Influence of Experimental Conditions on Electronic Tongue Results—Case of Valsartan Minitablets Dissolution
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Membrane Materials
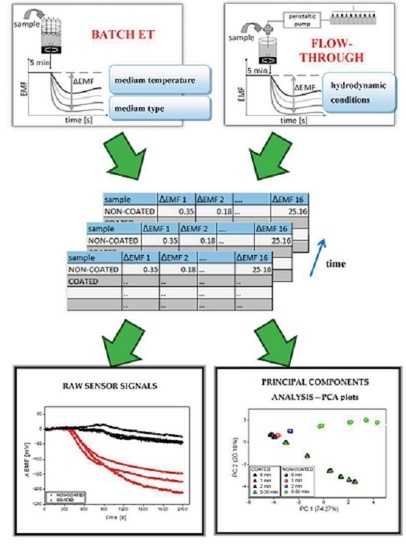
2.2. Sensor Arrays and Electronic Tongue Measurements
- batch measurements in two temperature values: room temperature (20–25 °C) or 37 °C;
- batch measurements in three medium types: deionized water, phosphate buffer pH 6.9 (prepared according to Ph.Eur., diluted to 1 mM), and artificial saliva pH 6.8 (0.17 M NaCl, 1.4 mM KH2PO4, 6.6 mM NaH2PO4);
