Ultrasensitive, Label Free, Chemiresistive Nanobiosensor Using Multiwalled Carbon Nanotubes Embedded Electrospun SU-8 Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
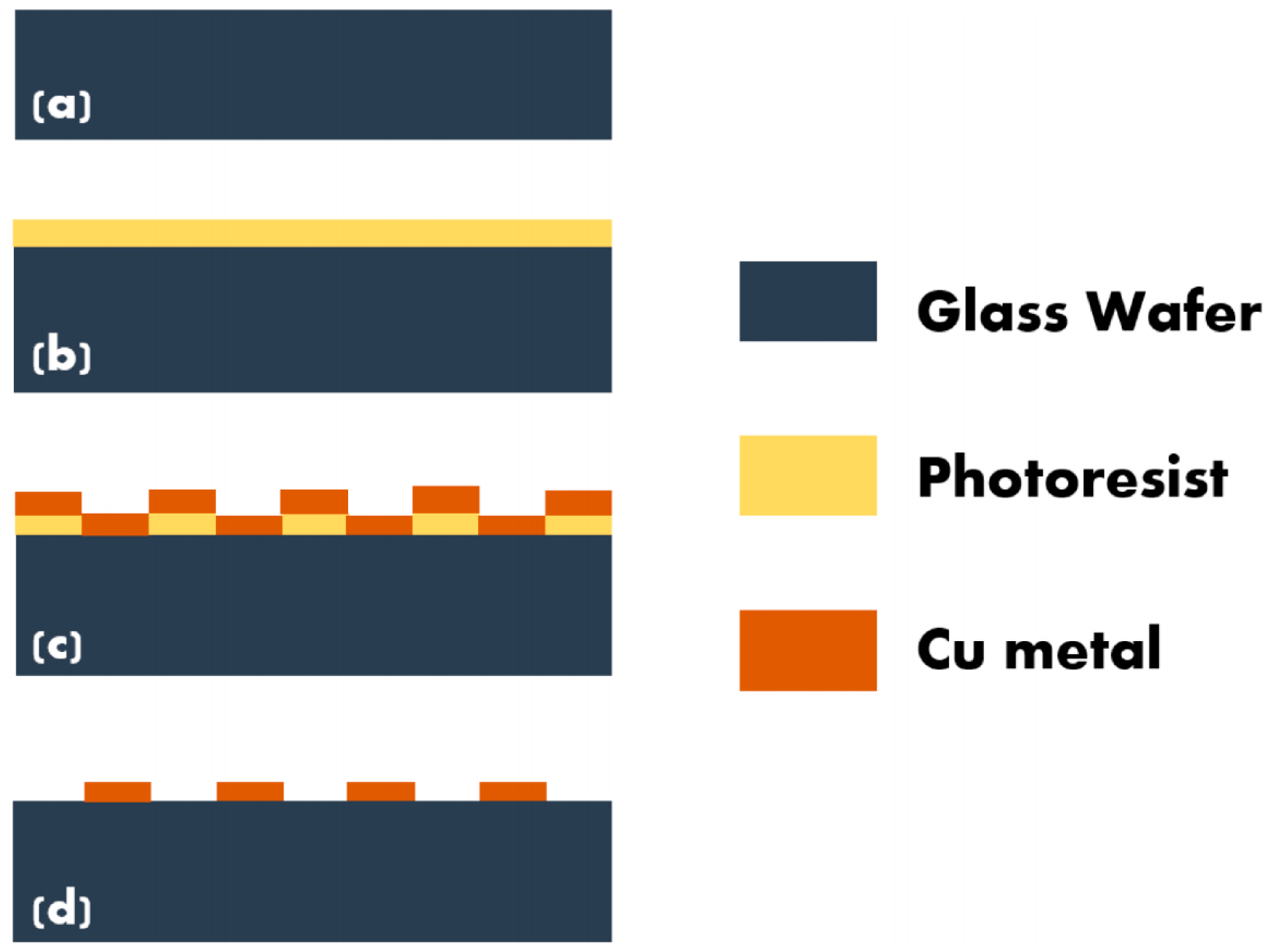
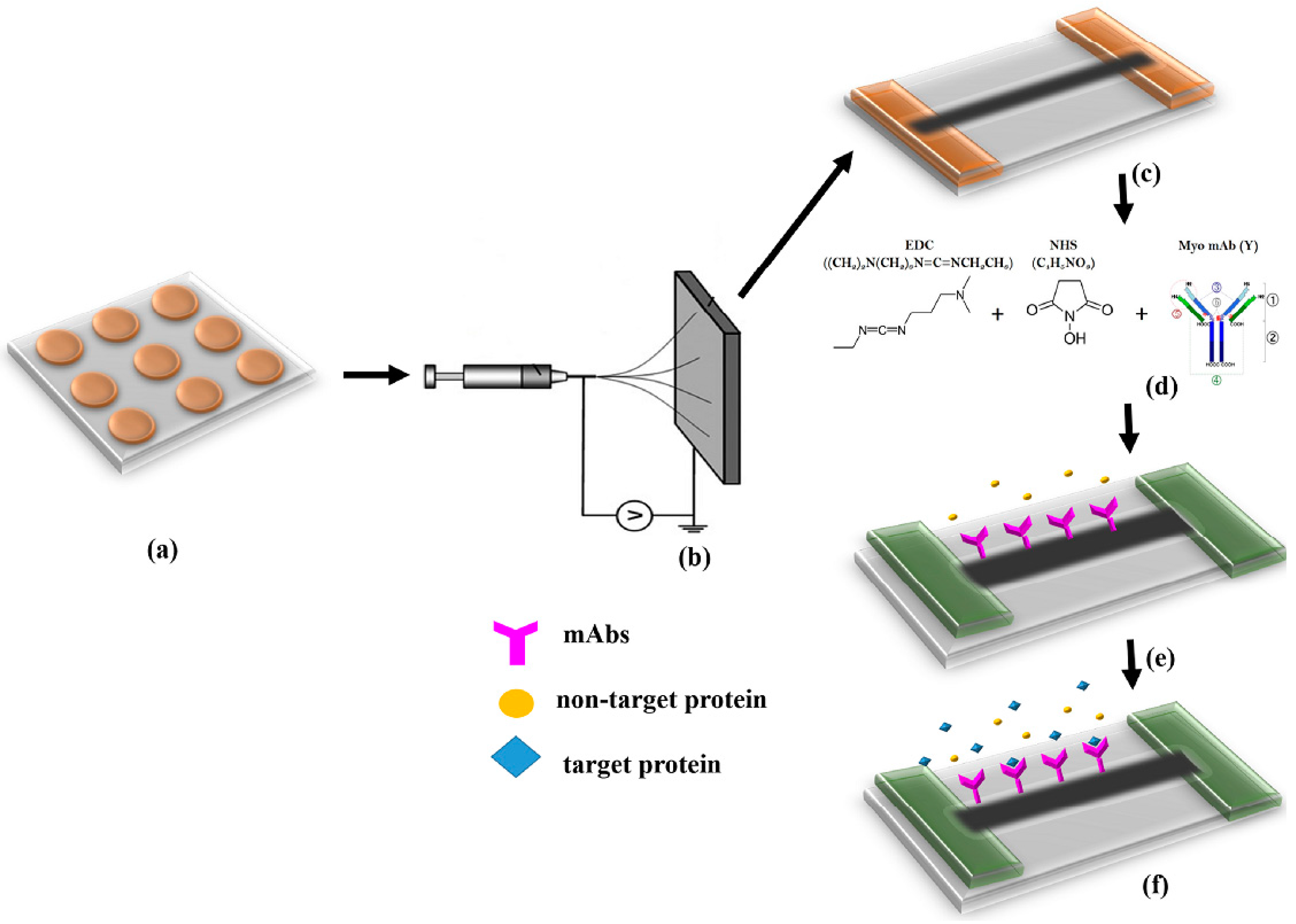
2.2. Fabrication of Microelectrode Array
2.3. Synthesis and Alignment of Nanofibers
2.4. Surface Functionalization
2.5. Experimentation Methodology
3. Results and Discussion
3.1. SEM Analysis
3.2. HRTEM Analysis
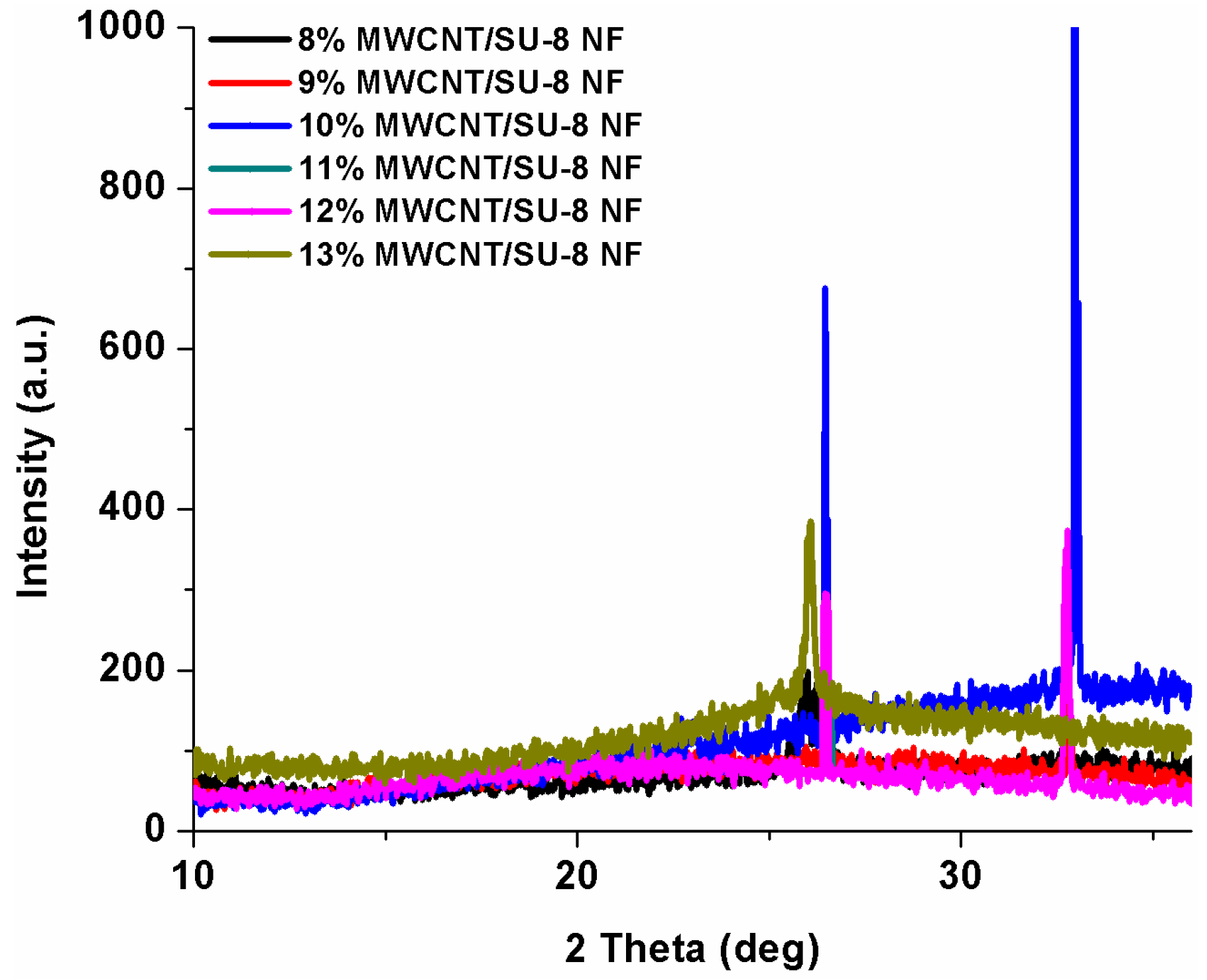
3.3. XRD Analysis
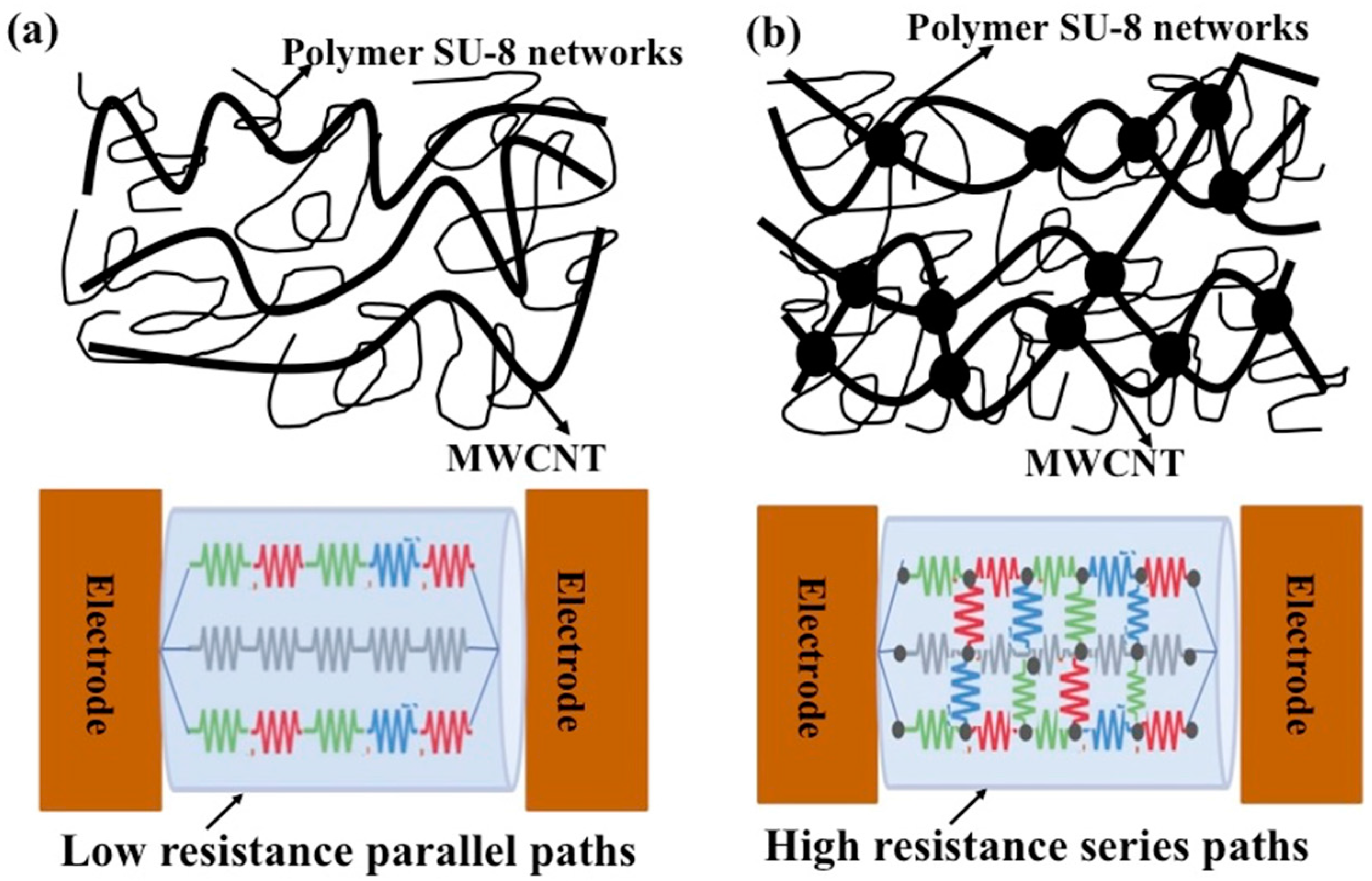
3.4. Conductivity Enhancement of SU-8 Using MWCNTs
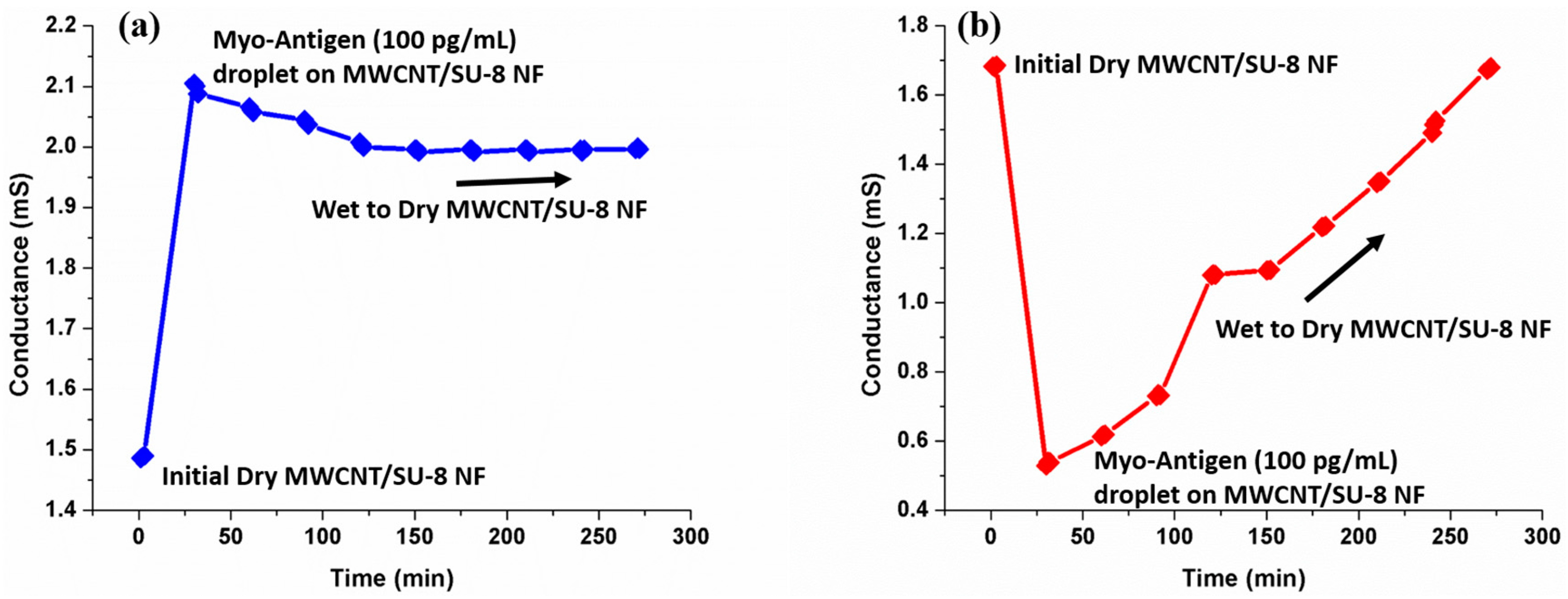
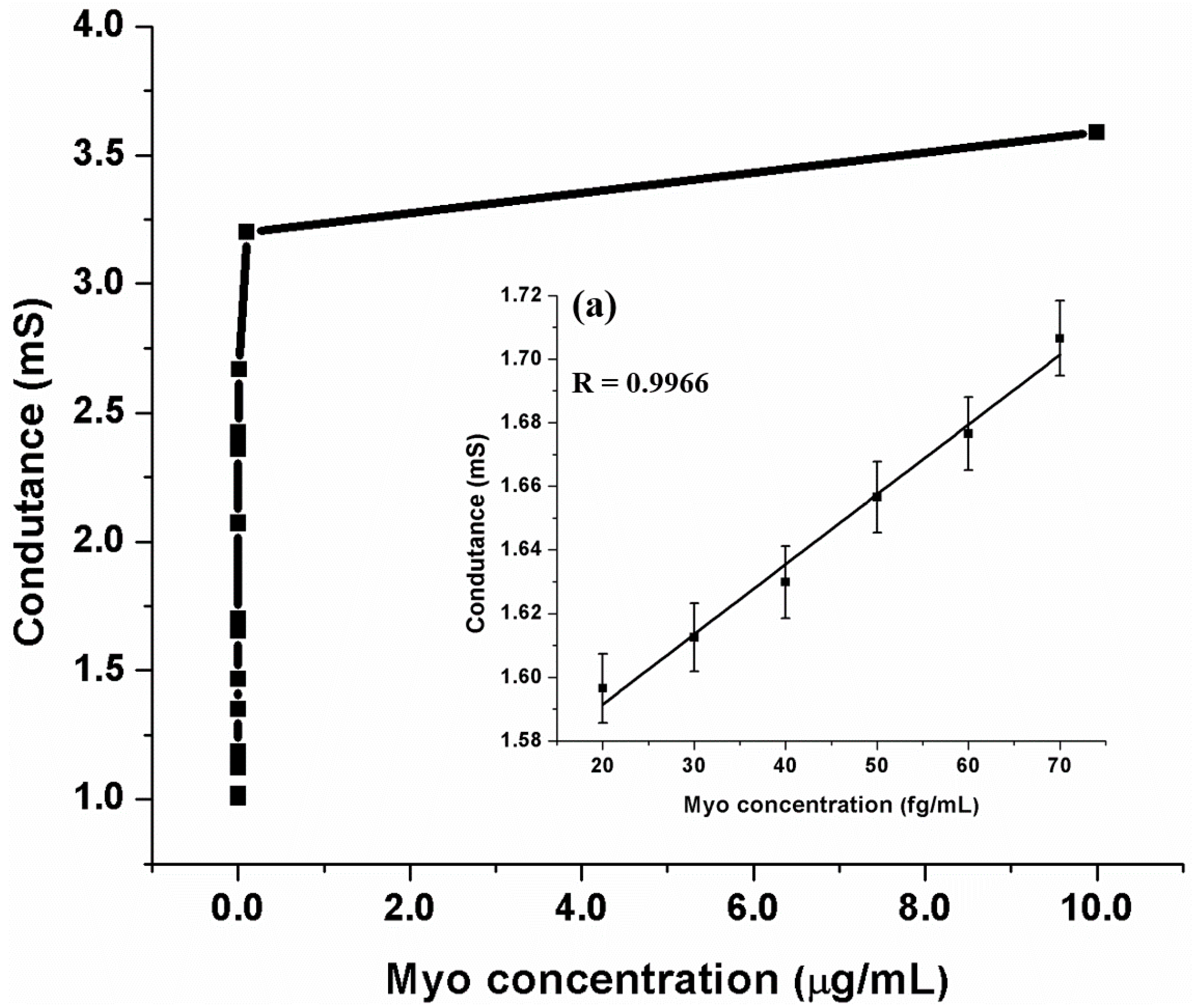
3.5. Detection of Myoglobin
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Wang, Q.; Liu, F.; Yang, X.; Wang, K.; Wang, H.; Deng, X. Sensitive point-of-care monitoring of cardiac biomarker myoglobin using aptamer and ubiquitous personal glucose meter. Biosens. Bioelectron. 2015, 64, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Boisen, A.; Dohn, S.; Keller, S.S.; Schmid, S.; Tenje, M. Cantilever-Like micromechanical sensors. Rep. Prog. Phys. 2011, 74. [Google Scholar] [CrossRef]
- Singamaneni, S.; LeMieux, M.C.; Lang, H.P.; Gerber, C.; Lam, Y.; Zauscher, S.; Datskos, P.G.; Lavrik, N.V.; Jiang, H.; Naik, R.R. Bimaterial microcantilevers as a hybrid sensing platform. Adv. Mater. 2008, 20, 653–680. [Google Scholar] [CrossRef]
- Fritz, J. Cantilever biosensors. Analyst 2008, 133, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Gossett, A.C.; Raj, M. Piezoelectric-Excited millimeter-sized cantilever (PEMC) sensors detect Bacillus anthracis at 300 spores/mL. Biosens. Bioelectron. 2006, 21, 1684–1692. [Google Scholar]
- Kishan, R.; Raj, M. PEMC-Based Method of Measuring DNA Hybridization at Femtomolar Concentration Directly in Human Serum and in the Presence of Copious Noncomplementary Strands. Anal. Chem. 2007, 79, 7392–7400. [Google Scholar]
