Pre-Clinical Tests of an Integrated CMOS Biomolecular Sensor for Cardiac Diseases Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
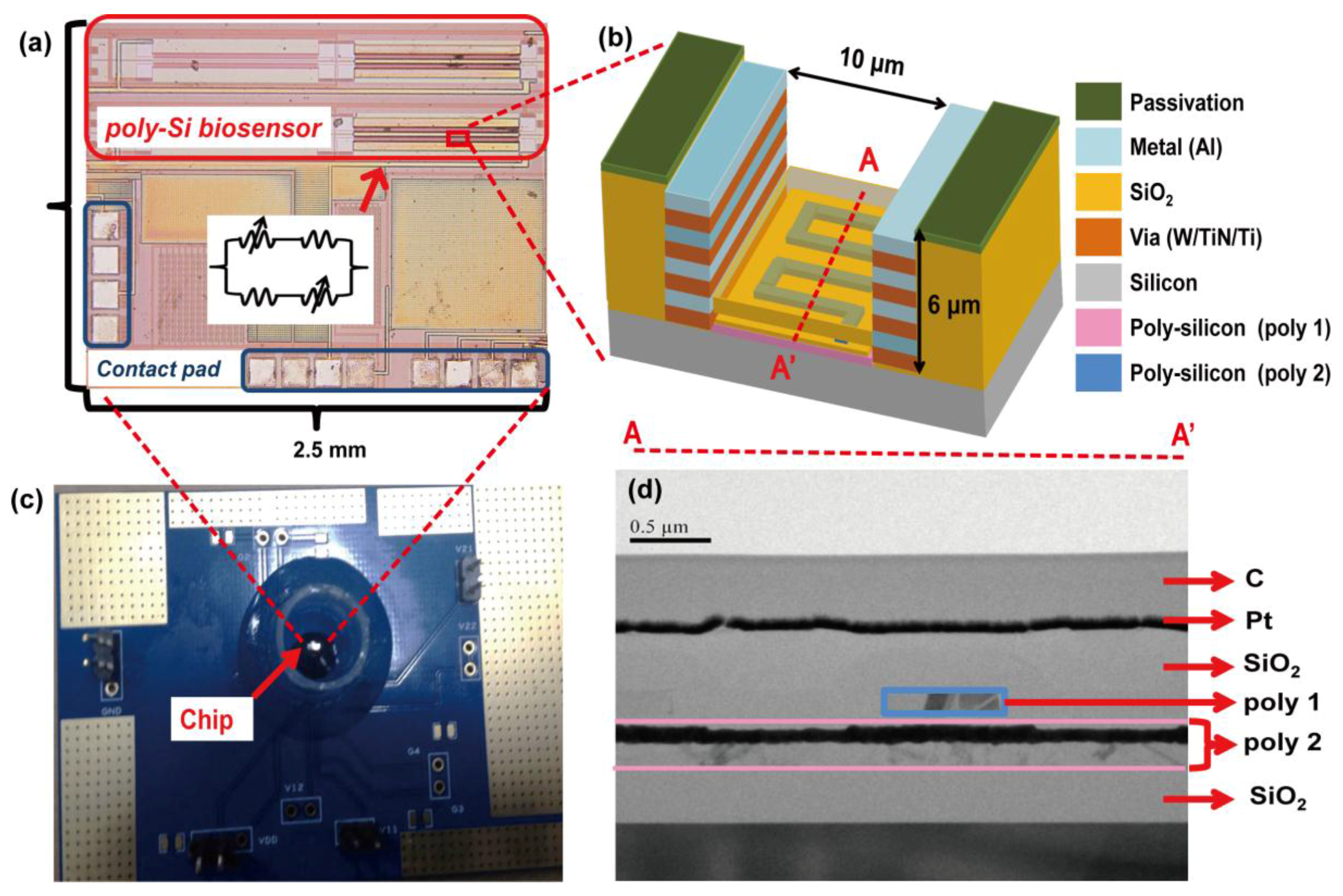
2.2. Design of CMOS Field-Effect Sensing Device
2.3. Surface Modification and Immobilization
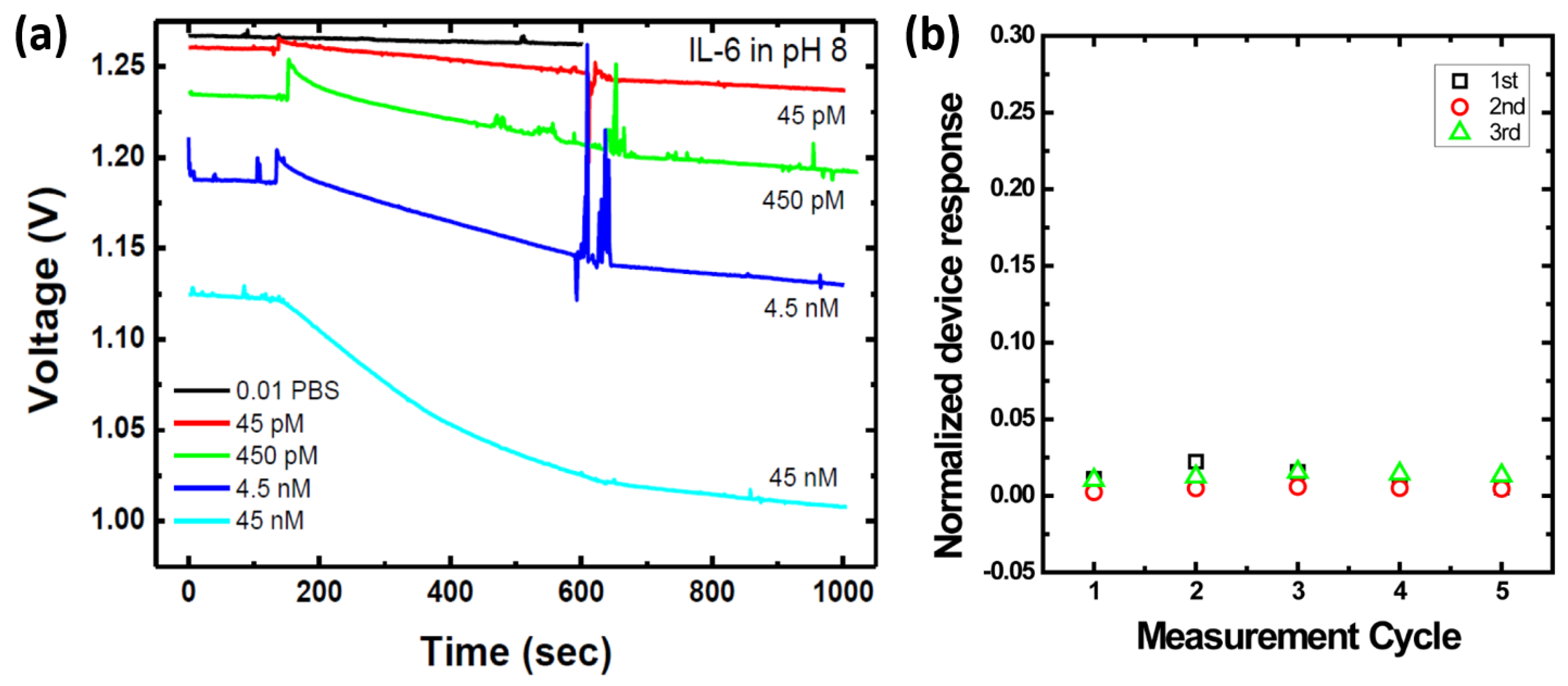
2.4. Measurement Methods
3. Results
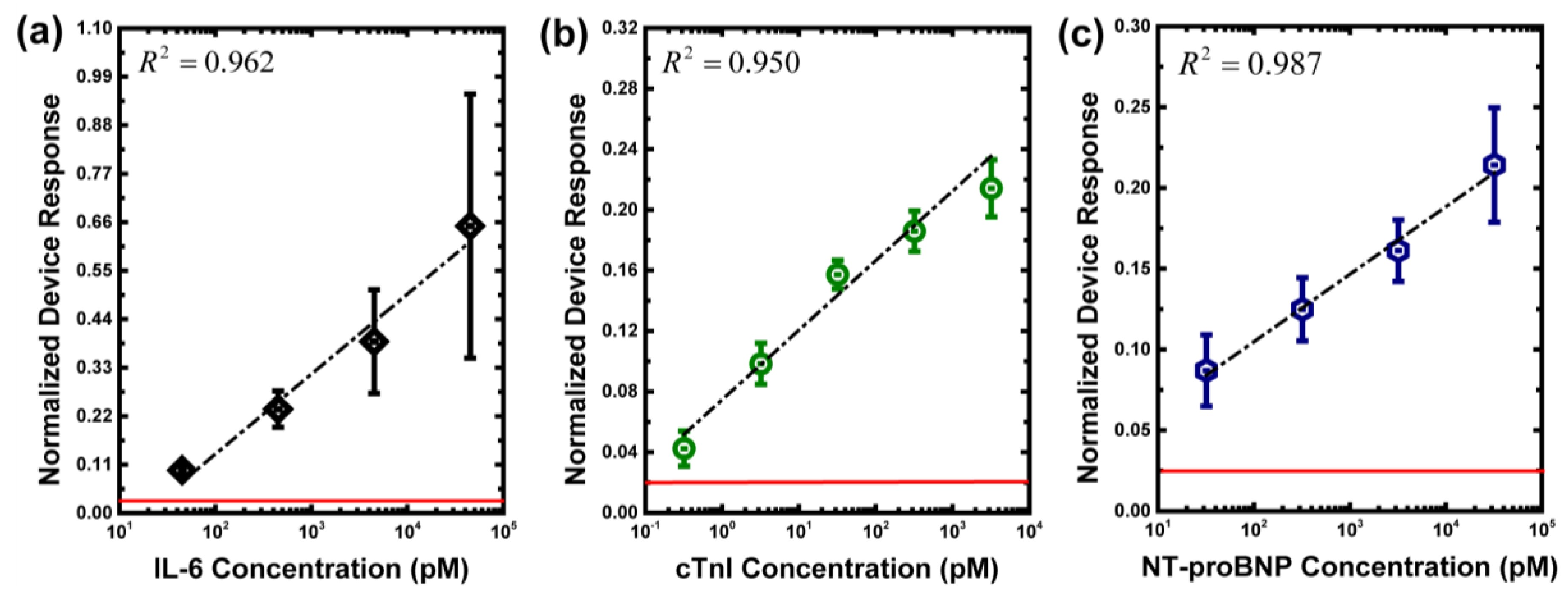
3.1. Measurement of Biomarkers in Buffer Solutions
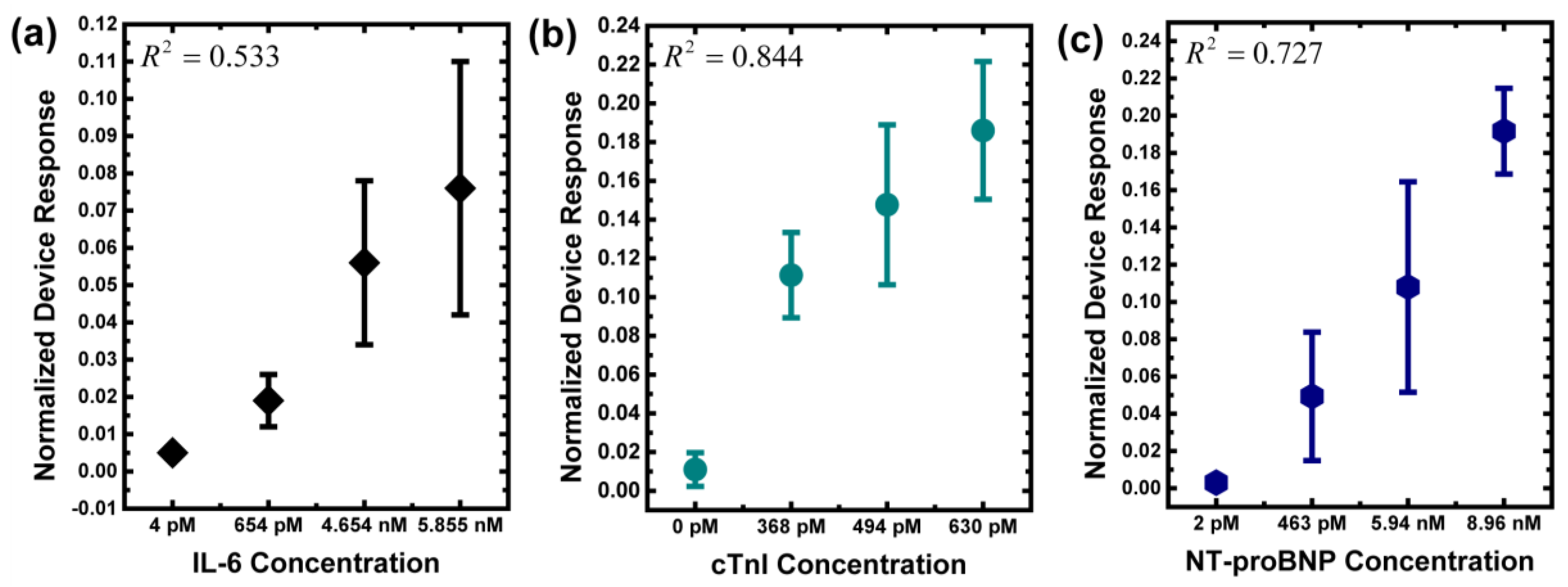
3.2. Measurement of Biomarkers in Human Serums
- Subject 1: healthy subject.
- Subject 2: subject with coronary artery disease; one-vessel disease; with medication control.
- Subject 3: subject with coronary artery disease; three-vessel disease; with medication control.
- Subject 4: subject with myocardial infarction; no shock; Killip Classification I: No evidence of heart failure.
- Subject 5: subject with myocardial infarction; no shock; Killip Classification II: Findings of mild to moderate heart failure and elevated jugular venous pressure.
- Subject 6: subject with congestive heart failure; outpatient clinic; NYHA functional class II: Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, and dyspnea.
- Subject 7: subject with congestive heart failure; ICU admission; needs heart transplant; NYHA functional class IV: Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases.
