Graphene Nanogrids FET Immunosensor: Signal to Noise Ratio Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Nanogrid Fabrication
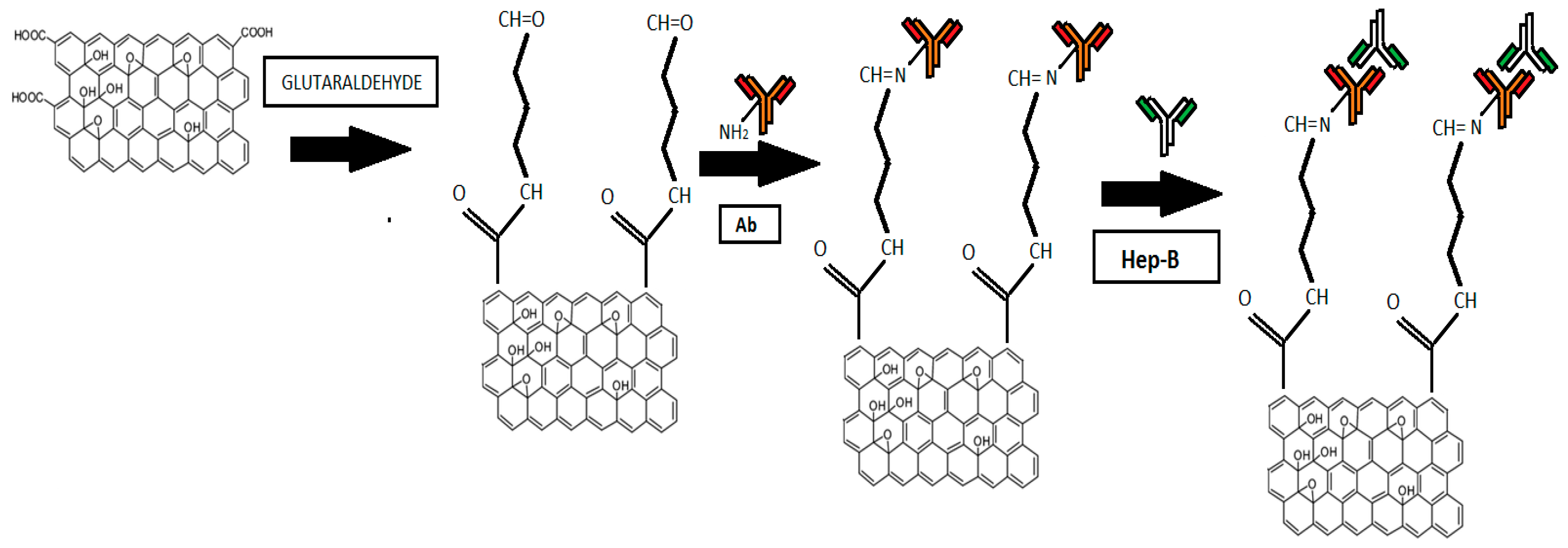
2.2. Immobilization Process
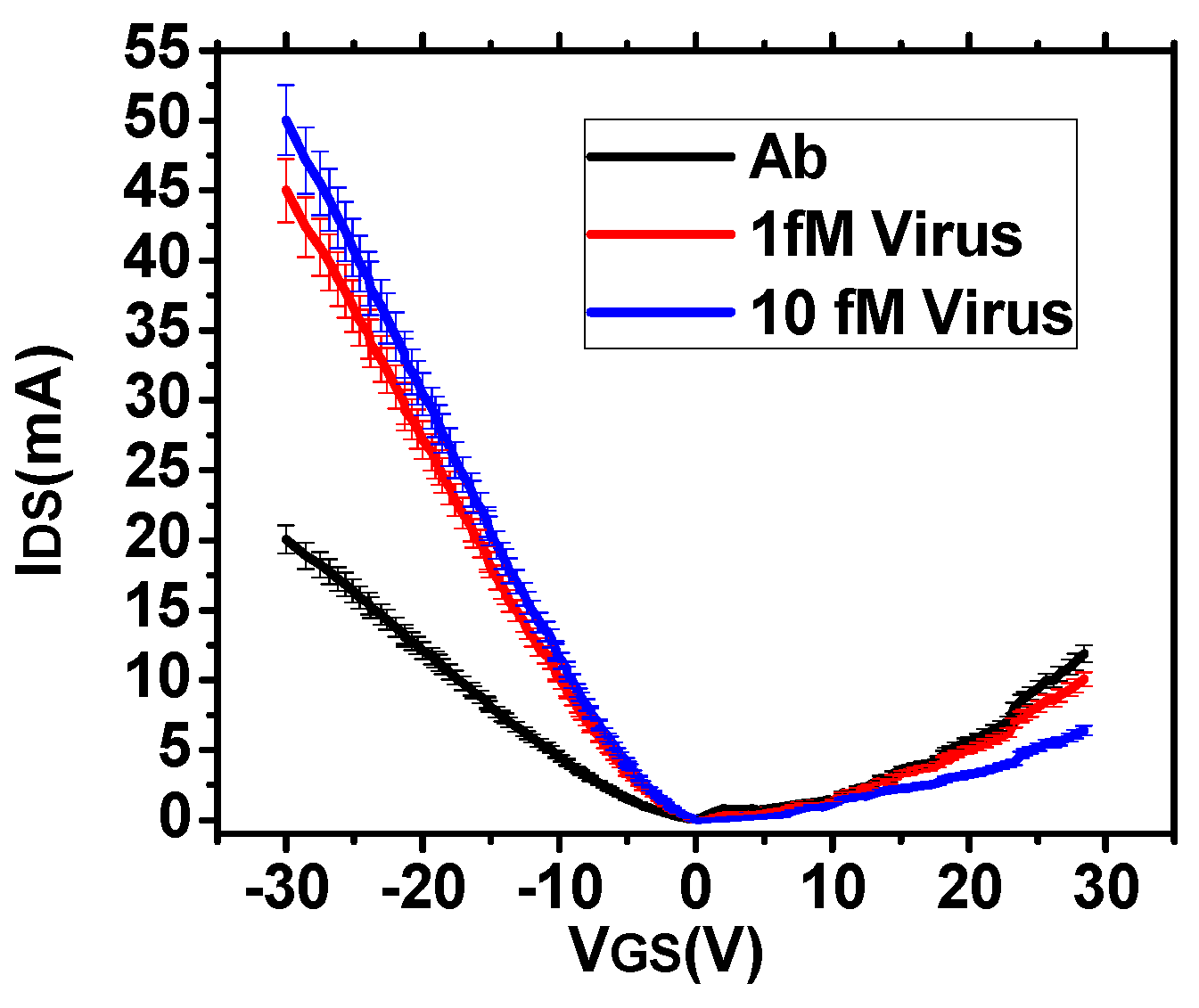
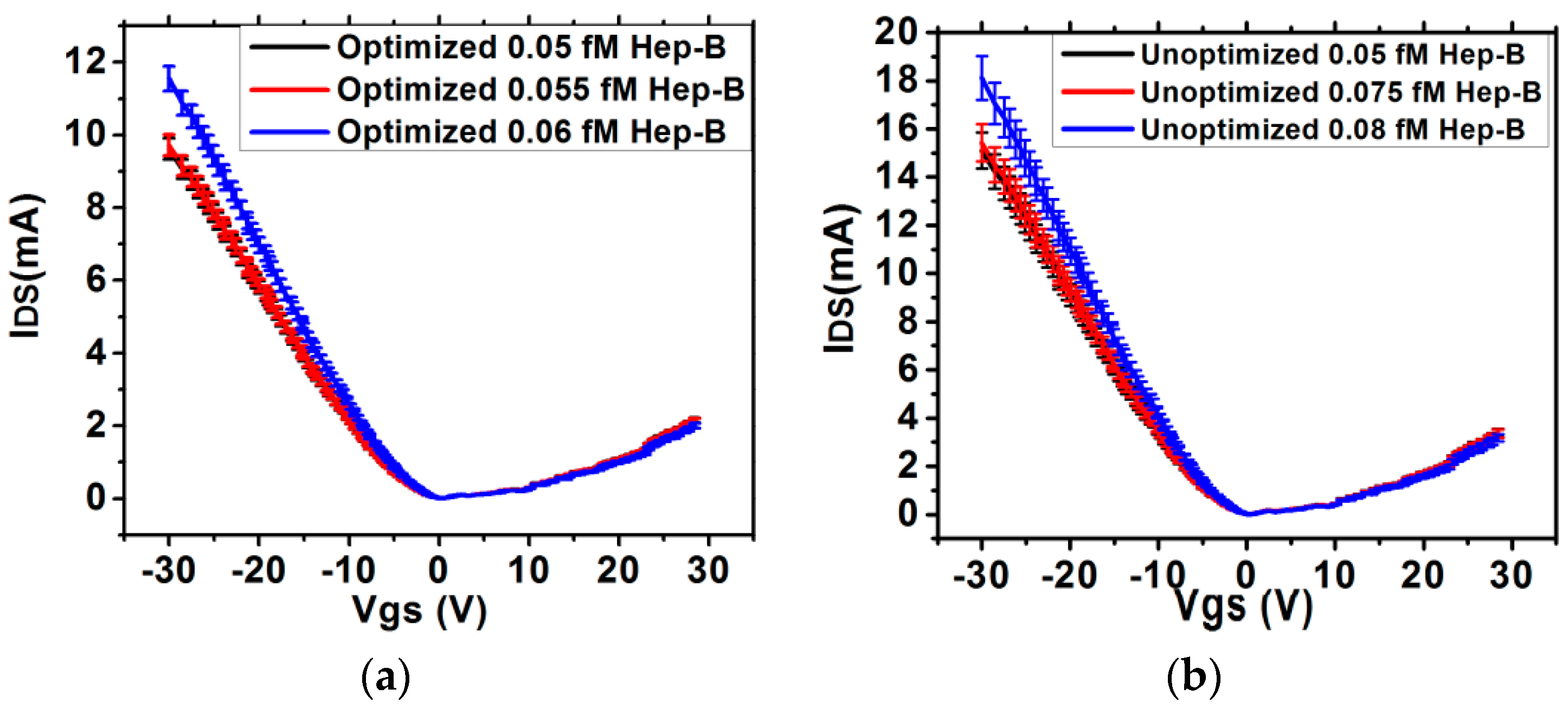
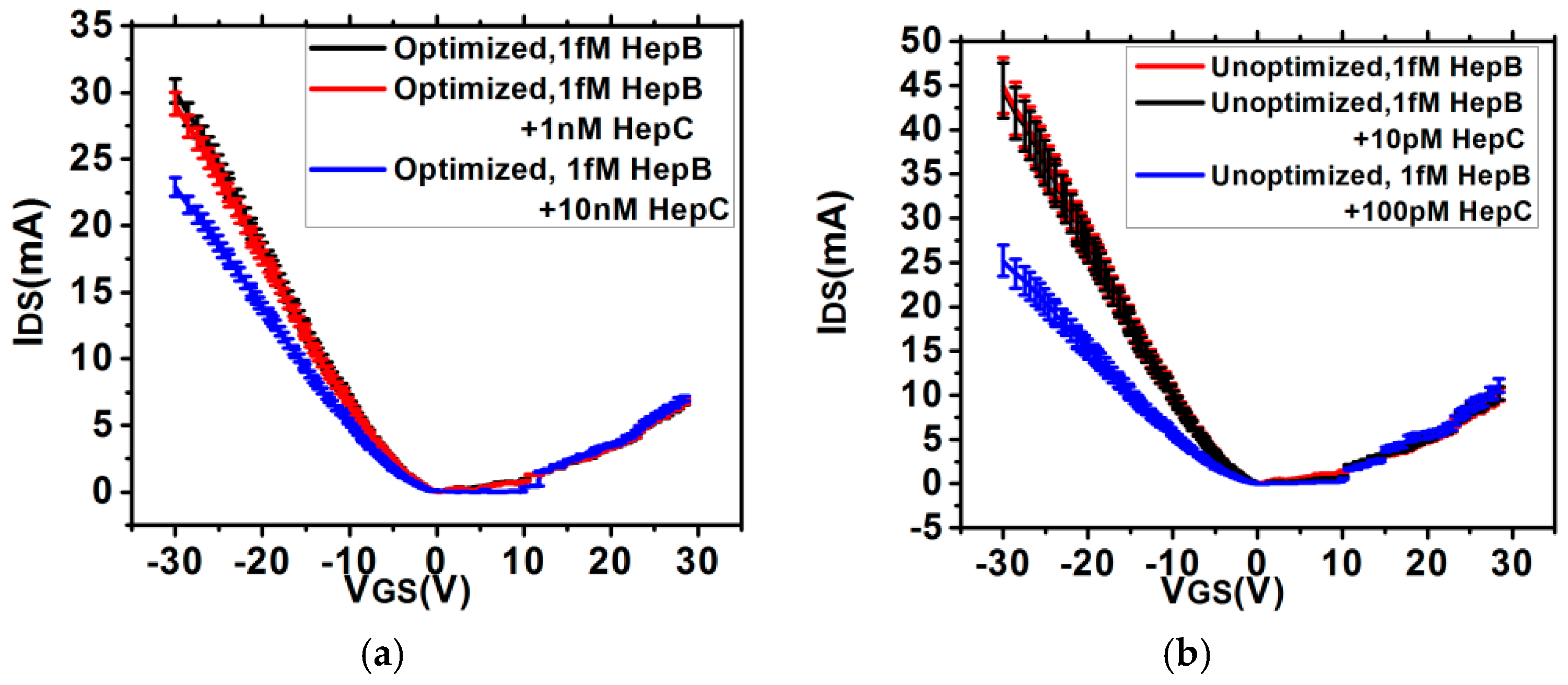
2.3. FET Measurement
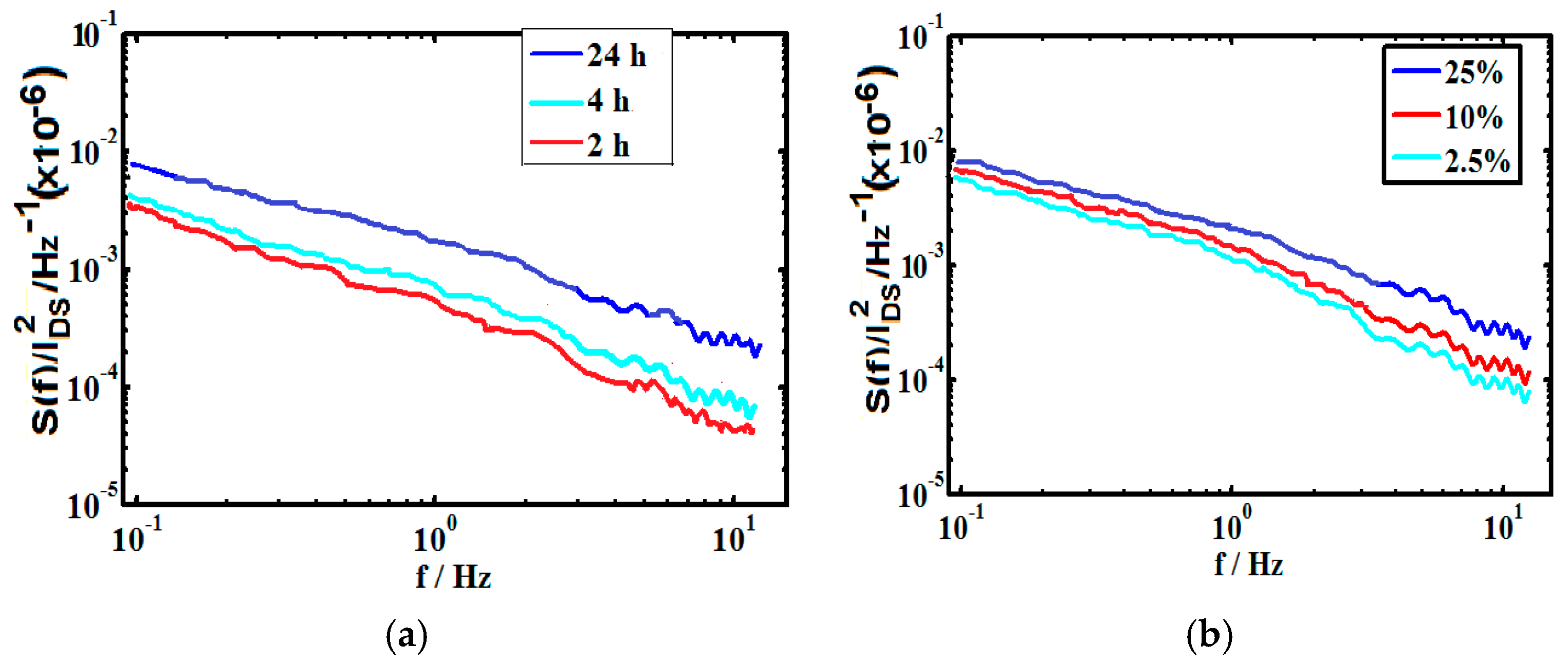
2.4. Power Spectral Density Measurement
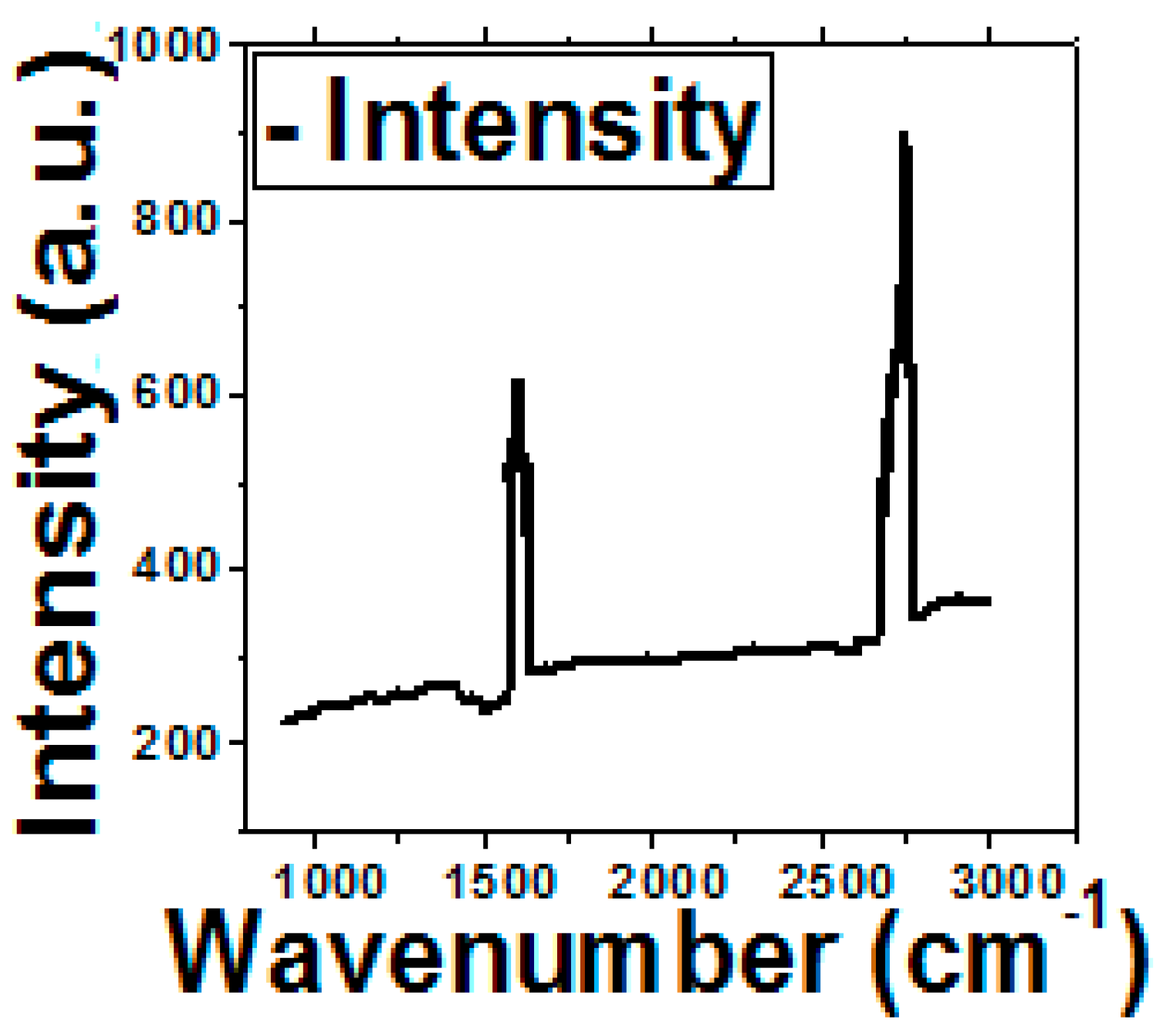
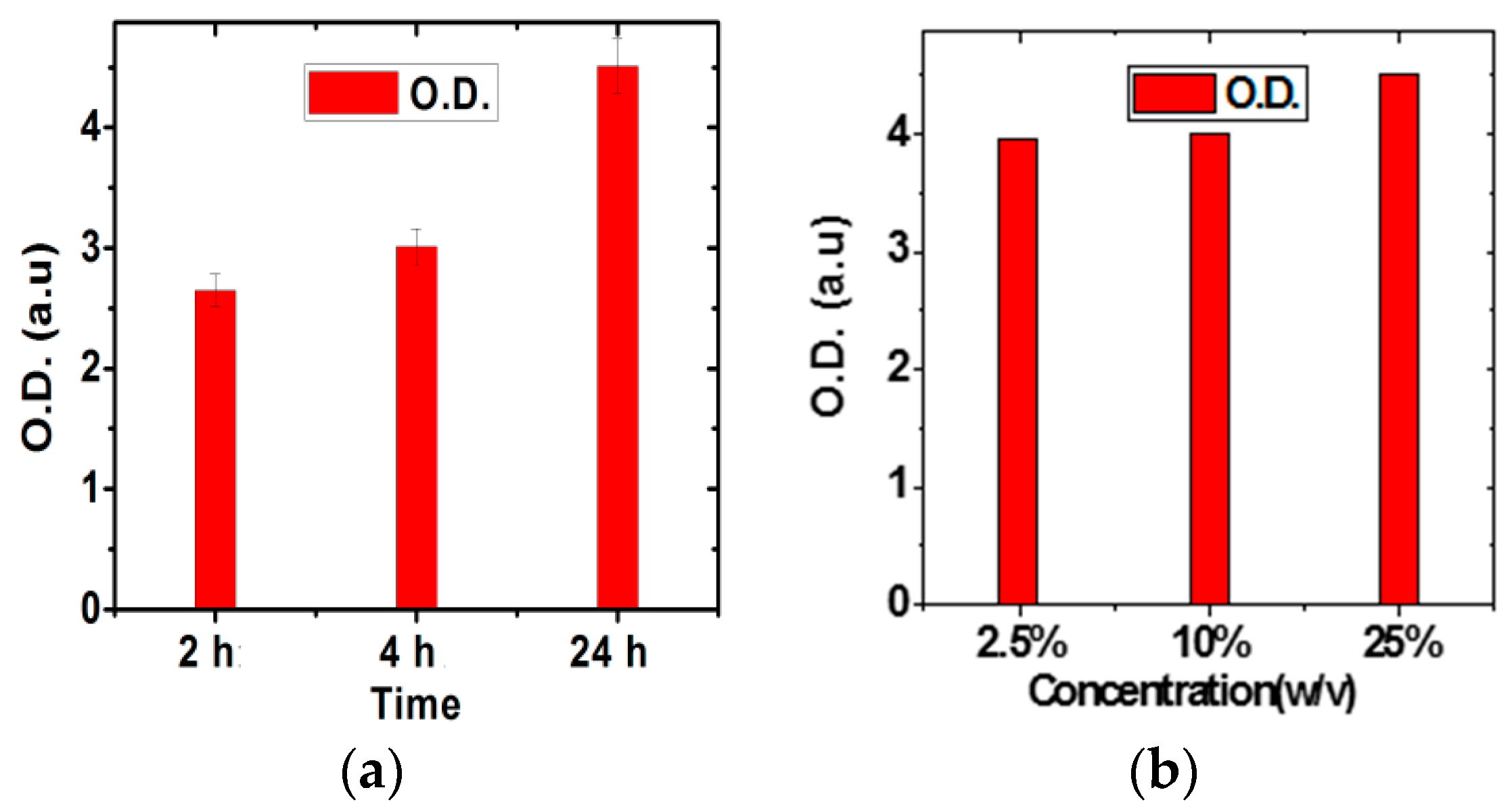
2.5. Optical Density and Raman Measurement
3. Results and Discussions
3.1. Graphene Characterization and Glutaraldehyde Optimization
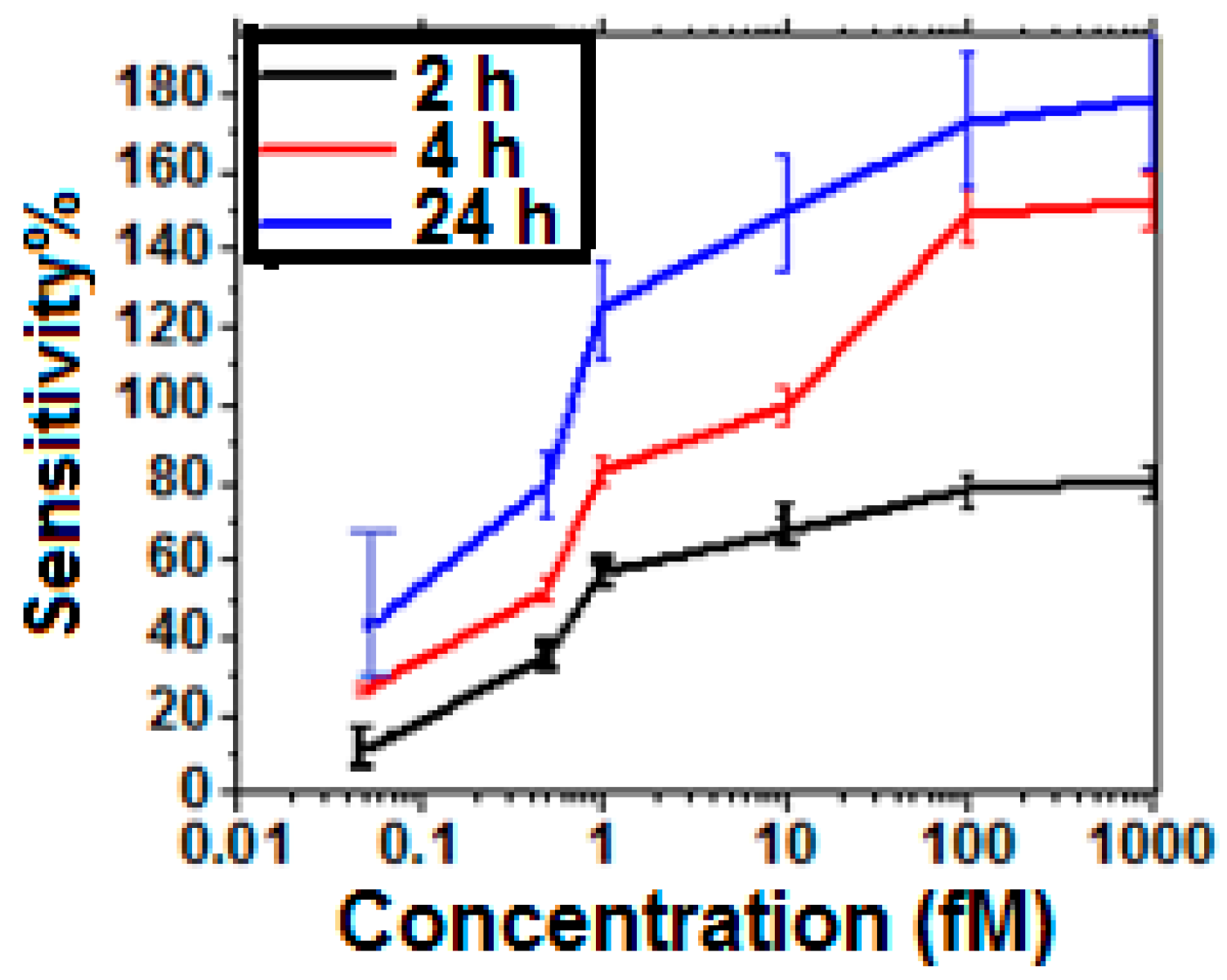
3.2. Estimation of Sensitivity and Noise
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect inatomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, X.D.; Chen, X.P.; Ding, X.; Li, X.Y. Subsecond response of humidity sensor based on graphene oxide quantum dots. IEEE Electron Device Lett. 2015, 36, 615–617. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Yu, K.; Chang, J.; Steeber, D.A.; Ocola, L.E.; Chen, J. Direct growth of vertically-oriented graphene for field-effect transistor biosensor. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Özyilmaz, B.; Zhang, Y.; Kim, P. Energy bandgap engineering of graphene nanoribbons. Phys. Rev. Lett. 2007, 98, 206805. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Su, C.H.; Wu, J.J. Synthesis of short graphene oxide nanoribbons for improved biomarker detection of Parkinson’s disease. Biosens. Bioelectron. 2015, 67, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Par, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar]
- Dong, X.; Long, Q.; Wang, J.; Chan-Park, M.B.; Huang, Y.; Huang, W.; Chen, P. A graphene nanoribbon network and its biosensing application. Nanoscale 2011, 3, 5156–5160. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; RoyChaudhuri, C. Attomolar Sensitivity of FET Biosensor Based on Smooth and Reliable Graphene Nanogrids. IEEE Electron Device Lett. 2016, 37, 492–495. [Google Scholar] [CrossRef]
- Rumyantsev, S.; Liu, G.; Stillman, W.; Shur, M.; Balandin, A.A. Electrical and Noise Characteristics of Graphene FETs: Ambient Effects, Noise Sources and Physical Mechanisms. J. Phys. Condens. Matter 2010, 22, 395302. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.R.; Bid, A. Effect of ambient on the resistance fluctuations of graphene. Appl. Phys. Lett. 2015, 106, 183105. [Google Scholar] [CrossRef]
- Rajan, N.K.; Brower, K.; Duan, X.; Reed, M.A. Limit of detection of field effect transistor biosensors: Effects of surface modification and size dependence. Appl. Phys. Lett. 2014, 104, 084106. [Google Scholar] [CrossRef]
