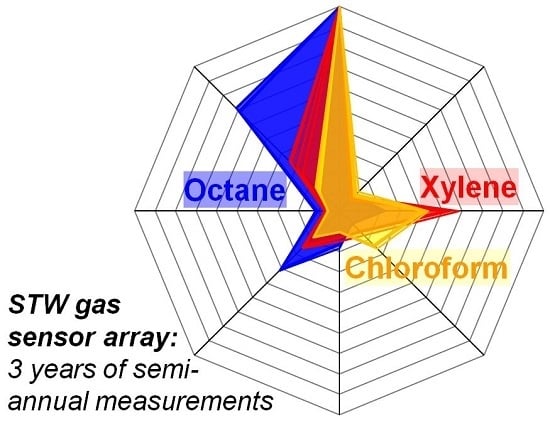
Long-Term Stability of Polymer-Coated Surface Transverse Wave Sensors for the Detection of Organic Solvent Vapors
Abstract
:1. Introduction
2. Materials and Methods
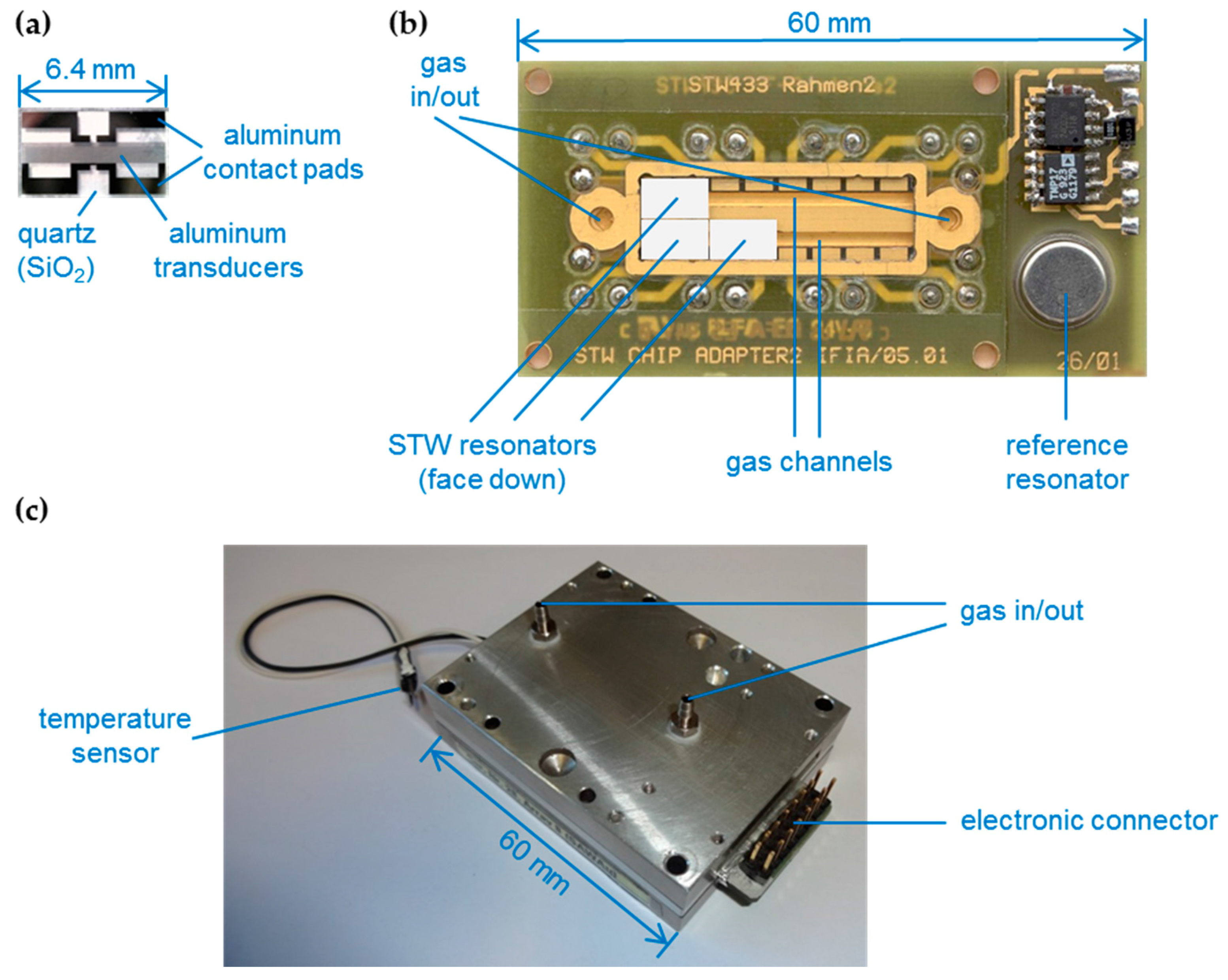
2.1. STW Device and Sensor Array
2.2. Device Coating
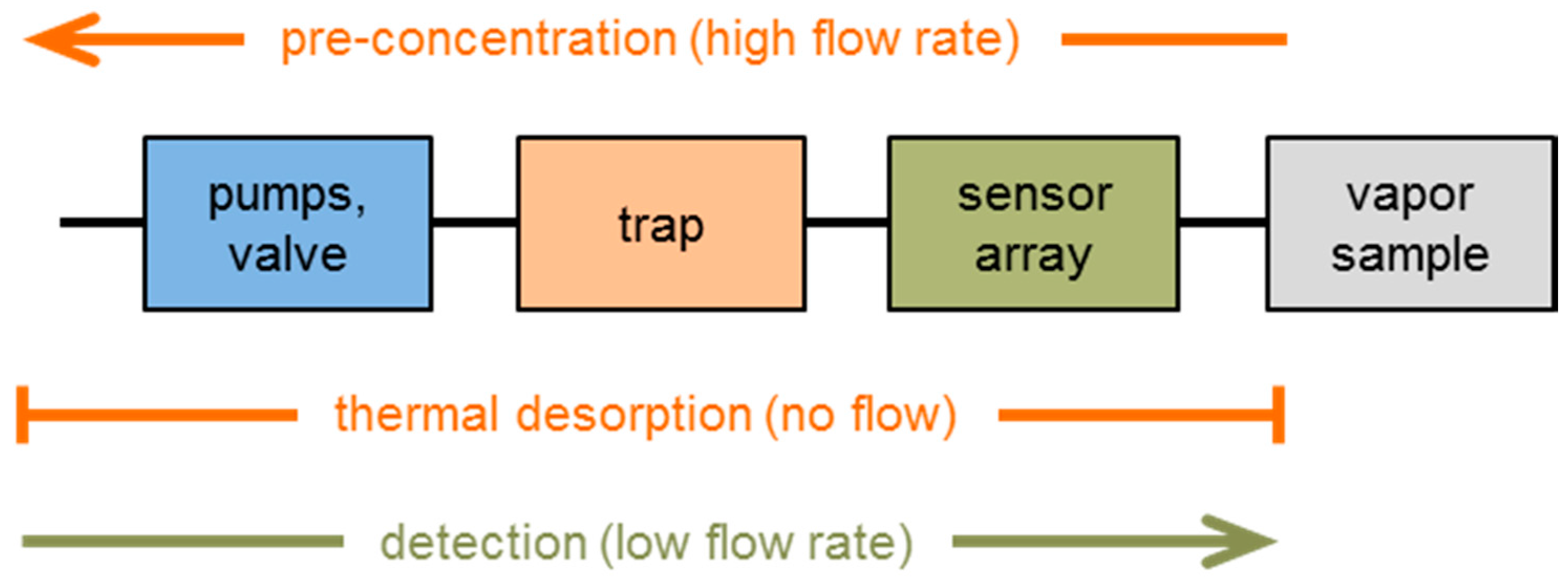
2.3. Samples, Measurement Setup and Measurement Protocol
2.4. Data Evaluation
3. Results and Discussion
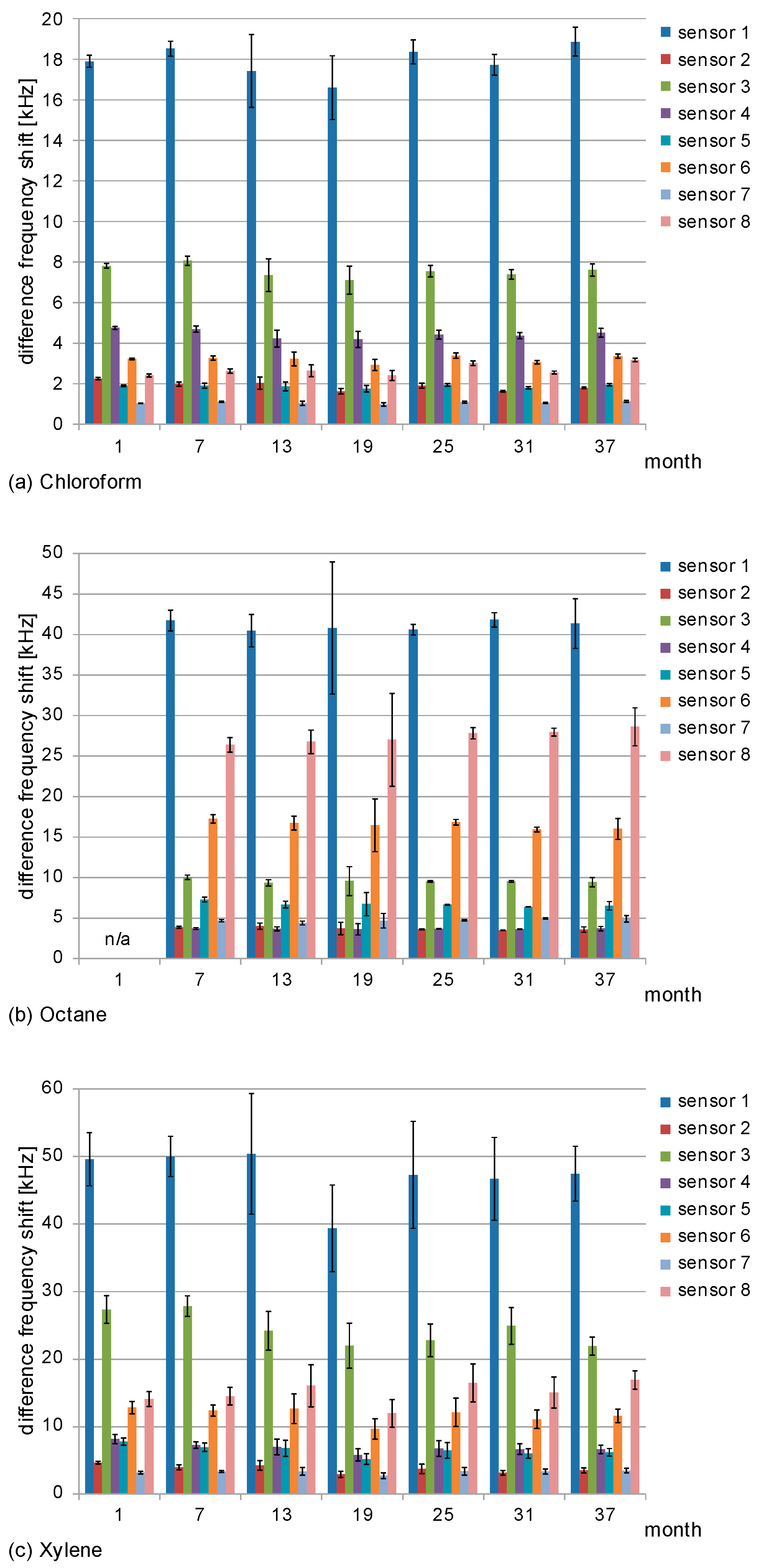
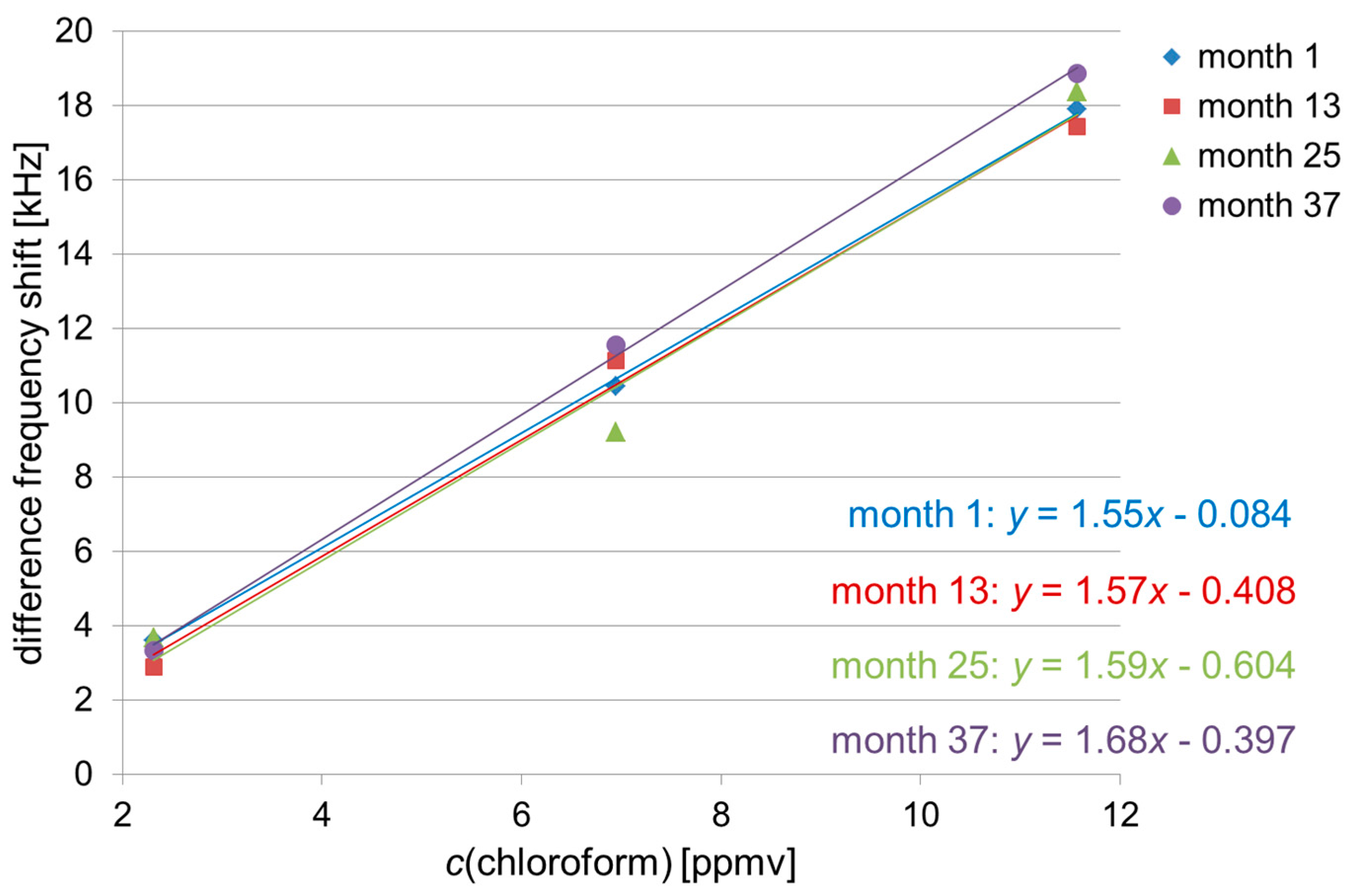
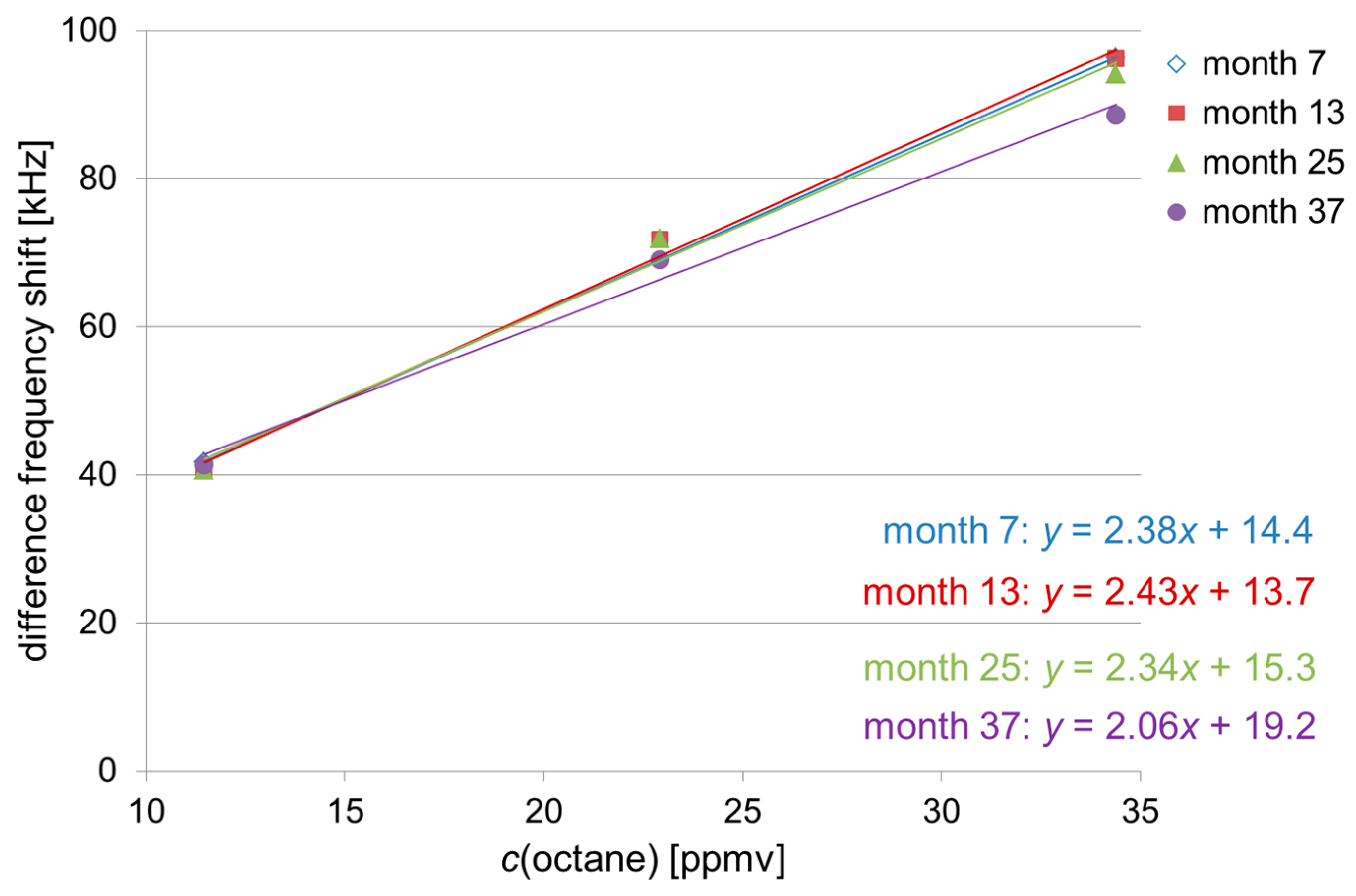
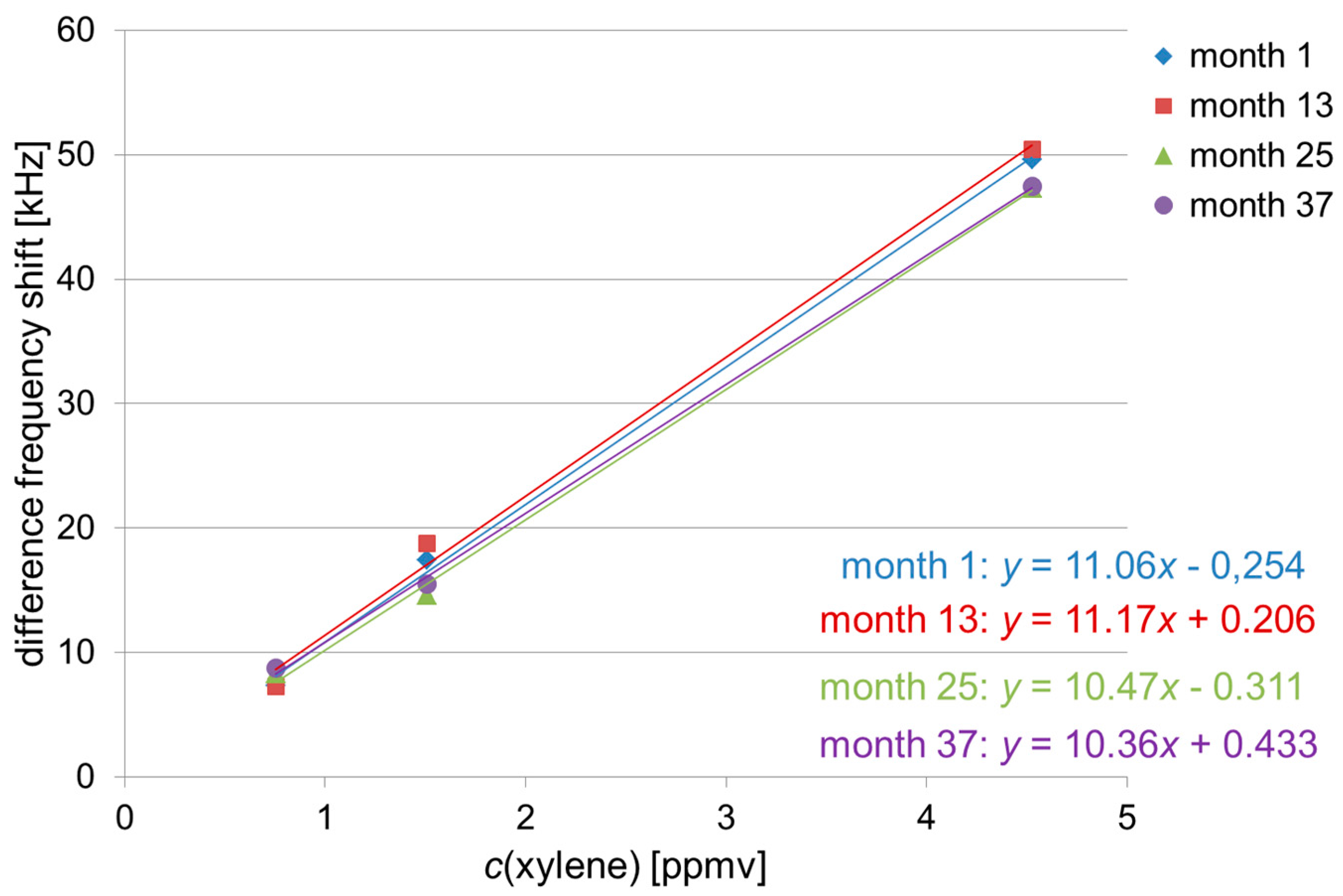
3.1. Absolute Difference Frequency Shifts
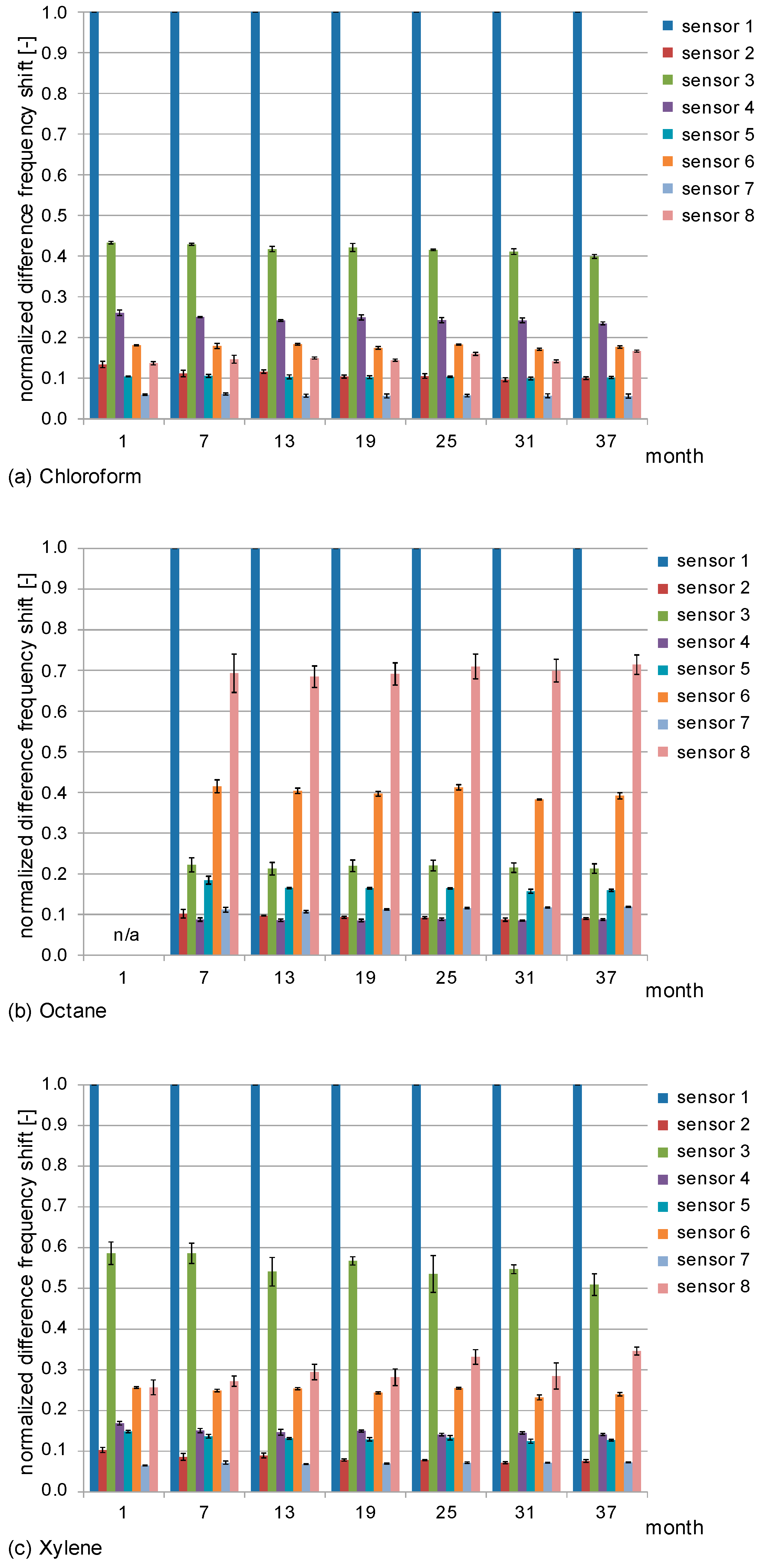
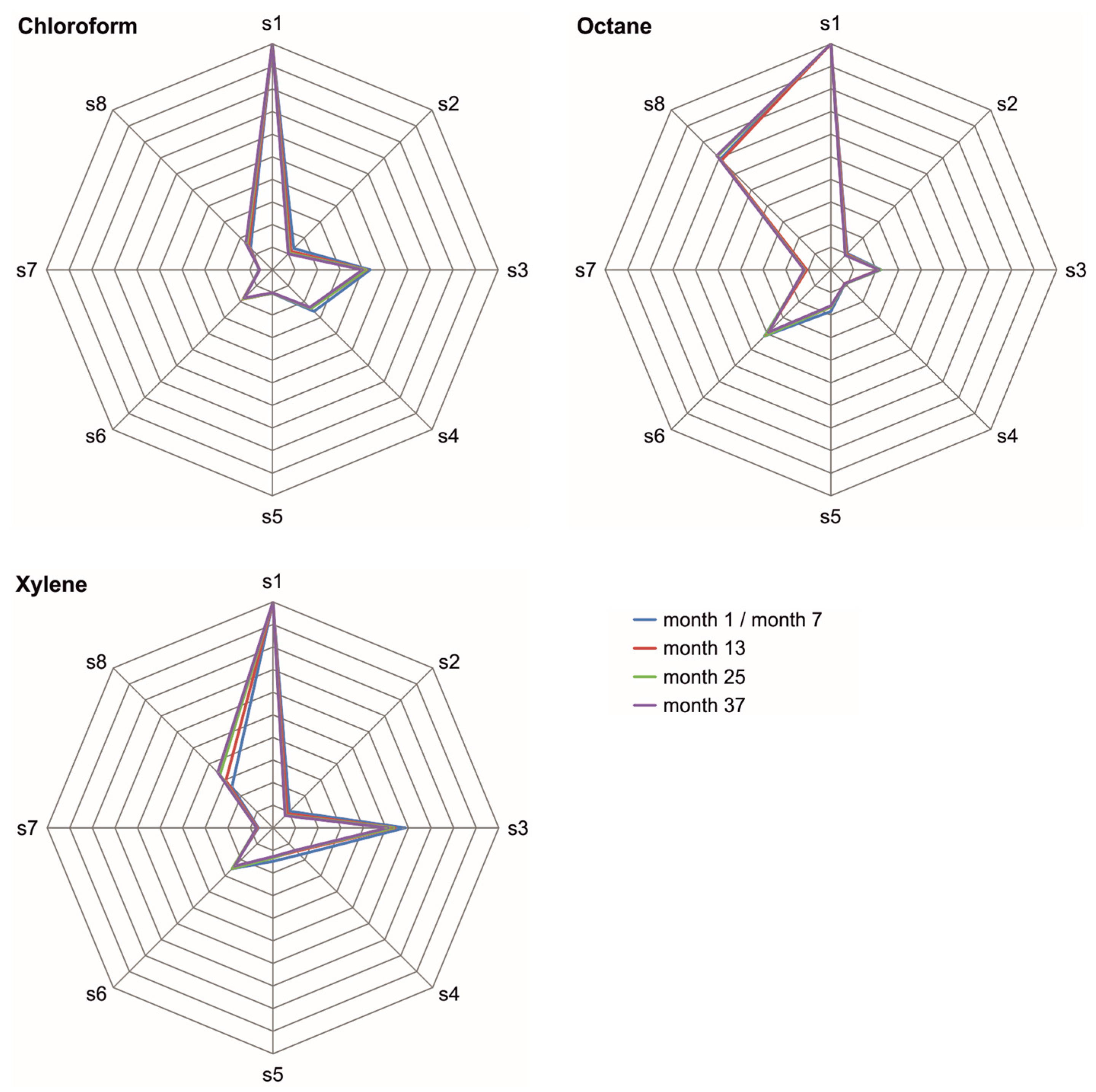
3.2. Normalized Difference Frequency Shifts and Signal Patterns
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW sensors for chemical vapors and gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Jakubik, W.P. Surface acoustic wave-based gas sensors. Thin