- Subject 8: subject with coronary artery disease; two-vessel disease; with medication control; admission due to myocardial infarction; shock with extracorporeal membrane oxygenation (ECMO); current congestive heart failure; ICU admission; Killip Classification IV: Cardiogenic shock defined as systolic blood pressure <90 and signs of hypoperfusion such as oliguria, cyanosis, and sweating.
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical Statement
References
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Christodoulidis, G.; Vittorio, T.J.; Fudim, M.; Lerakis, S.; Kosmas, C.E. Inflammation in coronary artery disease. Cardiol. Rev. 2014, 22, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Panja, M. High sensitive C-reactive protein: A novel biochemical markers and its role in coronary artery disease. J. Assoc. Phys. India 2005, 53, 25–32. [Google Scholar]
- Zakynthinos, E.; Pappa, N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 2009, 53, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Huang, L.; Liu, C.; Shan, S.; Li, Q.; Ke, L.; Cheng, B. The clinical value of inflammatory biomarkers in coronary artery disease: PTX3 as a new inflammatory marker. Exp. Gerontol. 2017, 97, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Sukhija, R.; Fahdi, I.; Garza, L.; Fink, L.; Scott, M.; Aude, W.; Pacheco, R.; Bursac, Z.; Grant, A.; Mehta, J.L. Inflammatory markers, angiographic severity of coronary artery disease, and patient outcome. Am. J. Cardiol. 