- Guo, Q.; Kong, T.; Su, R.; Zhang, Q.; Cheng, G. Noise spectroscopy as an equilibrium analysis tool for highly sensitive electrical biosensing. Appl. Phys. Lett. 2012, 101, 093704. [Google Scholar] [CrossRef]
- Heller, I.; Chatoor, S.; Männik, J.; Zevenbergen, M.A.G.; Oostinga, J.B.; Morpurgo, A.F.; Dekker, C.; Lemay, S.G. Charge Noise in Graphene Transistors. Nano Lett. 2010, 10, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Kochat, V.; Tiwary, C.S.; Biswas, T.; Ramalingam, G.; Hsieh, K.; Chattopadhyay, K.; Raghavan, S.; Jain, M.; Ghosh, A. Magnitude and Origin of Electrical Noise at Individual Grain Boundaries in Graphene. Nano Lett. 2016, 16, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.A.; Sabri, S.; Szkopek, T. Low-frequency noise and hysteresis in graphene field-effect transistors on oxide. Micro Nano Lett. 2010, 5, 37–41. [Google Scholar] [CrossRef]
- Kayyalha, M.; Chen, Y.P. Observation of reduced 1/f noise in Graphene field effect transistors on Boron Nitride substrates. Appl. Phys. Lett. 2015, 107, 113101. [Google Scholar] [CrossRef]