- Ebrahimi, A.; Dak, P.; Salm, E.; Dash, S.; Garimella, S.V.; Bashir, R.; Alam, M.A. Nanotextured superhydrophobic electrodes enable detection of attomolar-scale dna concentration within a droplet by non-faradaic impedance spectroscopy. Lab Chip 2013, 13, 4248–4256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, X.; Fan, W.; Du, X. Iminodiacetic acid-functionalized gold nanoparticles for optical sensing of myoglobin via Cu2+ coordination. Langmuir 2011, 27, 6504–6510. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Mirkin, C.A. Drivers of bio diagnostic development. Nature 2009, 462, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Vermesh, O.; Srivastava, A.; Yen, B.K.; Qin, L.; Ahmad, H.; Kwong, G.A.; Liu, C.C.; Gould, J.; Hood, L.; et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nat. Biotechnol. 2008, 26, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Miranda, O.R.; Chen, H.-T.; You, C.-C.; Mortenson, D.E.; Yang, X.-C.; Bunz, U.H.; Rotello, V.M. Enzyme-Amplified array sensing of proteins in solution and in biofluids. J. Am. Chem. Soc. 2010, 132, 5285–5289. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Mandal, S.; Yang, A.H.; Cordovez, B. Nanobiosensors: Optofluidic, electrical and mechanical approaches to biomolecular detection at the nanoscale. Microfluid. Nanofluid. 2008, 4, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Urban, G.A. Micro-and nanobiosensors—State of the art and trends. Meas. Sci. Technol. 2008, 20. [Google Scholar] [CrossRef]
- Wohlstadter, J.N.; Wilbur, J.L.; Sigal, G.B.; Biebuyck, H.A.; Billadeau, M.A.; Dong, L.; Fischer, A.B.; Gudibande, S.R.; Jameison, S.H.; Kenten, J.H.; et al. Carbon nanotube-based biosensor. Adv. Mater. 2003, 15, 1184–1187. [Google Scholar] [CrossRef]
- So, H.-M.; Won, K.; Kim, Y.H.; Kim, B.-K.; Ryu, B.H.; Na, P.S.; Kim, H.; Lee, J.-O. Single-Walled carbon nanotube biosensors using aptamers as molecular recognition elements. J. Am. Chem. Soc. 2005, 127, 11906–11907. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.-I.; Lieber, C.M. Direct ultrasensitive electrical detection of DNA and DNA sequence variations using nanowire nanosensors. Nano Lett. 2004, 4, 51–54. [Google Scholar] [CrossRef]