- two systems working in various hydrodynamic conditions: batch ET and flow–through ET.
2.3. Pharmaceutical Formulations and Reference Dissolution Studies
3. Results and Discussion
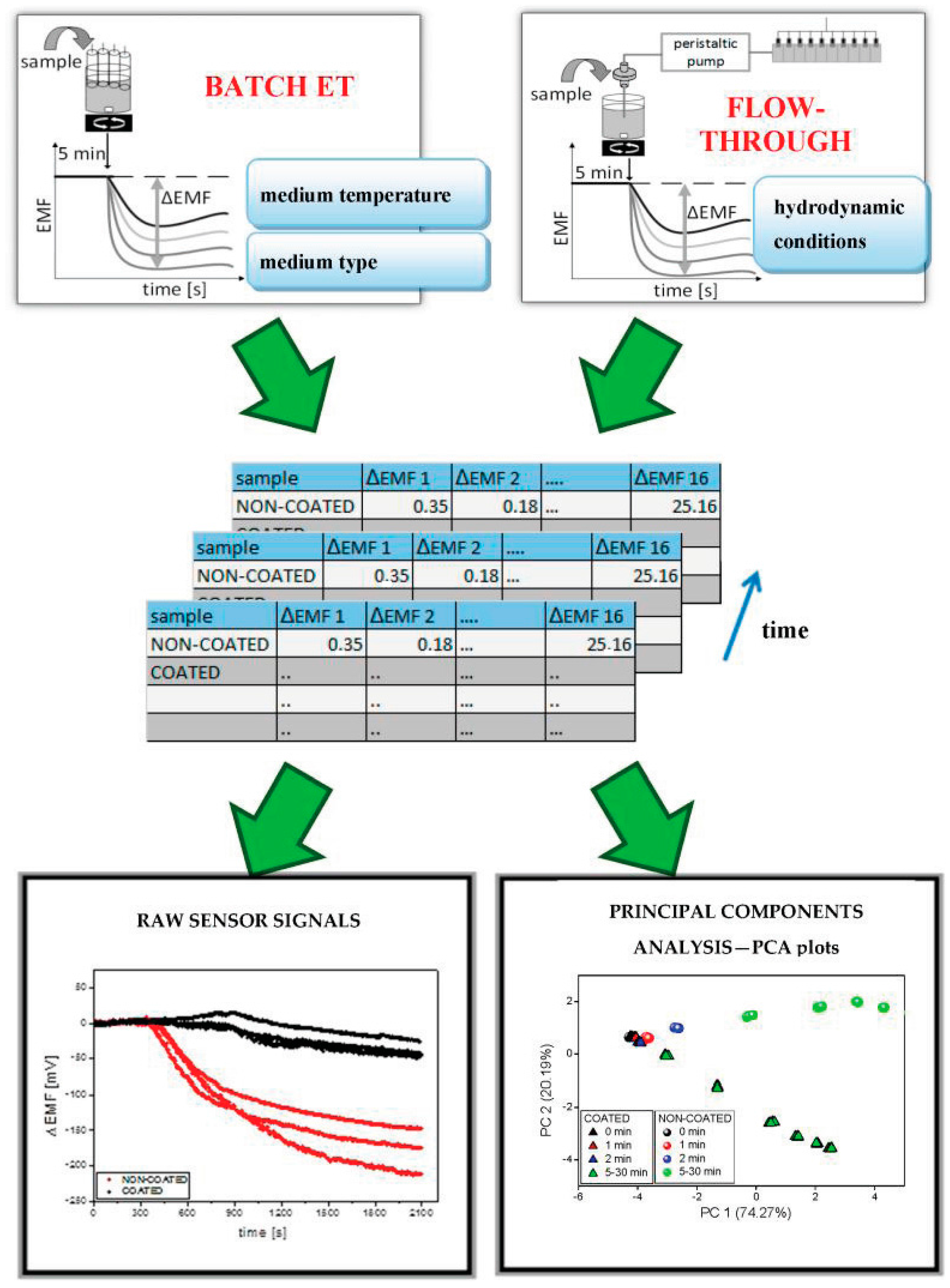
3.1. Reference Dissolution Studies
3.2. Performances of the Potentiometric Sensor Arrays
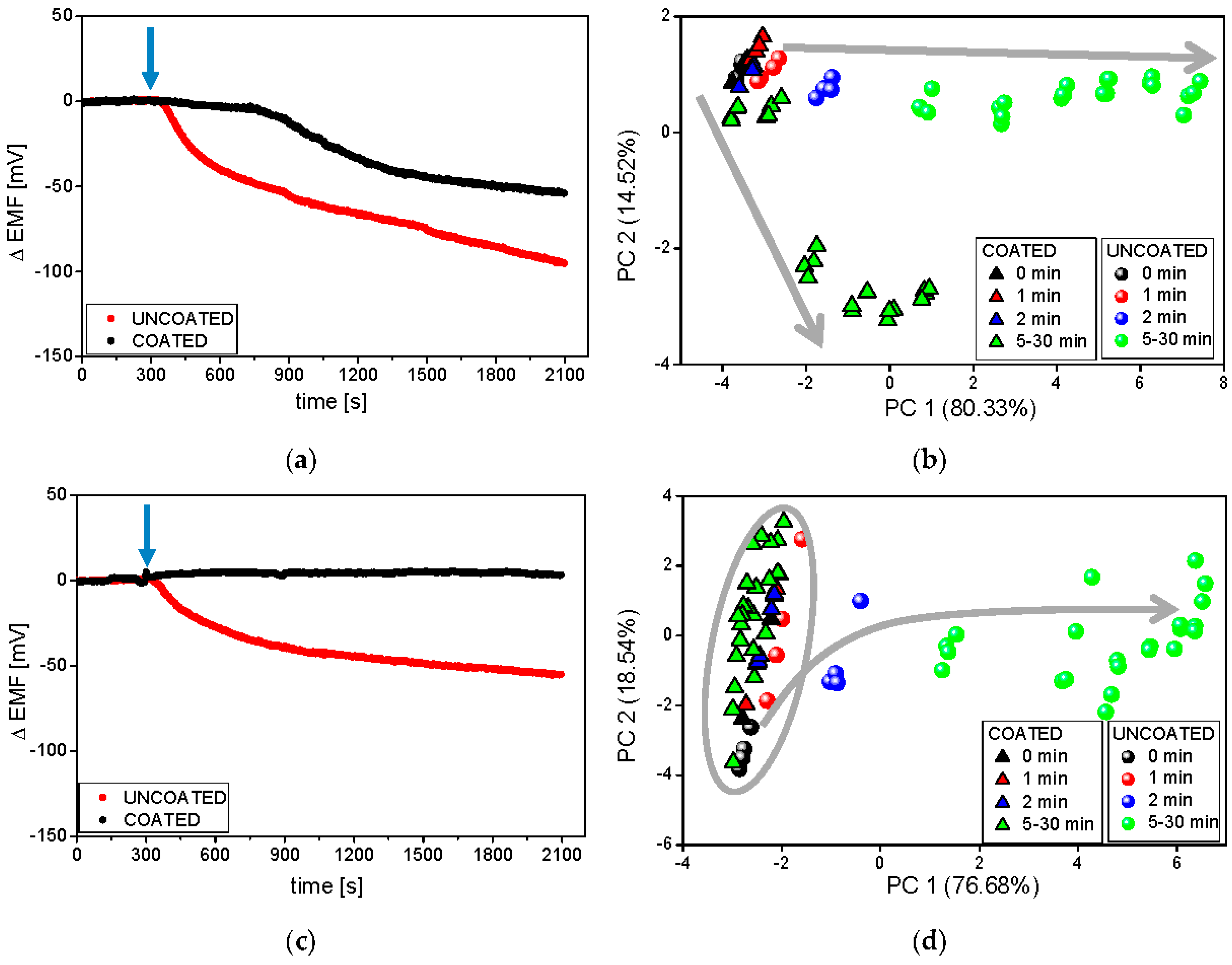
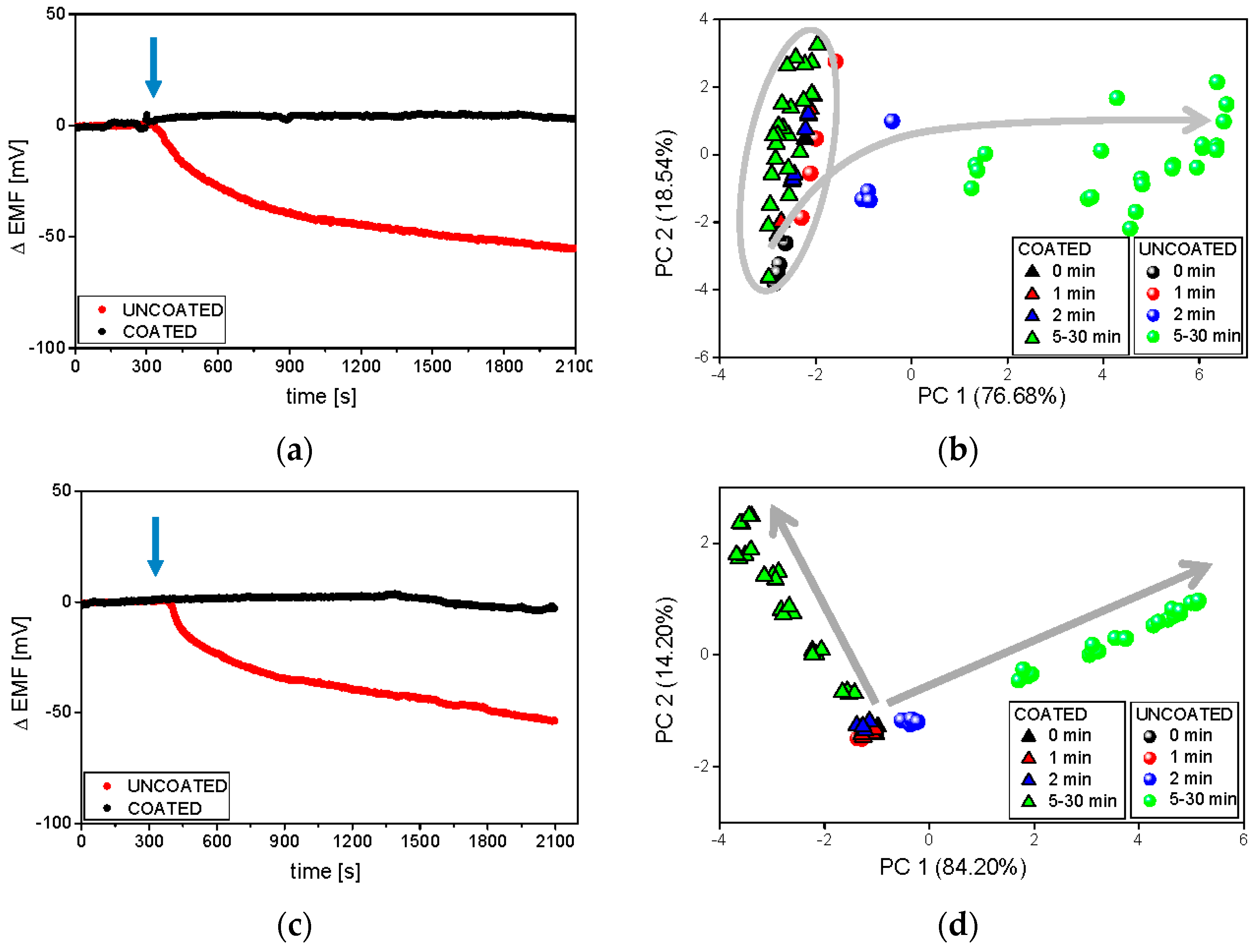
3.3. Electronic Tongue Dissolution Studies under Various Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guhmann, M.; Preis, M.; Gerber, F.; Pollinger, N.; Breitkreutz, J.; Weitschies, W. Design, development and in vitro eveluation of diclofenac taste-masked orodispersible tablet formulations. Drug Dev. Ind. Pharm. 2015, 41, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Loyd, V.A.; Popovich, N.G.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, 9th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- U.S. FDA/CDER. Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms; U.S. FDA: Silver Spring, MD, USA, 1997.