- Kumar, M.; Laitinen, A.; Cox, D.; Hakonen, P.J. Ultra low 1/f noise in suspended bilayer graphene. Appl. Phys. Lett. 2015, 106, 263505. [Google Scholar] [CrossRef]
- Tan, Z.B.; Puska, A.; Nieminen, T.; Duerr, F.; Gould, C.; Molenkamp, L.W.; Trauzettel, B.; Hakonen, P.J. Shot noise in lithographically patterned graphene nanoribbons. Phys. Rev. B 2013, 88, 245415. [Google Scholar] [CrossRef]
- Danneau, R.; Wu, F.; Tomi, M.Y.; Oostinga, J.B.; Morpurgo, A.F.; Hakonen, P.J. Shot noise suppression and hopping conduction in graphene nanoribbons. Phys. Rev. B 2010, 82, 161405. [Google Scholar] [CrossRef]
- Gopar, V. Shot noise fluctuations in disordered graphene nanoribbons near the Dirac point. Phys. E 2016, 77, 23–28. [Google Scholar] [CrossRef]
- Pal, A.N.; Ghosh, A. Resistance Noise in Electrically Biased Bilayer Graphene. Phys. Rev. Lett. 2009, 102, 126805. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Chen, J.-H.; Adam, S.; Williams, E.D.; Fuhrer, M.S. Charged impurity scattering in bilayer graphene. Phys. Rev. B 2010, 82, 041406. [Google Scholar] [CrossRef]
- Chang, J.; Mao, S.; Zhang, Y.; Cui, S.; Zhou, G.; Wu, X.; Yang, C.H.; Chen, J. Ultrasonic-assisted self-assembly of monolayer graphene oxide for rapid detection of Escherichia coli bacteria. Nanoscale 2013, 5, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Khalily, M.A.; Goktas, M.; Guler, M.O. Tuning viscoelastic properties of supramolecular peptide gels via dynamic covalent crosslinking. Org. Biomol. Chem. 2015, 13, 1983–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Zhao, Y.; Du, B.; Wua, D.; Li, H.; Yang, M. Ultrasensitive detection of kanamycin in animal derived foods by label-free electrochemical immunosensor. Food Chem. 2012, 134, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Cho, M.K.; Lee, E.J.; Ahn, K.Y.; Lee, K.E.; Jung, J.H.; Cho, Y.; Han, S.S.; Kim, Y.K.; Lee, J. A