Response Characterization of an Inexpensive Aerosol Sensor
Abstract
:1. Introduction
2. Methods
2.1. Prototype Aerosol Sensor (PAS)
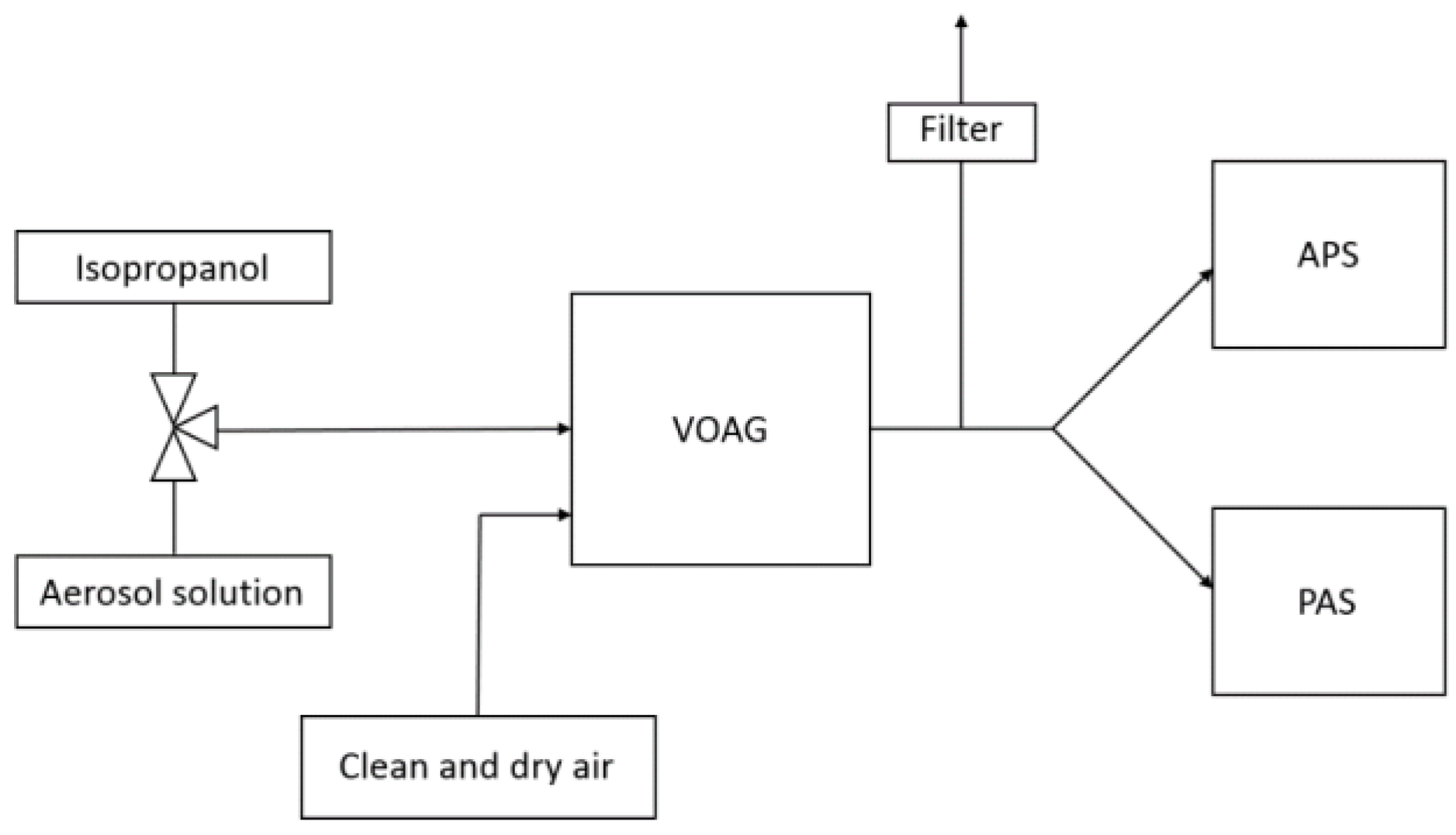
2.2. Novel Laboratory Evaluation Method
2.3. Field Validation
2.3.1. Reference Instrument and Configuration
2.3.2. Measurement Site
3. Results and Discussion
3.1. Laboratory Test Results
3.1.1. Transparent Liquid Dioctyl Sebacate (DOS)
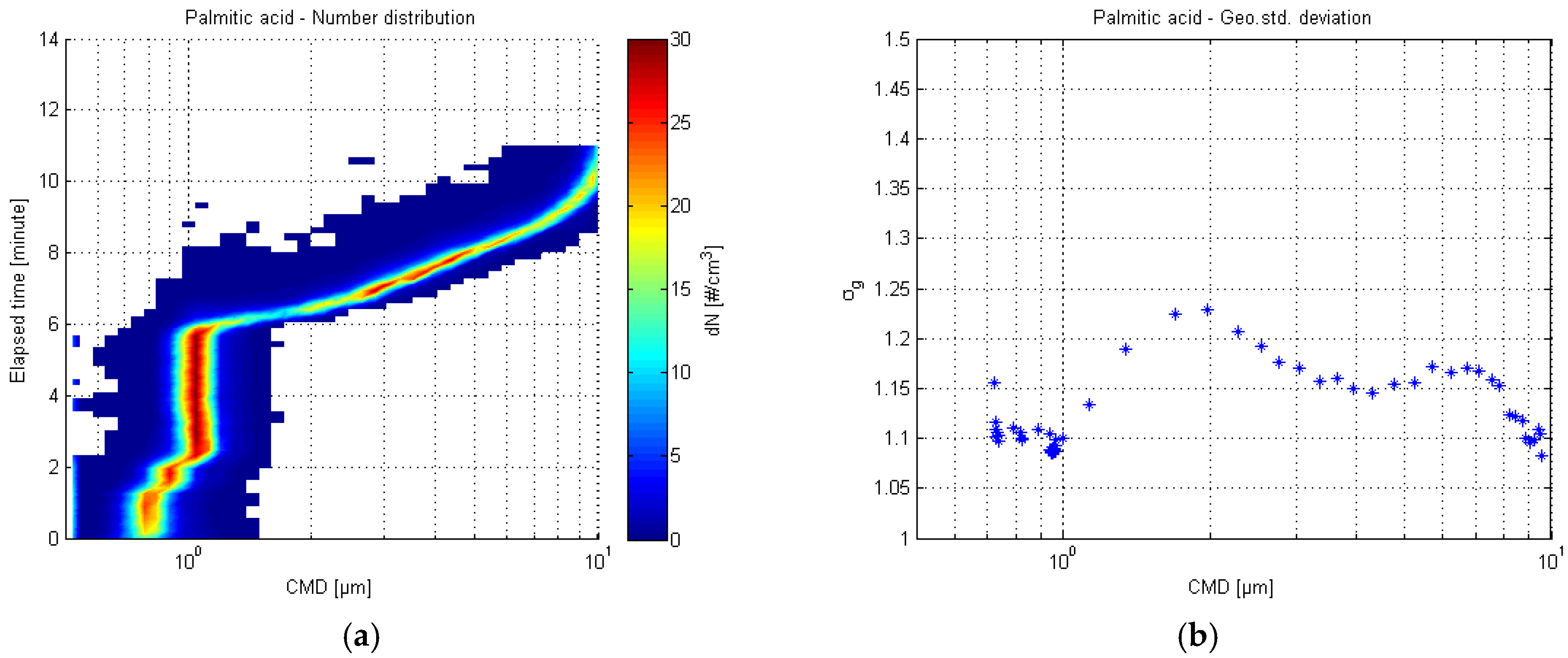
3.1.2. White Crystalline Palmitic Acid
3.2. Field Validation Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Official Journal of the European Union. Directive 2008/50/EC of the European Parliament and the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; Official Journal of the European Union: Brussels, Belgium, 2008. [Google Scholar]
- Heimann, I.; Bright, V.B.; McLeod, M.W.; Mead, M.I.; Popoola, O.A.M.; Stewart, G.B.; Jones, R.L. Source Attribution of Air Pollution by Spatial Scale Separation using High Spatial Density Networks of Low Cost Air Quality Sensors. Atmos. Environ. 2015, 113, 10–19. [Google Scholar] [CrossRef]
- Moltchanov, S.; Levy, I.; Etzion, Y.; Lerner, U.; Broday, D.M.; Fishbain, B. On the Feasibility of Measuring Urban Air Pollution by Wireless Distributed Sensor Networks. Sci. Total Environ. 2015, 502, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Rajasegarar, S.; Zhang, P.; Zhou, Y.; Karunasekera, S.; Leckie, C.; Palaniswami, M. High Resolution Spatio-Temporal Monitoring of Air Pollutants using Wireless Sensor Networks. In Proceedings of the 2014 IEEE Ninth International Conference on Intelligent Sensors, Sensor Networks and Information Processing (ISSNIP), Singapore, 21–24 April 2014; pp. 1–6. [Google Scholar]
- Jerrett, M.; Donaire-Gonzalez, D.; Popoola, O.; Jones, R.; Cohen, R.C.; Almanza, E.; de Nazelle, A.; Mead, I.; Carrasco-Turigas, G.; Cole-Hunter, T.; et al. Validating Novel Air Pollution Sensors to Improve Exposure Estimates for Epidemiological Analyses and Citizen Science. Environ. Res. 2017, 158, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Holstius, D.M.; Pillarisetti, A.; Smith, K.R.; Seto, E. Field Calibrations of a Low-Cost Aerosol Sensor at a Regulatory Monitoring Site in California. Atmos. Meas. Tech. 2014, 7, 1121–1131. [Google Scholar] [CrossRef]