- Maehashi, K.; Katsura, T.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-Free protein biosensor based on aptamer-modified carbon nanotube field-effect transistors. Anal. Chem. 2007, 79, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Cella, L.N.; Shirale, D.J.; Park, M.; Muñoz, F.J.; Yates, M.V.; Mulchandani, A. Carbon nanotubes-based chemiresistive biosensors for detection of microorganisms. Biosens. Bioelectron. 2010, 26, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, A.; Khare, S.; Mulchandani, A. Single-Walled carbon nanotubes based chemiresistive genosensor for label-free detection of human rheumatic heart disease. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Puri, N.; Niazi, A.; Biradar, A.M.; Mulchandani, A. Conducting polymer functionalized single-walled carbon nanotube based chemiresistive biosensor for the detection of human cardiac myoglobin. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterial 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Lee, I.; Park, H.I.; Park, S.; Kim, M.J.; Yun, M. Highly reproducible single polyaniline nanowire using electrophoresis method. Nano 2008, 3, 75–82. [Google Scholar] [CrossRef]
- Lee, I.; Luo, X.; Huang, J.; Cui, X.T.; Yun, M. Detection of cardiac biomarkers using single polyaniline nanowire-based conductometric biosensors. Biosensors 2012, 2, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An introduction to electrospinning and nanofibers. World Sci. 2005, 90, 342–382. [Google Scholar]
- Ramakrishna, S.; Lala, N.L.; Garudadhwaj, H.; Ramaseshan, R.; Ganesh, V. Polymer nanofibers for biosensor applications. In Molecular Building Blocks for Nanotechnology; Springer: New York, NY, USA, 2007; pp. 377–392. [Google Scholar]
- Luo, Y.; Nartker, S.; Wiederoder, M.; Miller, H.; Hochhalter, D.; Drzal, L.T.; Alocilja, E.C. Novel biosensor based on electrospun nanofiber and magnetic nanoparticles for the detection of E. coli O157:H7. IEEE Trans. Nanotechnol. 2012, 11, 676–681. [Google Scholar] [CrossRef]
