Upconversion Nanoparticle-Based Förster Resonance Energy Transfer for Detecting DNA Methylation
Abstract
:1. Introduction
2. Experimental Section
2.1. Tissue Specimens
2.2. Bisulfite Treatment
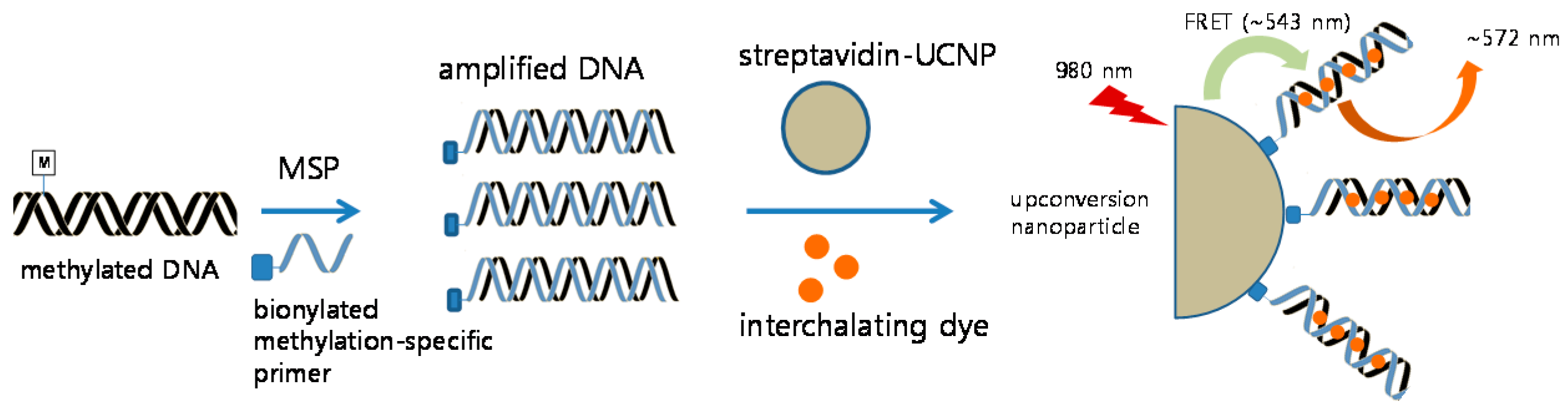
2.3. Detection of CDKN2A Methylation by Methylation-Specific PCR
2.4. Preparation of UCNPs
2.5. Detection of CDKN2A Methylation by Methylation-Specific UCNP-FRET
2.6. Detection of CDKN2A Methylation by Real-Time PCR
2.7. Detection of CDKN2A Methylation by Pyrosequencing
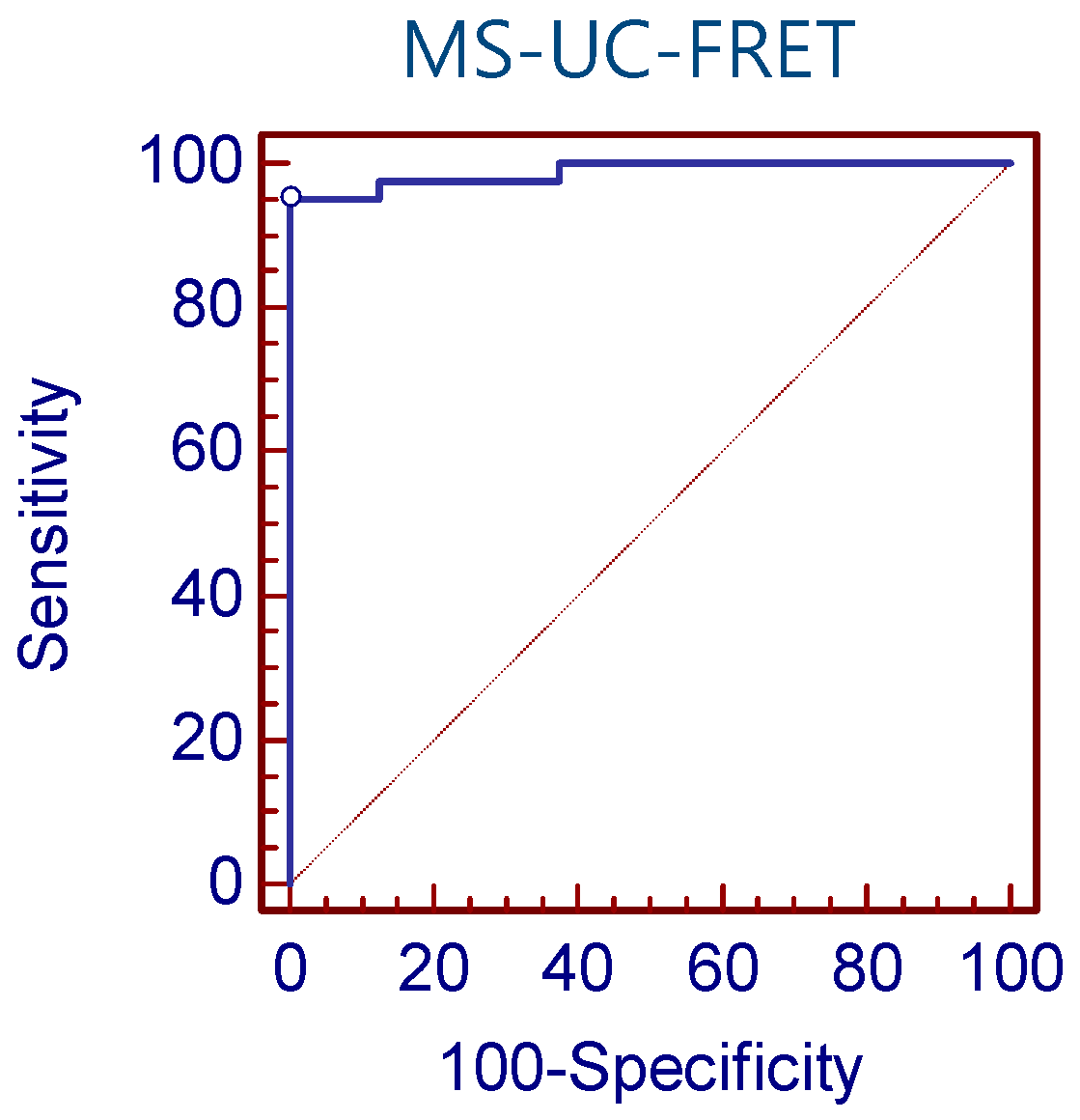
2.8. Detection Sensitivity of MS-UC-FRET
2.9. Detection of Methylated DNA in Lung Cancer Tissues
3. Results
3.1. Detection Sensitivity of MS-UC-FRET
3.2. CDKN2A Methylation in Lung Cancer Tissue by MS-UC-FRET
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, D.; Herman, J.G.; Guo, M. The clinical value of aberrant epigenetic changes of DNA damage repair genes in human cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Naemura, M.; Murasaki, C.; Inoue, Y.; Okamoto, H. Transcriptional Regulation of the p16 Tumor Suppressor Gene. Anticancer Res. 2015, 35, 4397–4401. [Google Scholar] [PubMed]
- Belinsky, S.A.; Nikula, K.J.; Palmisano, W.A.; Michels, R.; Saccomanno, G.; Gabrielson, E.; Baylin, S.B.; Herman, J.G. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc. Natl. Acad. Sci. USA 1998, 95, 11891–11896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.R.; Rong, Y.; Ming, L.; Xin, Y.; Feng, J.; Lin, X. The prognostic value of epigenetic silencing of p16 gene in NSCLC patients: A systematic review and meta-analysis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Yin, H.; Sun, B.; Zhou, Y.; Wang, M.; Xu, Z.; Fu, Z.; Ai, S. A new strategy for methylated DNA detection based on photoelectrochemical immunosensor using Bi2S3 nanorods, methyl bonding domain protein and anti-his tag antibody. Biosens. Bioelectron. 2014, 51, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.; Liu, F.; Wu, Z.; Lu, S.; Jin, Q.; Zhao, J.; Zhong, X.; Mao, H. Highly sensitive detection of DNA methylation levels by using a quantum dot-based FRET method. Nanoscale 2015, 7, 17547–17555. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Hansen, L.L. PCR-based methods for detecting single-locus DNA methylation biomarkers in cancer diagnostics, prognostics, and response to treatment. Clin. Chem. 2009, 55, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Graff, J.R.; Myohanen, S.; Nelkin, B.D.; Baylin, S.B. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 1996, 93, 9821–9826. [Google Scholar] [CrossRef] [PubMed]