2007, 99, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Wainstein, M.V.; Mossmann, M.; Araujo, G.N.; Gonçalves, S.C.; Gravina, G.L.; Sangalli, M.; Veadrigo, F.; Matte, R.; Reich, R.; Costa, F.G.; et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol. Metab. Syndr. 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Geng, G.Y.; Han, W.J.; Zhao, M.H.; Wu, L.; Liu, H.L. Interleukin-6 (IL-6)-174G/C genomic polymorphism contribution to the risk of coronary artery disease in a Chinese population. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155. [Google Scholar] [PubMed]
- McKie, P.M.; Burnett, J.C., Jr. NT-proBNP: The Gold Standard Biomarker in Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 2437–2439. [Google Scholar] [CrossRef] [PubMed]
- Van der Burg-de Graauw, N.; Cobbaert, C.M.; Middelhoff, C.J.; Bantje, T.A.; van Guldener, C. The additive value of N-terminal pro-B-type natriuretic peptide testing at the emergency department in patients with acute dyspnoea. Eur. J. Int. Med. 2009, 20, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, S.H.; Park, S.; Jee, S.H.; Hong, M.K.; Chung, N.; Cho, S.Y.; Jang, Y. The additive value of multiple biomarkers in prediction of premature coronary artery disease. Acta Cardiol. 2015, 70, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Guevara, C.; Swaminathan, V.V.; Reddy, B.; Huang, J.C.; Liu, Y.S.; Bashir, R. On-chip electrical detection of parallel loop-mediated isothermal amplification with DG-BioFETs for the detection of foodborne bacterial pathogens. RSC Adv. 2016, 6, 103872–103887. [Google Scholar] [CrossRef]