- Uzun, S.D.; Kayaci, F.; Uyar, T.; Timur, S.; Toppare, L. Bioactive surface design based on functional composite electrospun nanofibers for biomolecule immobilization and biosensor applications. ACS Appl. Mater. Interfaces 2014, 6, 5235–5243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, K.; Ali, M.A.; Agrawal, V.V.; Malhotra, B.D.; Sharma, A. Highly sensitive bio-functionalized mesoporous electrospun TiO2 nanofiber based interface for biosensing. ACS Appl. Mater. Interfaces 2014, 6, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.S.; Katepalli, H.; Sharma, A.; Madou, M. Fabrication and electrical conductivity of suspended carbon nanofiber arrays. Carbon 2011, 49, 1727–1732. [Google Scholar] [CrossRef]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun poly(vinyl alcohol)/glucose oxidase biocomposite membranes for biosensor applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Manesh, K.M.; Santhosh, P.; Gopalan, A.; Lee, K.P. Electrospun poly (vinylidene fluoride)/poly (aminophenylboronic acid) composite nanofibrous membrane as a novel glucose sensor. Anal. Biochem. 2007, 360, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, K.; Gouma, P.; Simon, S. Electrospun biocomposite nanofibers for urea biosensing. Sens. Actuators B Chem. 2005, 108, 585–588. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Jia, J.; Wang, J. Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sens. Actuators B Chem. 2012, 171, 580–587. [Google Scholar] [CrossRef]
- Su, X.; Ren, J.; Meng, X.; Rena, X.; Tang, F. A novel platform for enhanced biosensing based on the synergy effects of electrospun polymer nanofibers and graphene oxides. Analyst 2013, 138, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Yu, S.A.; Lin, S.L. Graphene modified electrospun poly(vinyl alcohol) nanofibrous membranes for glucose oxidase immobilization. Express Polym. Lett. 2014, 8, 565–573. [Google Scholar] [CrossRef]
- Ning, N.; Bai, X.; Yang, D.; Zhang, L.; Lu, Y.; Nishi, T.; Tian, M. Dramatically improved dielectric properties of polymer composites by controlling the alignment of carbon nanotubes in matrix. RSC Adv. 2014, 4, 4543–4551. [Google Scholar] [CrossRef]