Solid Films 2011, 520, 986–993. [Google Scholar] [CrossRef]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Gaso, M.I.; March-Iborra, C.; Montoya-Baides, A.; Arnau-Vives, A. Surface generated acoustic wave biosensors for the detection of pathogens: A review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef] [PubMed]
- Vellekoop, M.J. Acoustic wave sensors and their technology. Ultrasonics 1998, 36, 7–14. [Google Scholar] [CrossRef]
- Bender, F.; Länge, K.; Barié, N.; Kondoh, J.; Rapp, M. On-line monitoring of polymer deposition for tailoring the waveguide characteristics of love-wave biosensors. Langmuir 2004, 20, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Iqbal, N.; Mujahid, A.; Schirhagl, R. Advanced vapor recognition materials for selective and fast responsive surface acoustic wave sensors: A review. Anal. Chim. Acta 2013, 787, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Avramov, I.D.; Kurosawa, S.; Rapp, M.; Krawczak, P.; Radeva, E.I. Investigations on plasma–polymer-coated SAW and STW resonators for chemical gas-sensing applications. IEEE Trans. Microw. Theory Tech. 2001, 49, 827–837. [Google Scholar] [CrossRef]
- Yantchev, V.M.; Strashilov, V.L.; Rapp, M.; Stahl, U.; Avramov, I.D. Theoretical and experimental mass-sensitivity analysis of polymer-coated SAW and STW resonators for gas sensing applications. IEEE Sens. J. 2002, 2, 307–313. [Google Scholar] [CrossRef]
- Otto, M. Chemometrics: Statistics and Computer Application in Analytical Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Bender, F.; Barié, N.; Romoudis, G.; Voigt, A.; Rapp, M. Development of a preconcentration unit for a SAW sensor micro array and its use for indoor air quality monitoring. Sens. Actuators B Chem. 2003, 93, 135–141. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G. Application of principal component analysis and artificial neural networks to recognize the individual VOCs of methanol/2-propanol in a binary mixture by SAW multi-sensor array. Sens. Actuators B Chem. 2003, 89, 269–284. [Google Scholar] [CrossRef]