highly sensitive and selective diagnostic assay based on virus nanoparticles. Nat. Nanotechnol. 2009, 4, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Das, R.D.; Maji, S.; Das, S.; RoyChaudhuri, C. Optimization of covalent antibody immobilization on macroporous silicon solid supports. Appl. Surf. Sci. 2010, 256, 5867–5875. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Wu, Z.; Liu, Y.; Liu, S.; Zou, Z.; Chen, S.H.; Qu, C. Immunizations with hepatitis B viral antigens and a TLR7/8 agonist adjuvant induce antigen-specific immune responses in HBV-transgenic mice. Int. J. Infect. Dis. 2014, 29, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mendez, E.E.; Du, X. Mobility-Dependent Low-Frequency Noise in Graphene Field-Effect Transistors. ACS Nano 2011, 5, 8124–8130. [Google Scholar] [CrossRef] [PubMed]


| Incubation Time (h) | Concentrations of Glutaraldehyde | (×106) for 1 fM Hep-B | (×106) for 10 fM Hep-B |
|---|---|---|---|
| 24 | 25% | 327.3684 | 6279.0336 |
| 10% | 312.0282 | 6189.3021 | |
| 2.5% | 311.0994 | 6176.0563 | |
| 4 | 25% | 461.1333 | 7389.6296 |
| 10% | 448.3117 | 7312.2811 | |
| 2.5% | 446.3157 | 7261.6071 | |
| 2 | 25% | 317.8089 | 5259.6899 |
| 10% | 310.6622 | 5227.6785 | |
| 2.5% | 303.9864 | 5168.1818 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, J.; RoyChaudhuri, C. Graphene Nanogrids FET Immunosensor: Signal to Noise Ratio Enhancement. Sensors 2016, 16, 1481. https://doi.org/10.3390/s16101481
Basu J, RoyChaudhuri C. Graphene Nanogrids FET Immunosensor: Signal to Noise Ratio Enhancement. Sensors. 2016; 16(10):1481. https://doi.org/10.3390/s16101481
Chicago/Turabian StyleBasu, Jayeeta, and Chirasree RoyChaudhuri. 2016. "Graphene Nanogrids FET Immunosensor: Signal to Noise Ratio Enhancement" Sensors 16, no. 10: 1481. https://doi.org/10.3390/s16101481