- Jiao, W.; Hagler, G.; Williams, R.; Sharpe, R.; Brown, R.; Garver, D.; Judge, R.; Caudill, M.; Rickard, J.; Davis, M.; et al. Community Air Sensor Network (CAIRSENSE) Project: Evaluation of Low-Cost Sensor Performance in a Suburban Environment in the Southeastern United States. Atmos. Meas. Tech. 2016, 9, 5281–5292. [Google Scholar] [CrossRef]
- Gao, M.; Cao, J.; Seto, E. A Distributed Network of Low-Cost Continuous Reading Sensors to Measure Spatiotemporal Variations of PM2.5 in Xi’an, China. Environ. Pollut. 2015, 199, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.C.; Kumar, P.; Pilla, F.; Skouloudis, A.N.; Di Sabatino, S.; Ratti, C.; Yasar, A.; Rickerby, D. End-User Perspective of Low-Cost Sensors for Outdoor Air Pollution Monitoring. Sci. Total Environ. 2017, 607, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Morawska, L.; Martani, C.; Biskos, G.; Neophytou, M.; Di Sabatino, S.; Bell, M.; Norford, L.; Britter, R. The Rise of Low-Cost Sensing for Managing Air Pollution in Cities. Environ. Int. 2015, 75, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, P.A.; Willeke, K. Aerosol Measurement: Principles, Techniques, and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Hinds, W.C. Aerosol Technology: Properties, Behaviour, and Measurement of Airborne Particles, 2nd ed.; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Kelly, K.E.; Whitaker, J.; Petty, A.; Widmer, C.; Dybwad, A.; Sleeth, D.; Martin, R.; Butterfield, A. Ambient and Laboratory Evaluation of a Low-Cost Particulate Matter Sensor. Environ. Pollut. 2017, 221, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Jing, H.; Zhang, Q.; Jiang, J.; Biswas, P. Laboratory Evaluation and Calibration of Three Low-Cost Particle Sensors for Particulate Matter Measurement. Aerosol Sci. Technol. 2015, 49, 1063–1077. [Google Scholar] [CrossRef]