- Ford, E.N.J.; Suthiwangcharoen, N.; D’Angelo, P.A.; Nagarajan, R. Role of Single-Walled Carbon Nanotubes on Ester Hydrolysis and Topography of Electrospun Bovine Serum Albumin/Poly(vinyl alcohol) Membranes. Appl. Mater. Interfaces 2014, 6, 11741–11748. [Google Scholar] [CrossRef] [PubMed]
- Kalaoglu-Altan, O.I.; Sanyal, R.; Sanyal, A. “Clickable” Polymeric Nanofibers through Hydrophilic-Hydrophobic Balance: Fabrication of Robust Biomolecular Immobilization Platforms. Biomacromolecules 2015, 16, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Sapountzi, E.; Braiek, M.; Farre, C.; Arab, M.; Chateaux, J.F.; Renault, N.J.; Lagarde, F. One-Step Fabrication of Electrospun Photo-Cross-Linkable Polymer Nanofibers Incorporating Multiwall Carbon Nanotubes and Enzyme for Biosensing. J. Electrochem. Soc. 2015, 162, B275–B281. [Google Scholar] [CrossRef]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef]
- Wouters, K.; Puers, R. Diffusing and swelling in su-8: Insight in material properties and processing. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- El Fissi, L.; Friedt, J.-M.; Ch’erioux, F.; Ballandras, S. Amine functionalized SU-8 layer guiding love mode surface acoustic wave. Sens. Actuators B Chem. 2010, 144, 23–26. [Google Scholar] [CrossRef]
- Wang, Y.; Pai, J.-H.; Lai, H.-H.; Sims, C.E.; Bachman, M.; Li, G.; Allbritton, N.L. Surface graft polymerization of su-8 for bio-mems applications. J. Micromech. Microeng. 2007, 17, 1371–1380. [Google Scholar] [CrossRef]
- Xue, P.; Bao, J.; Chuah, Y.J.; Menon, N.V.; Zhang, Y.; Kang, Y. Protein covalently conjugated SU-8 surface for the enhancement of mesenchymal stem cell adhesion and proliferation. Langmuir 2014, 30, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.L.; Popat, K.C.; Norman, J.J.; Desai, T.A. Surface modification of SU-8 for enhanced biofunctionality and nonfouling properties. Langmuir 2008, 24, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Kale, N.; Lal, R.; Rao, V.R.; Mukherji, S. A novel dry method for surface modification of SU-8 for immobilization of biomolecules in Bio-MEMS. Biosens. Bioelectron. 2007, 22, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Pinto, R.; Rao, V.R.; Mukherji, S. Silanization and antibody immobilization on SU-8. Appl. Surf. Sci. 2007, 253, 3127–3132. [Google Scholar] [CrossRef]