- Eads, C.A.; Danenberg, K.D.; Kawakami, K.; Saltz, L.B.; Blake, C.; Shibata, D.; Danenberg, P.V.; Laird, P.W. MethyLight: A high-throughput assay to measure DNA methylation. Nucleic. Acids. Res. 2000, 28. [Google Scholar] [CrossRef]
- Colella, S.; Shen, L.; Baggerly, K.A.; Issa, J.P.; Krahe, R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. BioTechniques 2003, 35, 146–150. [Google Scholar] [PubMed]
- Bailey, V.J.; Easwaran, H.; Zhang, Y.; Griffiths, E.; Belinsky, S.A.; Herman, J.G.; Baylin, S.B.; Carraway, H.E.; Wang, T.H. MS-qFRET: A quantum dot-based method for analysis of DNA methylation. Genome Res. 2009, 19, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Dadmehr, M.; Hosseini, M.; Hosseinkhani, S.; Ganjali, M.R.; Khoobi, M.; Behzadi, H.; Hamedani, M.; Sheikhnejad, R. DNA methylation detection by a novel fluorimetric nanobiosensor for early cancer diagnosis. Biosens. Bioelectron. 2014, 60, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kolpashchikov, D.M. Binary probes for nucleic acid analysis. Chem. Rev. 2010, 110, 4709–4723. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Gnanasammandhan, M.K.; Zhang, Y. Small upconverting fluorescent nanoparticles for biomedical applications. Small 2010, 6, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Auzel, F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 39–73. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Schafer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. Engl. 2011, 50, 5808–5829. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, L.; Ma, W.; Wu, X.; Sun, M.; Kuang, H.; Wang, L.; Kotov, N.A.; Xu, C. Dual-Mode Ultrasensitive Quantification of MicroRNA in Living Cells by Chiroplasmonic Nanopyramids Self-Assembled from Gold and Upconversion Nanoparticles. J. Am. Chem. Soc. 2016, 138, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lu, F.; Wu, X.C.; Zhu, J.J. An upconversion fluorescent resonant energy transfer biosensor for hepatitis B virus (HBV) DNA hybridization detection. Analyst 2015, 140, 7622–7628. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Joshi, P.; Alazemi, A.; Zhang, P. Upconversion nanoparticle-based ligase-assisted method for specific and sensitive detection of T790M mutation in epidermal growth factor receptor. Biosens. Bioelectron. 2014, 62, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Im, S.G.; Sung, H.; Hah, S.S.; Cong, V.T.; Lee, D.H.; Son, S.J.; Oh, H.B. Upconversion nanoparticle-based Forster resonance energy transfer for detecting the IS6110 sequence of Mycobacterium tuberculosis complex in sputum. Biosens. Bioelectron. 2014, 53, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kim, K.U.; Kim, J.E.; Kim, H.H.; Lee, M.K.; Lee, C.H.; Lee, S.Y.; Oh, T.; An, S. Detection of HOXA9 gene methylation in tumor tissues and induced sputum samples from primary lung cancer patients. Clin. Chem. Lab. Med. 2011, 49, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Im, S.G.; Hah, S.S.; Cong, V.T.; Lee, E.J.; Lee, Y.S.; Lee, G.K.; Lee, D.H.; Son, S.J. Effects of upconversion nanoparticles on polymerase chain reaction. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Kawasaki, T.; Brahmandam, M.; Cantor, M.; Kirkner, G.J.; Spiegelman, D.; Makrigiorgos, G.M.; Weisenberger, D.J.; Laird, P.W.; Loda, M.; et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J. Mol. Diagn. 2006, 8, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Irahara, N.; Shima, K.; Kure, S.; Kirkner, G.J.; Schernhammer, E.S.; Hazra, A.; Hunter, D.J.; Quackenbush, J.; Spiegelman, D.; et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hwang, S.H.; Song, E.J.; Shin, H.J.; Jung, J.S.; Lee, E.Y. Level of HOXA5 hypermethylation in acute myeloid leukemia is associated with short-term outcome. Korean J. Lab. Med. 2010, 30, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Basheer, N.B.; Amirthalingam, L.; Muthukumar, H.; Kaliaperumal, R.; Shanmugam, K. Conventional and nanotechniques for DNA methylation profiling. J. Mol. Diagn. 