- Barié, N.; Bücking, M.; Stahl, U.; Rapp, M. Detection of coffee flavour ageing by solid-phase microextraction/surface acoustic wave sensor array technique (SPME/SAW). Food Chem. 2015, 176, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Zeynali, S. Electronic nose based on the polymer coated SAW sensors array for the warfare agent simulants classification. Sens. Actuators B Chem. 2008, 129, 412–423. [Google Scholar] [CrossRef]
- Bender, F.; Skrypnik, A.; Voigt, A.; Marcoll, J.; Rapp, M. Selective detection of HFC and HCFC refrigerants using a surface acoustic wave sensor system. Anal. Chem. 2003, 75, 5262–5266. [Google Scholar] [CrossRef]
- Barié, N.; Rapp, M.; Ache, H.J. UV crosslinked polysiloxanes as new coating materials for SAW devices with high long-term stability. Sens. Actuators B Chem. 1998, 46, 97–103. [Google Scholar] [CrossRef]
- Adhikari, P.; Alderson, L.; Bender, F.; Ricco, A.J.; Josse, F. Investigation of polymer-plasticizer blends as SH-SAW sensor coatings for detection of benzene in water with high sensitivity and long-term stability. ACS Sens. 2017, 2, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Pestov, D.; Guney-Altay, O.; Levit, N.; Tepper, G. Improving the stability of surface acoustic wave (SAW) chemical sensor coatings using photopolymerization. Sens. Actuators B Chem. 2007, 126, 557–561. [Google Scholar] [CrossRef]
- Barié, N.; Voigt, A.; Rapp, M.; Marcoll, J. Fast SAW based sensor system for real-time analysis of volatile anesthetic agents. In Proceedings of the 2007 IEEE Sensors, Atlanta, GA, USA, 28–31 October 2007; pp. 958–961. [Google Scholar]
- Länge, K.; Blaess, G.; Voigt, A.; Götzen, R.; Rapp, M. Integration of a surface acoustic wave biosensor in a microfluidic polymer chip. Biosens. Bioelectron. 2006, 22, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Reibel, J.; Voigt, A.; Balzer, M.; Bülow, O. New miniaturized SAW-sensor array for organic gas detection driven by multiplexed oscillators. Sens. Actuators B Chem. 2000, 65, 169–172. [Google Scholar] [CrossRef]