- Deepu, A.; Sai, V.V.; Mukherji, S. Simple surface modification techniques for immobilization of biomolecules on SU-8. J. Mater. Sci. Mater. Med. 2009, 20, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Blagoi, G.; Boisen, A. Polymeric cantilever-based biosensors with integrated readout. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef] [Green Version]
- Nordström, M.; Keller, S.; Lillemose, M.; Johansson, A.; Dohn, S.; Haefliger, D.; Blagoi, G.; Havsteen-Jakobsen, M.; Boisen, A. SU-8 cantilevers for bio/chemical sensing; fabrication, characterisation and development of novel read-out methods. Sensors 2008, 8, 1595–1612. [Google Scholar] [CrossRef]
- Calleja, M.; Nordström, M.; Álvarez, M.; Tamayo, J.; Lechuga, L.M.; Boisen, A. Highly sensitive polymer-based cantilever-sensors for DNA detection. Ultramicroscopy 2005, 105, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Seena, V.; Rajorya, A.; Pant, P.; Mukherji, S.; Rao, V.R. Polymer microcantilever biochemical sensors with integrated polymer composites for electrical detection. Solid State Sci. 2009, 11, 1606–1611. [Google Scholar] [CrossRef]
- Holgado, M.; Barrios, C.A.; Ortega, F.J.; Sanza, F.J.; Casquel, R.; Laguna, M.F.; Bañuls, M.J.; López-Romero, D.; Puchades, R.; Maquieira, A. Label-Free biosensing by means of periodic lattices of high aspect ratio SU-8 nano-pillars. Biosens. Bioelectron. 2010, 25, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Shew, B.Y.; Kuo, C.H.; Huang, Y.C.; Tsai, Y.H. UV-LIGA interferometer biosensor based on the SU-8 optical waveguide. Sens. Actuators A Phys. 2005, 120, 383–389. [Google Scholar] [CrossRef]
- Esinenco, D.; Psoma, S.D.; Kusko, M.; Schneider, A.; Muller, R. SU-8 micro-biosensor based on Mach-Zehnder interferometer. Rev. Adv. Mater. Sci. 2005, 10, 295–299. [Google Scholar]