2013, 15, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wen, Y.; Zhu, S.; Hua, F.; Zhao, H.; Xu, H.; You, J.; Sun, L.; Wang, W.; Chen, J.; et al. Association between P(16INK4a) promoter methylation and non-small cell lung cancer: a meta-analysis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Liu, Y.; An, Q.; Li, L.; Zhang, D.; Huang, J.; Feng, X.; Cheng, S.; Gao, Y. Hypermethylation of p16INK4a in Chinese lung cancer patients: biological and clinical implications. Carcinogenesis 2003, 24, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Hawes, S.E.; Stern, J.E.; Wiens, L.; Lu, H.; Dong, Z.M.; Jordan, C.D.; Kiviat, N.B.; Vesselle, H. DNA methylation in tumor and matched normal tissues from non-small cell lung cancer patients. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Sul, H.J.; Son, J.W. Promoter Methylation of CDKN2A, RARβ, and RASSF1A in Non-Small Cell Lung Carcinoma: Quantitative Evaluation Using Pyrosequencing. Tuberc. Respir. Dis. 2012, 73, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A. Developments in real-time PCR research and molecular diagnostics. Expert. Rev. Mol. Diagn. 2010, 10, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Guo, Y.; Zhang, P. Highly sensitive and selective oligonucleotide sensor for sickle cell disease gene using photon upconverting nanoparticles. Biosens. Bioelectron. 2009, 24, 1522–1526. [Google Scholar] [CrossRef] [PubMed]


| Methylation% | 0 | 0.01 | 0.05 | 0.1 | 1 | 5 | 10 | 20 | 50 | 100 |
| No. positive/No. tested | ||||||||||
| UC-MS-FRET | NEG | 4/10 | 2/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| RQ-PCR | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| pyrosequencing | NEG | ND | ND | ND | ND | 2/2 | 2/2 | 2/2 | 2/2 | ND |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Hwang, S.-H.; Im, S.-G.; Lee, M.-K.; Lee, C.-H.; Son, S.J.; Oh, H.-B. Upconversion Nanoparticle-Based Förster Resonance Energy Transfer for Detecting DNA Methylation. Sensors 2016, 16, 1259. https://doi.org/10.3390/s16081259
Kim S, Hwang S-H, Im S-G, Lee M-K, Lee C-H, Son SJ, Oh H-B. Upconversion Nanoparticle-Based Förster Resonance Energy Transfer for Detecting DNA Methylation. Sensors. 2016; 16(8):1259. https://doi.org/10.3390/s16081259
Chicago/Turabian StyleKim, Seockjune, Sang-Hyun Hwang, Su-Gyeong Im, Min-Ki Lee, Chang-Hun Lee, Sang Jun Son, and Heung-Bum Oh. 2016. "Upconversion Nanoparticle-Based Förster Resonance Energy Transfer for Detecting DNA Methylation" Sensors 16, no. 8: 1259. https://doi.org/10.3390/s16081259