- Masson, J.F.; Battaglia, T.M.; Khairallah, P.; Beaudoin, S.; Booksh, K.S. Quantitative measurement of cardiac markers in undiluted serum. Anal. Chem. 2007, 79, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Agafonova, L.E.; Shumyantseva, V.V.; Archakov, A.I. Quartz crystal microbalance for the cardiac markers/antibodies binding kinetic measurements in the plasma samples. Chem. Phys. Lett. 2014, 604, 5–9. [Google Scholar] [CrossRef]
- Billah, M.M.; Hays, H.C.W.; Hodges, C.S.; Ponnambalam, S.; Vohra, R.; Millner, P.A. Mixed self-assembled monolayer (mSAM) based impedimetric immunosensors for cardiac troponin i (cTnI) and soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1). Sens. Act. B Chem. 2012, 173, 361–366. [Google Scholar] [CrossRef]
- Huang, C.W.; Huang, Y.J.; Yen, P.W.; Tsai, H.H.; Liao, H.H.; Juang, Y.Z.; Lu, S.S.; Lin, C.T. A CMOS wireless biomolecular sensing system-on-chip based on polysilicon nanowire technology. Lab Chip 2013, 13, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Kuan, D.H.; Wang, I.S.; Lin, J.R.; Yang, C.-H.; Huang, C.-H.; Lin, C.-T.; Huang, N.-T. A microfluidic device integrating dual CMOS polysilicon nanowire sensors for on-chip whole blood processing and simultaneous detection of multiple analytes. Lab Chip 2016, 16, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Chee, R.E.; Agarwal, A.; Wong, S.M.; Zhang, G.J. Label-free