- Barié, N.; Skrypnik, A.; Voigt, A.; Rapp, M.; Marcoll, J. Work place monitoring using a high sensitive surface acoustic wave based sensor system. In Proceedings of the TRANSDUCERS 2007—2007 International Solid-State Sensors, Actuators and Microsystems Conference, Lyon, France, 10–14 June 2007; pp. 1003–1006. [Google Scholar]

| Sensor No. | Polymer | Supplier | Frequency Shift by Spray Coating [MHz] |
|---|---|---|---|
| 1 | PBMA: Poly(butyl methacrylate) | Aldrich, Milwaukee, WI, USA | −1.1138 |
| 2 | Polyurethane alkyd resin with trace isocyanates | RS Components, Corby, UK | −0.7400 |
| 3 | PECH: Polyepichlorohydrin | Aldrich, Milwaukee, WI, USA | −1.3271 |
| 4 | Silar-10C | Alltech, Deerfield, IL, USA | −0.4404 |
| 5 | PCFV: Poly(chlorotrifluoro ethylene-co-vinylidene fluoride) | Aldrich, Milwaukee, WI, USA | −1.0000 |
| 6 | L grease | Apiezon, London, UK | −0.6004 |
| 7 | PDMS: Polydimethylsiloxane | Macherey-Nagel, Düren, Germany | −0.4304 |
| 8 | PIB: Polyisobutylene | Aldrich, Milwaukee, WI, USA | −1.1233 |
| Solvent | Molecular Formula | Relative Molar Mass Mr | Boiling Point [°C] | Density [g/cm³] |
|---|---|---|---|---|
| Chloroform | CHCl3 | 119.38 | 61 | 1.48 |
| n-Octane | C8H18 | 114.23 | 125–126 | 0.70 |
| Xylene 1 | C8H10 | 106.17 | 137–143 | 0.86–0.88 |
| Solvent | Drop Volumes [µL] | Concentrations [mg/m3] | Concentrations [ppmv] |
|---|---|---|---|
| Chloroform | 1; 3; 5 | 11.4; 34.2; 57.0 | 2.3; 6.9; 11.6 |
| n-Octane | 10; 20; 30 | 54.1; 108.2; 162.2 | 11.5; 22.9; 34.4 |
| Xylene 1 | 0.5; 1; 3 | 3.3; 6.6; 19.8 | 0.8; 1.5; 4.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stahl, U.; Voigt, A.; Dirschka, M.; Barié, N.; Richter, C.; Waldbaur, A.; Gruhl, F.J.; Rapp, B.E.; Rapp, M.; Länge, K. Long-Term Stability of Polymer-Coated Surface Transverse Wave Sensors for the Detection of Organic Solvent Vapors. Sensors 2017, 17, 2529. https://doi.org/10.3390/s17112529
Stahl U, Voigt A, Dirschka M, Barié N, Richter C, Waldbaur A, Gruhl FJ, Rapp BE, Rapp M, Länge K. Long-Term Stability of Polymer-Coated Surface Transverse Wave Sensors for the Detection of Organic Solvent Vapors. Sensors. 2017; 17(11):2529. https://doi.org/10.3390/s17112529
Chicago/Turabian StyleStahl, Ullrich, Achim Voigt, Marian Dirschka, Nicole Barié, Christiane Richter, Ansgar Waldbaur, Friederike J. Gruhl, Bastian E. Rapp, Michael Rapp, and Kerstin Länge. 2017. "Long-Term Stability of Polymer-Coated Surface Transverse Wave Sensors for the Detection of Organic Solvent Vapors" Sensors 17, no. 11: 2529. https://doi.org/10.3390/s17112529
APA StyleStahl, U., Voigt, A., Dirschka, M., Barié, N., Richter, C., Waldbaur, A., Gruhl, F. J., Rapp, B. E., Rapp, M., & Länge, K. (2017). Long-Term Stability of Polymer-Coated Surface Transverse Wave Sensors for the Detection of Organic Solvent Vapors. Sensors, 17(11), 2529. https://doi.org/10.3390/s17112529