- Anand, O.; Yu, L.X.; Conner, D.P.; Davit, B.M. Dissolution testing for generic drugs: An FDA perspective. AAPS J. 2011, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Particle Sciences—Drug Development Services. In Vitro Dissolution Testing for Solid Oral Dosage Forms; Technical Brief; Particle Sciences: Bethlehem, PA, USA, 2010; Volume 5. [Google Scholar]
- European Pharmacopoeia Commission. European Pharmacopoeia, 6th ed.; Council of Europe: Strasbourg, France, 2007; pp. 263–275. [Google Scholar]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing—Electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Namieśnik, J.; Wardencki, W. Food analysis using artificial senses. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef]
- Ha, D.; Sun, Q.; Su, K.; Wan, H.; Li, H.; Xu, N.; Sun, F.; Zhuang, L.; Hu, N.; Wang, P. Recent achievements in electronic tongue and bioelectronic tongue as taste sensors. Sens. Actuators B Chem. 2015, 207, 1136–1146. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróbewski, W. Potentiometric and hybrid electronic tongues for bioprocess monitoring—An overview. Anal. Methods 2015, 7, 3958–3966. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Taste sensing systems (electronic tongues) for pharmaceutical applications. Int. J. Pharm. 2011, 417, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Rational development of taste masked oral liquids guided by an electronic tongue. Int. J. Pharm. 2010, 400, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, N.A.; Nam, T.S.; Lee, S.; Jeong, S.H. Evaluation of taste-masking effects of pharmaceutical sweeteners with an electronic tongue system. Drug Dev. Ind. Pharm. 2014, 40, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Jańczyk, M.; Kutyła, A.; Sollohub, K.; Wosicka, H.; Cal, K.; Ciosek, P. Electronic tongue for the detection of taste-masking microencapsulation of active pharmaceutical substances. Bioelectrochemistry 2010, 80, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.S.; Bonnefille, M.; Aranyos, A.; Mitchell, J.C.; Douroumis, D. Taste masking of paracetamol by hot-melt extrusion: An in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Krause, J. Novel Paediatric Formulations for the Drug Sodium Benzoate. Ph.D. Thesis, Heinrich-Heine-University Dusseldorf, Dusseldorf, Germany, 2008. [Google Scholar]
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Kluk, A.; Sznitowska, M.; Brandt, A.; Sznurkowska, K.; Plata-Nazar, K.; Mysliwiec, M.; Kaminska, B.; Kotlowska, H. Can preschool-aged children swallow several minitablets at a time? Results from a clinical pilot study. Int. J. Pharm. 2015, 485, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Douroumis, D. An in vitro–in-vivo taste assessment of bitter drug: Comparative electronic tongues study. J. Pharm. Pharmacol. 