- Sousan, S.; Koehler, K.; Thomas, G.; Park, J.H.; Hillman, M.; Halterman, A.; Peters, T.M. Inter-Comparison of Low-Cost Sensors for Measuring the Mass Concentration of Occupational Aerosols. Aerosol Sci. Technol. 2016, 50, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Sousan, S.; Koehler, K.; Hallett, L.; Peters, T.M. Evaluation of the Alphasense Optical Particle Counter (OPC-N2) and the Grimm Portable Aerosol Spectrometer (PAS-1.108). Aerosol Sci. Technol. 2016, 50, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Zikova, N.; Hopke, P.K.; Ferro, A.R. Evaluation of New Low-Cost Particle Monitors for PM2.5 Concentrations Measurements. J. Aerosol Sci. 2017, 105, 24–34. [Google Scholar] [CrossRef]
- Shinyei Technology Co., Ltd. Technical Specifications Sheet: PPD42NS; Shinyei Technology Co., Ltd.: Kobe, Japan, 2010. [Google Scholar]
- Shinyei Technology Co., Ltd. Technicial Specifications Sheet: PPD60PV; Shinyei Technology Co., Ltd.: Kobe, Japan, 2010. [Google Scholar]
- Peters, T.M. Comparison of the Grimm 1.108 and 1.109 Portable Aerosol Spectrometer to the TSI 3321 Aerodynamic Particle Sizer for Dry Particles. Ann. Occup. Hyg. 2006, 50, 843–850. [Google Scholar] [PubMed]
- Vesala, T.; Järvi, L.; Launiainen, S.; Sogachev, A.; Rannik, U.; Mammarella, I.; Siivola, E.; Keronen, P.; Rinne, J.; Riikonen, A.; et al. Surface-Atmosphere Interactions Over Complex Urban Terrain in Helsinki, Finland. Tellus Ser. B Chem. Phys. Meteorol. 2008, 60, 188–199. [Google Scholar] [CrossRef]
- Järvi, L.; Rannik, U.; Mammarella, I.; Sogachev, A.; Aalto, P.P.; Keronen, P.; Siivola, E.; Kulmala, M.; Vesala, T. Annual Particle Flux Observations Over a Heterogeneous Urban Area. Atmos. Chem. Phys. 2009, 9, 7847–7856. [Google Scholar] [CrossRef] [Green Version]
- Timonen, H.; Aurela, M.; Carbone, S.; Saarnio, K.; Frey, A.; Saarikoski, S.; Teinilä, K.; Kulmala, M.; Hillamo, R. Seasonal and Diurnal Changes in Inorganic Ions, Carbonaceous Matter and Mass in Ambient Aerosol Particles at an Urban, Background Area. Boreal Environ. Res. 2014, 19, 71–86. [Google Scholar]
- Sillanpää, M.; Hillamo, R.; Saarikoski, S.; Frey, A.; Pennanen, A.; Makkonen, U.; Spolnik, Z.; Van Grieken, R.; Branis, M.; Brunekreef, B.; et al. Chemical Composition and Mass Closure of Particulate Matter at Six Urban Sites in Europe. Atmos. Environ. 2006, 40, 212–223. [Google Scholar] [CrossRef]