electrical detection of cardiac biomarker with CMOS-compatible silicon nanowire sensor arrays. Anal. Chem. 2009, 81, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, J.; Choi, B.; Yoon, J.; Kim, J.Y.; Choi, Y.K.; Kim, D.M.; Kim, D.H.; Choi, S.J. A highly responsive silicon nanowire/amplifier MOSFET hybrid biosensor. Sci. Rep. 2015, 5, 12286. [Google Scholar] [CrossRef] [PubMed]
- Peronnet, E.; Becquart, L.; Martinez, J.; Charrier, J.P.; Jolivet-Reynaud, C. Isoelectric point determination of cardiac troponin I forms present in plasma from patients with myocardial infarction. Clin. Chim. Acta 2007, 377, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Zheng, H.; Huang, B.; Cai, L. Interleukin-6 gene cloning, expression and purification. Zhongguo Yi Xue Ke Xue Yuan Xue Bao Acta Acad. Med. Sin. 1998, 20, 185–190. [Google Scholar]
- Hideshima, S.; Sato, R.; Kuroiwa, S.; Osaka, T. Fabrication of stable antibody-modified field effect transistors using electrical activation of Schiff base cross-linkages for tumor marker detection. Biosens. Bioelectron. 2011, 26, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Retna, R.C.; Ohsaka, T. Voltammetric detection of uric acid in the presence of ascorbic acid at a gold electrode modified with a self-assembled monolayer of heteroaromatic thiol. J. Electroanal. Chem. 2003, 540, 69–77. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.I.; Iqbal, Z.; Shah, Y.; Ahmad, L.; Nazir, S.; Watson, D.G.; Khan, J.A.; Nasir, F.; Khan, A. A new HPLC method for the simultaneous determination of ascorbic acid and aminothiols in human plasma and erythrocytes using electrochemical detection. Talanta 2011, 84, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Lee, J.K.; Chiang, F.T.; Yang, C.H.; Huang, S.W.; Hwang, J.J.; Lin, J.L.; Tseng, C.D.; Chen, J.J.; Tsai, C.T. Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit. Care Med. 2011, 39, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wu, C.K.; Yeong, E.K.; Lin, H.H.; Huang, Y.T.; Lee, J.K.; Lin, Y.H.; Chiang, F.T.; Tang, Y.B.; Tsai, C.T. Prognostic significance of left ventricular diastolic function in burn patients. Shock 2012, 37, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Cannon, C.P.; Jesse, R.L.; Newby, L.K.; Ravkilde, J.; Storrow, A.B.; Wu, A.H.B.; Christenson, R.H. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical Characteristics and Utilization of Biochemical Markers in Acute Coronary Syndromes. J. Clin. Chem. 2007, 53, 552–574. [Google Scholar]
- Maisel, A.; Mueller, C.; Adams, K., Jr.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.F.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef] [PubMed]
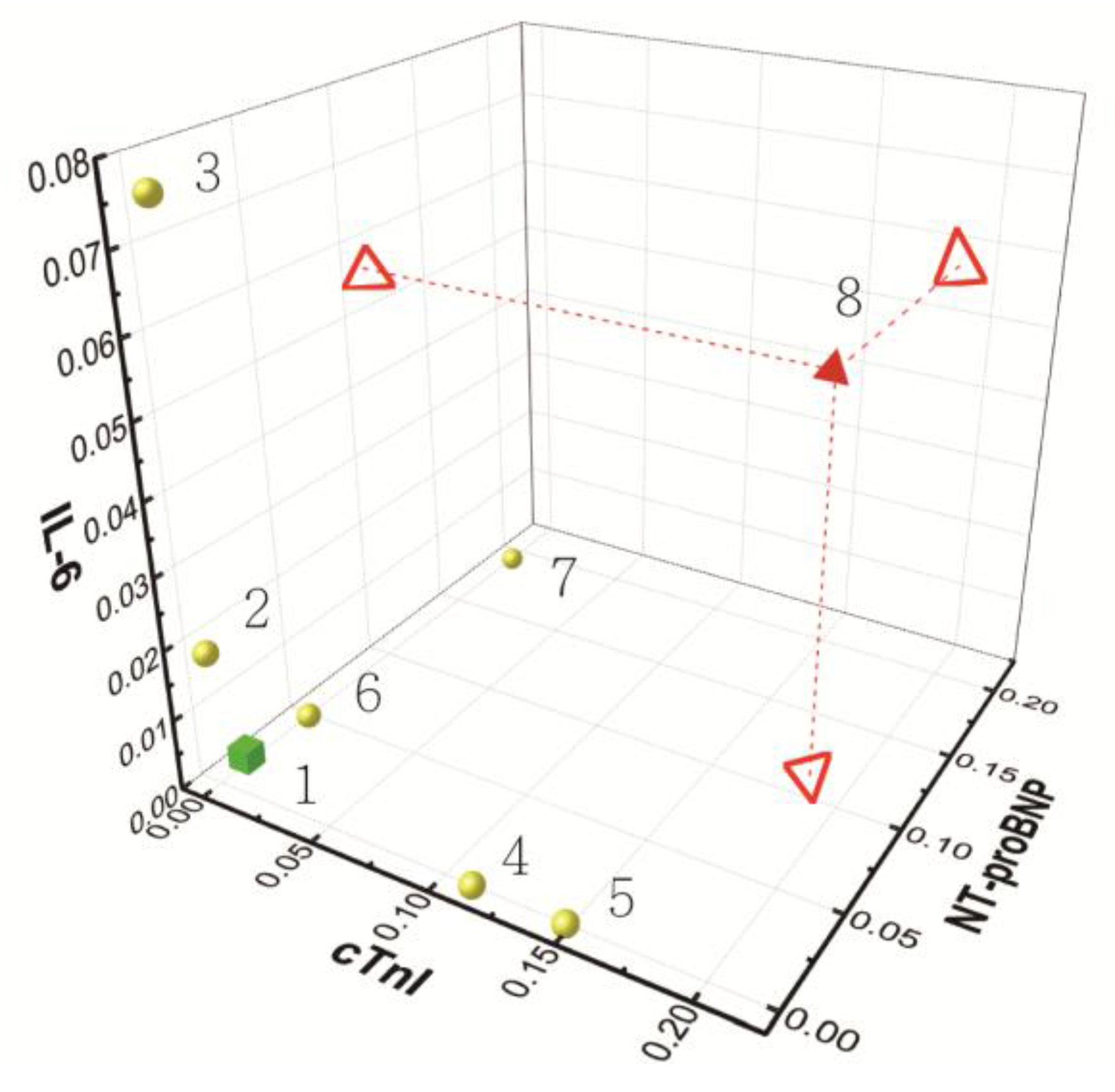
| Sub. No. | ELISA (pM) | CMOS Biosensor (NDR) | Note (Symptoms) | ||||
|---|---|---|---|---|---|---|---|
| IL-6 | cTnI | NT-proBNP | IL-6 | cTnI | NT-proBNP | ||