2014, 67, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Gondongwe, X.D.; Habara, M.; Winzenburg, G. Interlaboratory testing of Insent e-tongues. Int. J. Pharm. 2014, 469, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Guhnmann, M.; Preis, M.; Gerber, F.; Pollinger, N.; Breitkreutz, J. Development of oral taste masked diclofenac formulations using a taste sensing systems. Int. J. Pharm. 2012, 438, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.J.; Kim, J.Y.; Rhee, Y.S.; Kim, S.H.; Lee, H.J.; Park, C.W.; Park, E.S. Preparation of sildenafil citrate microcapsules and in vitro/in vivo evaluation of taste masking efficiency. Int. J. Pharm. 2014, 466, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Kirsanov, D.; Ciosek, P.; del Valle, M.; Yaroshenko, I.; Wesoły, M.; Zabadaj, M.; Gonzalez-Calabuig, A.; Wróblewski, W.; Legin, A. Independent comparison study of six different electronic tongues applied for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2015, 114, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Grother, L.; Axe, P.; Breitkreutz, J. In-vitro and in vivo evaluation of taste-masked cetirizine hydrochloride formulated in oral lyophilisates. Int. J. Pharm. 2015, 491, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhang, B.; Hu, L.; Zhuang, L.; Hu, N.; Wang, P. A novel bioelectronic tongue in vivo for highly sensitive bitterness detection with brain-machine interface. Biosens. Bioelectron. 2016, 78, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.C. Taste-masking assessment of solid oral dosage forms—A critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, E.; Matsunaga, C.; Yoshida, K.; Mifsud, J.C.; Irie, T.; Yoshida, M.; Uchida, T. Famotidine orally disintegrating tablets: Bitterness comparison of original and generic products. Chem. Pharm. Bull. 2009, 57, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Guffon, N.; Kibleur, Y.; Copalu, W.; Tissen, C.; Breitkreutz, J. Developing a new formulation of sodium phenylbutyrate. Arch. Dis. Child. 2012, 97, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.G.; Kovacs, Z.; Szollosi, D.; Fekete, A. Temperature correction of electronic tongue measurements results. Acta Aliment. Hung. 2013, 42, 37–44. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Performance qualification of an electronic tongue based on ICH guideline Q2 Measurements. J. Pharm. Biomed. Anal. 2010, 51, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Eckert, C.; Preis, M.; Breitkreutz, J. New protocol for αAstree electronic tongue enabling full performance qualification according to ICH Q2. J. Pharm. Biomed. Anal. 2013, 83, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, P.; Wesoły, M.; Zabadaj, M.; Lisiecka, J.; Sołłohub, K.; Cal, K.; Wróblewski, W. Towards flow-through/flow injection electronic tongue for the analysis of pharmaceuticals. Sens. Actuators B Chem. 2015, 207, 1087–1094. [Google Scholar] [CrossRef]