- Sillanpää, M.; Frey, A.; Hillamo, R.; Pennanen, A.S.; Salonen, R.O. Organic, Elemental and Inorganic Carbon in Particulate Matter of Six Urban Environments in Europe. Atmos. Chem. Phys. 2005, 5, 2869–2879. [Google Scholar] [CrossRef]
- Sillanpää, M.; Saarikoski, S.; Hillamo, R.; Pennanen, A.; Makkonen, U.; Spolnik, Z.; Van Grieken, R.; Koskentalo, T.; Salonen, R.O. Chemical Composition, Mass Size Distribution and Source Analysis of Long-Range Transported Wildfire Smokes in Helsinki. Sci. Total Environ. 2005, 350, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Niemi, J.V.; Tervahattu, H.; Vehkamäki, H.; Martikanen, J.; Laakso, L.; Kulmala, M.; Aarnio, P.; Koskentalo, T.; Sillanpää, M.; Makkonen, U. Characterization of Aerosol Particle Episodes in Finland Caused by Wildfires in Eastern Europe. Atmos. Chem. Phys. 2005, 5, 2299–2310. [Google Scholar] [CrossRef]
- Karppinen, A.; Härkönen, J.; Kukkonen, J.; Aarnio, P.; Koskentalo, T. Statistical Model for Assessing the Portion of Fine Particulate Matter Transported Regionally and Long Range to Urban Air. Scand. J. Work Environ. Health 2004, 30, 47–53. [Google Scholar] [PubMed]
- Saarikoski, S.; Sillanpää, M.; Sofiev, M.; Timonen, H.; Saarnio, K.; Teinilä, K.; Karppinen, A.; Kukkonen, J.; Hillamo, R. Chemical Composition of Aerosols during a Major Biomass Burning Episode Over Northern Europe in Spring 2006: Experimental and Modelling Assessments. Atmos. Environ. 2007, 41, 3577–3589. [Google Scholar] [CrossRef]
- Timonen, H.; Saarikoski, S.; Tolonen-Kivimäki, O.; Aurela, M.; Saarnio, K.; Petäjä, T.; Aalto, P.P.; Kulmala, M.; Pakkanen, T.; Hillamo, R. Size Distributions, Sources and Source Areas of Water-Soluble Organic Carbon in Urban Background Air. Atmos. Chem. Phys. 2008, 8, 5635–5647. [Google Scholar] [CrossRef]
- Saarnio, K.; Teinilä, K.; Aurela, M.; Timonen, H.; Hillamo, R. High-Performance Anion-Exchange Chromatography-Mass Spectrometry Method for Determination of Levoglucosan, Mannosan, and Galactosan in Atmospheric Fine Particulate Matter. Anal. Bioanal. Chem. 2010, 398, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Saarikoski, S.; Timonen, H.; Saarnio, K.; Aurela, M.; Järvi, L.; Keronen, P.; Kerminen, V.; Hillamo, R. Sources of Organic Carbon in Fine Particulate Matter in Northern European Urban Air. Atmos. Chem. Phys. 2008, 8, 6281–6295. [Google Scholar] [CrossRef]
