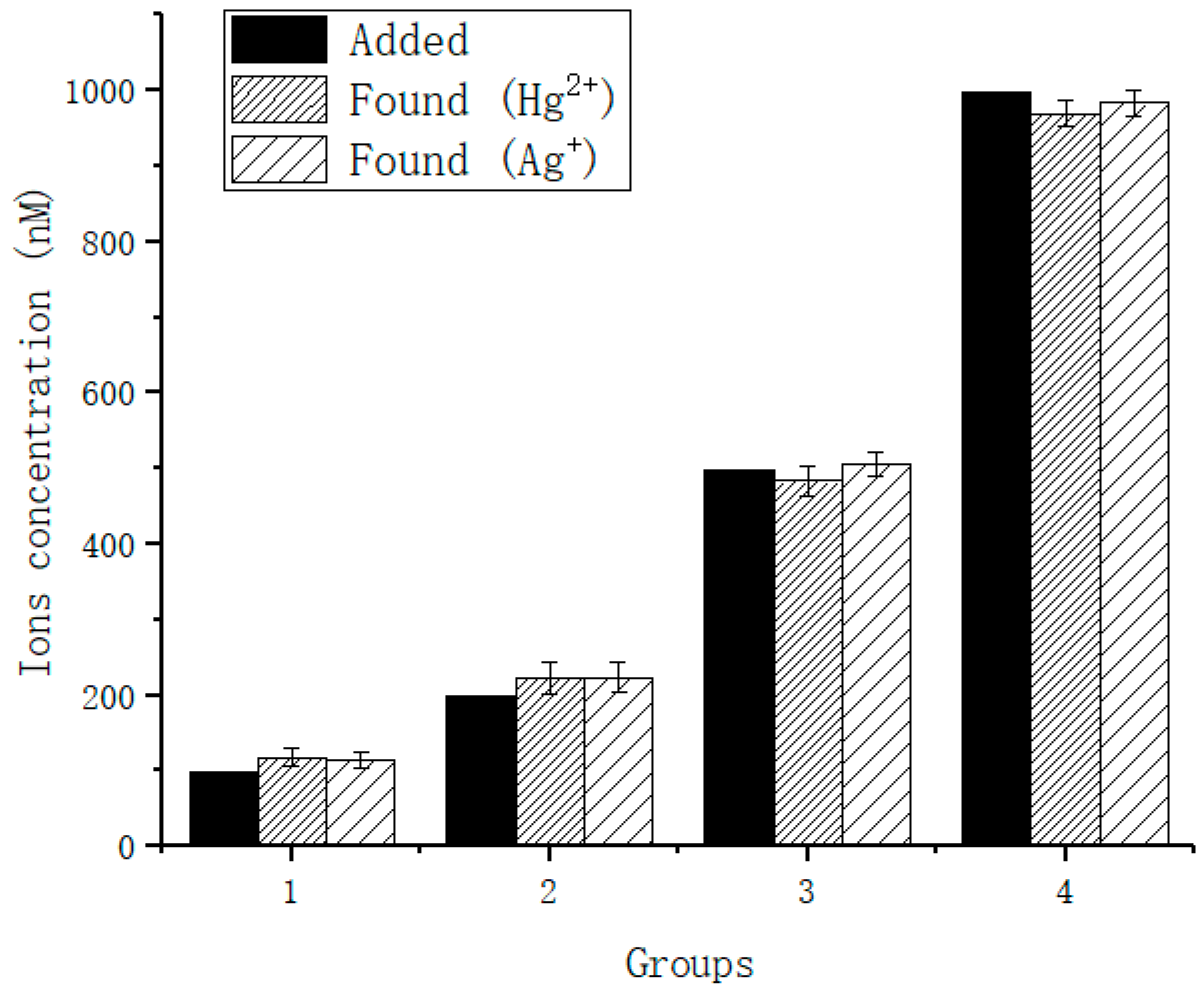
| Added | Detected | Recovery (%) | SD | |
|---|---|---|---|---|
| Hg2+ | 100 nM | 118 nM | 118 | 12.4 |
| 200 nM | 222 nM | 111 | 21.8 | |
| 500 nM | 483 nM | 96.6 | 19.4 | |
| 1000 nM | 969 nM | 96.9 | 16.7 | |
| Ag+ | 100 nM | 114 nM | 114 | 11.4 |
| 200 nM | 223 nM | 115 | 20.3 | |
| 500 nM | 505 nM | 101 | 15.4 | |
| 1000 nM | 983 nM | 98.3 | 17.9 |
| Detection Method | LOD | Analytical Range (nM) | Reaction and Incubation Time | Ref. |
|---|---|---|---|---|
| Colorimetric | 10 pM | 1.0 × 10−2–1 | 45 min | [40] |
| 1 nM | 1–1.0 × 103 | 6 day | [41] | |
| 2 nM | 2–1.0 × 102 | 3 h | [51] | |
| Electrochemical | 0.2 nM | 0.5–1.0 × 103 | 50 min | [52] |
| 0.12 pM | 0.2 × 10−3–35 | 2 h | [38] | |
| 0.1 nM | 0.1–1 × 104 | 5 day | [53] | |
| Fluorometric | 30 nM | 0–117.0 × 106 | 1 h | [21] |
| 9.5 nM | 32–1.8 × 103 | 30 min | [54] | |
| 3 nM | 5–1.0 × 103 | 1 min | [55] | |
| 35 pM | 0–1.6 × 103 | 15 min | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, Y.; Dong, Y. A Multifunctional Molecular Probe for Detecting Hg2+ and Ag+ Based on Ion-Mediated Base Mismatch. Sensors 2018, 18, 3280. https://doi.org/10.3390/s18103280
Wang L, Zhang Y, Dong Y. A Multifunctional Molecular Probe for Detecting Hg2+ and Ag+ Based on Ion-Mediated Base Mismatch. Sensors. 2018; 18(10):3280. https://doi.org/10.3390/s18103280
Chicago/Turabian StyleWang, Luhui, Yingying Zhang, and Yafei Dong. 2018. "A Multifunctional Molecular Probe for Detecting Hg2+ and Ag+ Based on Ion-Mediated Base Mismatch" Sensors 18, no. 10: 3280. https://doi.org/10.3390/s18103280