- Lee, H.J.; An, S.; Hwang, J.H.; Jung, S.-G.; Jo, H.S.; Kim, K.N.; Sim, Y.S.; Park, C.H.; Yoon, S.S.; Park, Y.W.; et al. Novel composite layer based on electrospun polymer nanofibers for efficient light scattering. ACS Appl. Mater. Interfaces 2015, 7, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, L.; Zeng, Y.; Zhang, L.; Li, F.; Liu, P.; Liu, C. Comparison of electrical properties between multi-walled carbon nanotube and graphene nanosheet/high density polyethylene composites with a segregated network structure. Carbon 2011, 49, 1094–1100. [Google Scholar] [CrossRef]
- Da Silva Leite Coelho, P.H.; Marchesin, M.S.; Morales, A.R.; Bartoli, J.R. Electrical percolation, morphological and dispersion properties of MWCNT/PMMA nanocomposites. Mater. Res. 2014, 17, 127–132. [Google Scholar] [CrossRef]
- Chen, R.; Das, S.R.; Jeong, C.; Khan, M.R.; Janes, D.B.; Alam, M.A. Copercolating graphene-wrapped silver nanowire network for high performance, highly stable, transparent conducting electrodes. Adv. Funct. Mater. 2013, 23, 5150–5158. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for cardiac biomarkers detection: A review. Sens. Actuators B Chem. 2012, 171, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Warwick, M.J. Immunoassay: A Practical Guide; Taylor & Francis Ltd.: London, UK, 2005. [Google Scholar]
- Wu, W.Y.; Bian, Z.P.; Wang, W.; Zhu, J.J. PDMS gold nanoparticle composite film-based silver enhanced colorimetric detection of cardiac troponin I. Sens. Actuators B Chem. 2010, 147, 298–303. [Google Scholar] [CrossRef]
- Cho, I.H.; Paek, E.H.; Kim, Y.K.; Kim, J.H.; Paek, S.H. Chemiluminometric enzyme-linked immunosorbent assays (ELISA)-on-a-chip biosensor based on cross-flow chromatography. Anal. Chim. Acta 2009, 632, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.R.; Martins, T.B. The flow cytometric analysis of cytokines using multi-analyte fluorescence microarray technology. Methods 2006, 38, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.F.; Battaglia, T.M.; Khairallah, P.; Beaudoin, S.; Booksh, K.S. Quantitative measurement of cardiac markers in undiluted