- Dzubay, T.G.; Hines, L.E.; Stevens, R.K. Particle Bounce Errors in Cascade Impactors. Atmos. Environ. 1976, 10, 229–234. [Google Scholar] [CrossRef]
- Pirinen, P.; Simola, H.; Aalto, J.; Kaukoranta, J.; Karlsson, P.; Ruuhela, R. Tilastoja Suomen Ilmastosta 1981–2010; Finnish Meteorological Institute: Helsinki, Finland, 2012. [Google Scholar]









| Model | PPD42NS | PPD60PV |
|---|---|---|
| Origin | Shinyei Technology Co., Ltd., Kobe, Japan | Shinyei Technology Co., Ltd., Kobe, Japan |
| Dimensions (mm) | 59 × 42 × 22 | 88 × 60 × 22 |
| Weight (g) | 24 | 36 |
| Power consumption (W) | 0.45 | 0.7 |
| Supply voltage (VDC) | 5 | 5 |
| Particle size detection (µm) | >1.0 | >0.5 |
| Wavelength (nm) | 940 | 940 |
| Type (-) | Forward angle, photometer | Forward angle, photometer |
| Output signal | Pulse width modulation | Pulse width modulation |
| Operating temperature (°C) | 0–45 | 0–45 |
| Operating humidity (%) | <95% | <95% |
| Price (€) | ~70 | ~150 |
| Parameter | Value |
|---|---|
| Dilution air flow rate | 20 L/min |
| Dispersion air flow rate | 1500 cc/min |
| Liquid feed rate | ~2.98 mm3/s |
| Droplet breakup frequency | 45 kHz |
| VOAG orifice diameter | 20 µm |
| DOS solution | 10 g/L 2-propanol |
| Palmitic acid solution | 10 g/L 2-propanol |
| 2-propanol | Max. evaporation residual 0.0001% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuula, J.; Mäkelä, T.; Hillamo, R.; Timonen, H. Response Characterization of an Inexpensive Aerosol Sensor. Sensors 2017, 17, 2915. https://doi.org/10.3390/s17122915
Kuula J, Mäkelä T, Hillamo R, Timonen H. Response Characterization of an Inexpensive Aerosol Sensor. Sensors. 2017; 17(12):2915. https://doi.org/10.3390/s17122915
Chicago/Turabian StyleKuula, Joel, Timo Mäkelä, Risto Hillamo, and Hilkka Timonen. 2017. "Response Characterization of an Inexpensive Aerosol Sensor" Sensors 17, no. 12: 2915. https://doi.org/10.3390/s17122915