| 1 | 4 ± 1.8 | 0 ± 0.0 | 2 ± 1.0 | 0.005 ± 0.001 | 0.011 ± 0.009 | 0.003 ± 0.001 | Healthy people |
| 2 | 654 ± 3.8 | - | - | 0.019 ± 0.007 | - | - | coronary artery disease |
| 3 | 5855 ± 5.9 | - | - | 0.076 ± 0.034 | - | - | |
| 4 | - | 368 ± 0.6 | - | - | 0.111 ± 0.022 | - | myocardial infarction |
| 5 | - | 494 ± 1.1 | - | - | 0.148 ± 0.041 | - | |
| 6 | - | - | 463 ± 0.2 | - | - | 0.049 ± 0.034 | congestive heart failure |
| 7 | - | - | 8960 ± 24.4 | - | - | 0.192 ± 0.023 | |
| 8 | 4654 ± 5.9 | 630 ± 6.4 | 5940 ± 24.6 | 0.056 ± 0.022 | 0.186 ± 0.036 | 0.108 ± 0.057 | coronary artery disease & congestive heart failure & myocardial infarction |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-K.; Wang, I.-S.; Huang, C.-H.; Chen, Y.-F.; Huang, N.-T.; Lin, C.-T. Pre-Clinical Tests of an Integrated CMOS Biomolecular Sensor for Cardiac Diseases Diagnosis. Sensors 2017, 17, 2733. https://doi.org/10.3390/s17122733
Lee J-K, Wang I-S, Huang C-H, Chen Y-F, Huang N-T, Lin C-T. Pre-Clinical Tests of an Integrated CMOS Biomolecular Sensor for Cardiac Diseases Diagnosis. Sensors. 2017; 17(12):2733. https://doi.org/10.3390/s17122733
Chicago/Turabian StyleLee, Jen-Kuang, I-Shun Wang, Chi-Hsien Huang, Yih-Fan Chen, Nien-Tsu Huang, and Chih-Ting Lin. 2017. "Pre-Clinical Tests of an Integrated CMOS Biomolecular Sensor for Cardiac Diseases Diagnosis" Sensors 17, no. 12: 2733. https://doi.org/10.3390/s17122733