serum. Anal. Chem. 2007, 79, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Tweedie, M.; Subramanian, R.; Lemoine, P.; Craig, I.; McAdams, E.T.; McLaughlin, J.A.; Maccraith, B.; Kent, N. Fabrication of impedimetric sensors for label-free point-of-care immunoassay cardiac marker systems, with passive microfluidic delivery. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 4610–4614.
- Shumyantseva, V.; Bulko, T.; Vagin, M.Y.; Suprun, E.; Archakov, A. Electrochemical immunoanalysis of cardiac myoglobin. Biochem. Suppl. Ser. B Biomed. Chem. 2010, 4, 237–242. [Google Scholar] [CrossRef]




| Transduction Platform | Detection Range | Ref. |
|---|---|---|
| ELISA | 20–230 ng·mL−1 | [66] |
| LOD—16 ng·mL−1 | ||
| Chemiluminescence | 10–104 ng·mL−1 | [67] |
| LOD—1.2 ng·mL−1 | ||
| Fluorescence | 0.01–10 ng·mL−1 | [68] |
| LOD—0.01 ng·mL−1 | ||
| Surface plasmon resonance (SPR) | 0.1–200 ng·mL−1 | [69] |
| LOD—below 1 ng·mL−1 | ||
| Electrochemical Impedance Spectroscopy/interdigitated electrodes | 0.5–500 ng·mL−1 | [70] |
| LOD—100 ng·mL−1 | ||
| Electrochemical/nanoparticles modified electrodes | 17.8–1780 ng·mL−1 | [71] |
| LOD—5 ng·mL−1 | ||
| Chemiresistive (Present work) | 20–70 fg·mL−1 | |
| LOD—6 fg·mL−1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durga Prakash, M.; Vanjari, S.R.K.; Sharma, C.S.; Singh, S.G. Ultrasensitive, Label Free, Chemiresistive Nanobiosensor Using Multiwalled Carbon Nanotubes Embedded Electrospun SU-8 Nanofibers. Sensors 2016, 16, 1354. https://doi.org/10.3390/s16091354
Durga Prakash M, Vanjari SRK, Sharma CS, Singh SG. Ultrasensitive, Label Free, Chemiresistive Nanobiosensor Using Multiwalled Carbon Nanotubes Embedded Electrospun SU-8 Nanofibers. Sensors. 2016; 16(9):1354. https://doi.org/10.3390/s16091354
Chicago/Turabian StyleDurga Prakash, Matta, Siva Rama Krishna Vanjari, Chandra Shekhar Sharma, and Shiv Govind Singh. 2016. "Ultrasensitive, Label Free, Chemiresistive Nanobiosensor Using Multiwalled Carbon Nanotubes Embedded Electrospun SU-8 Nanofibers" Sensors 16, no. 9: 1354. https://doi.org/10.3390/s16091354