- Nesrin, K.R.; Heba, M.M.; Azza, A.M. Potentiometric determination of amlodipine besilate and valsartan using microsized and polymeric matrix membrane sensors. Port. Electrochim. Acta 2011, 30, 15–29. [Google Scholar]
- Park, Y.J.; Lee, H.-K.; Im, Y.B.; Lee, W.; Han, H.-K. Improved pH-independent dissolution and oral absorption of valsartan via the preparation of solid dispersion. Arch. Pharm. Res. 2010, 33, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Raja Rajeswari, K.; Abbulu, K.; Sudhakar, M. Development, characterization and solubility study of solid dispersion of Valsartan. J. Chem. Pharm. Res. 2011, 3, 180–187. [Google Scholar]
- Dey, P.; Maiti, S. Orodispersible tablets: A new trend in drug delivery. J. Nat. Sci. Biol. Med. 2010, 1, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lvova, L.; Shin, J.H.; Oh, S.H.; Lee, C.S.; Kim, B.H.; Cha, G.S.; Nam, H. Determination of oceanic carbon dioxide using a carbonate-selective electrode. Anal. Chem. 2002, 74, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Lingenfelter, P.; Bedlechowicz-Sliwakowska, I.; Sokalski, T.; Maj-Żurawska, M.; Lewenstam, A. Time-dependent phenomena in the potential response of ion-selective electrodes treated by the Nernst-Planck-Poisson mode. 1. Intramembrane processes and selectivity. Anal. Chem. 2008, 78, 6783–6791. [Google Scholar] [CrossRef] [PubMed]
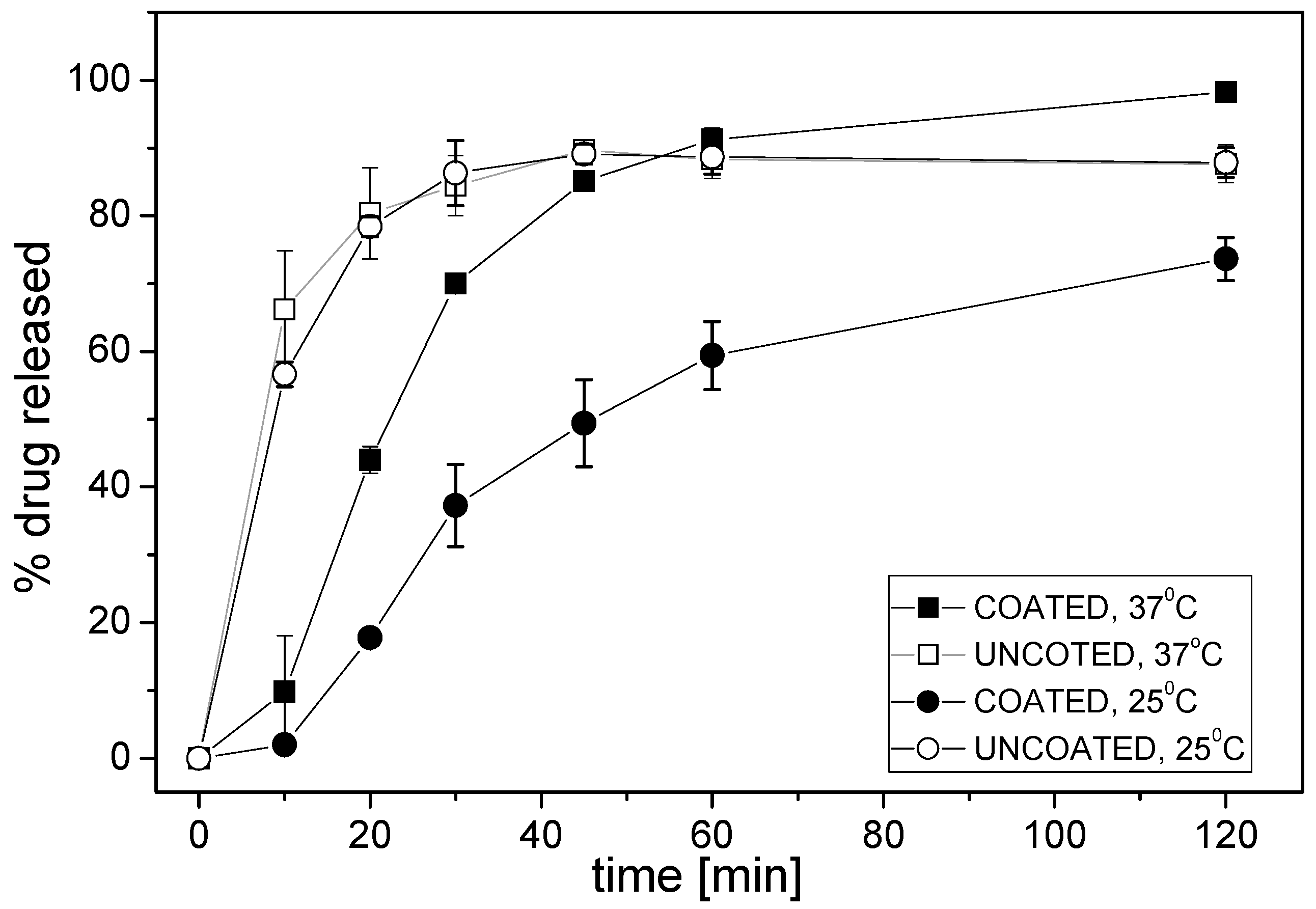

| Electrode Type | Electroactive Component | Plasticizer | Batch ET | Flow-Through ET | |
|---|---|---|---|---|---|
| CS-D | KTFPB | DOS | + | − | |
| CS-N | KTFPB | o-NPOE | + | + | |
| AS-D | TDMAC | DOS | + | − | |
| AS-N | TDMAC | o-NPOE | + | + | |
| PS-D | TBHDPB | DOS | + | − | |
| PS-N | TBHDPB | o-NPOE | + | + | |
| CARB-B | Carbonate ionophore VII | TDMAC | BOA | + | + |
| CARB-N | Carbonate ionophore VII | TDMAC | o-NPOE | + | + |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesoły, M.; Kluk, A.; Sznitowska, M.; Ciosek, P.; Wróblewski, W. Influence of Experimental Conditions on Electronic Tongue Results—Case of Valsartan Minitablets Dissolution. Sensors 2016, 16, 1353. https://doi.org/10.3390/s16091353
Wesoły M, Kluk A, Sznitowska M, Ciosek P, Wróblewski W. Influence of Experimental Conditions on Electronic Tongue Results—Case of Valsartan Minitablets Dissolution. Sensors. 2016; 16(9):1353. https://doi.org/10.3390/s16091353
Chicago/Turabian StyleWesoły, Małgorzata, Anna Kluk, Małgorzata Sznitowska, Patrycja Ciosek, and Wojciech Wróblewski. 2016. "Influence of Experimental Conditions on Electronic Tongue Results—Case of Valsartan Minitablets Dissolution" Sensors 16, no. 9: 1353. https://doi.org/10.3390/s16091353
APA StyleWesoły, M., Kluk, A., Sznitowska, M., Ciosek, P., & Wróblewski, W. (2016). Influence of Experimental Conditions on Electronic Tongue Results—Case of Valsartan Minitablets Dissolution. Sensors, 16(9), 1353. https://doi.org/10.3390/s16091353