A Simple BODIPY-Based Viscosity Probe for Imaging of Cellular Viscosity in Live Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Fluorescence Lifetime Detection
2.2. Cell Incubation and Imaging
2.3. Synthesis of BTV
3. Results
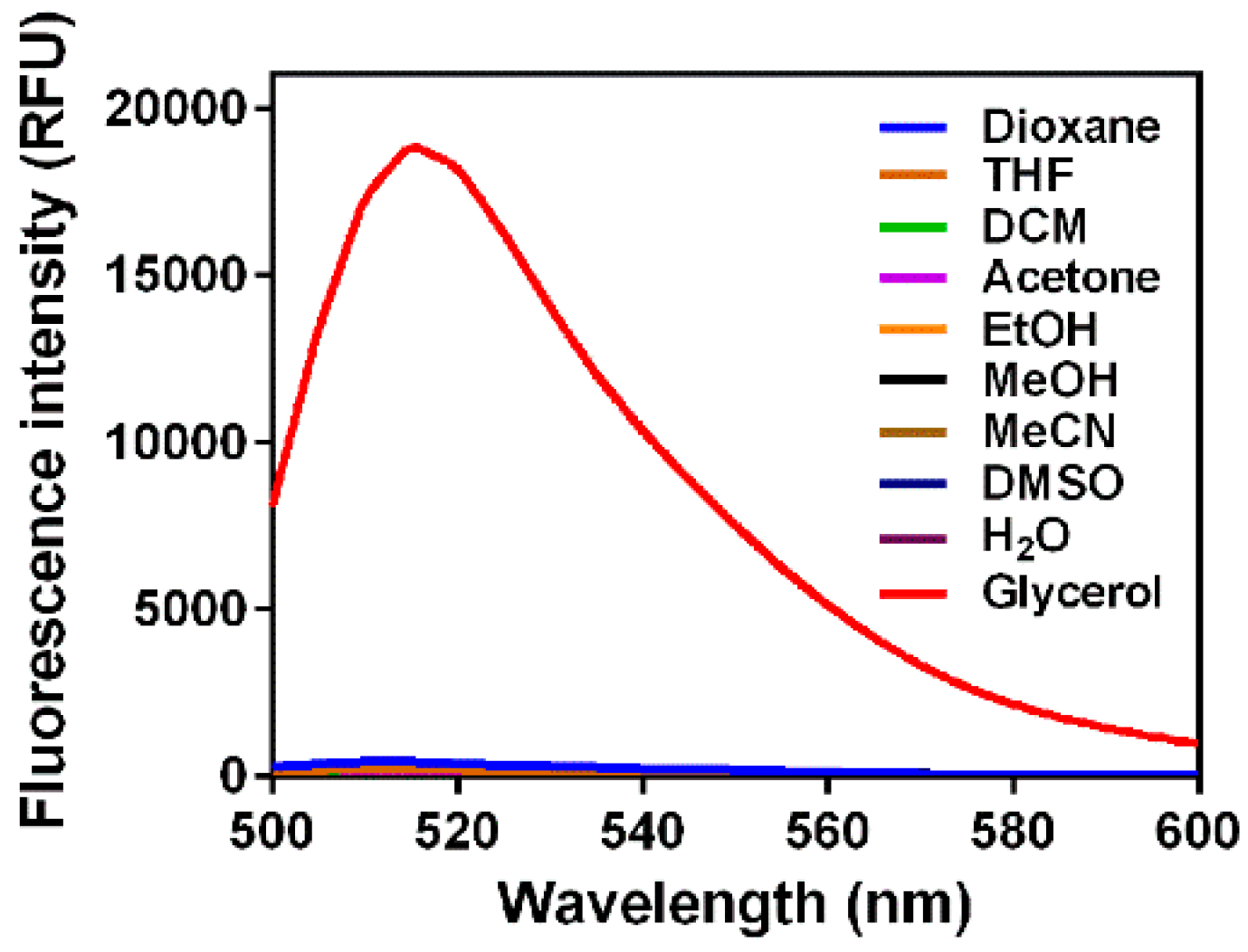
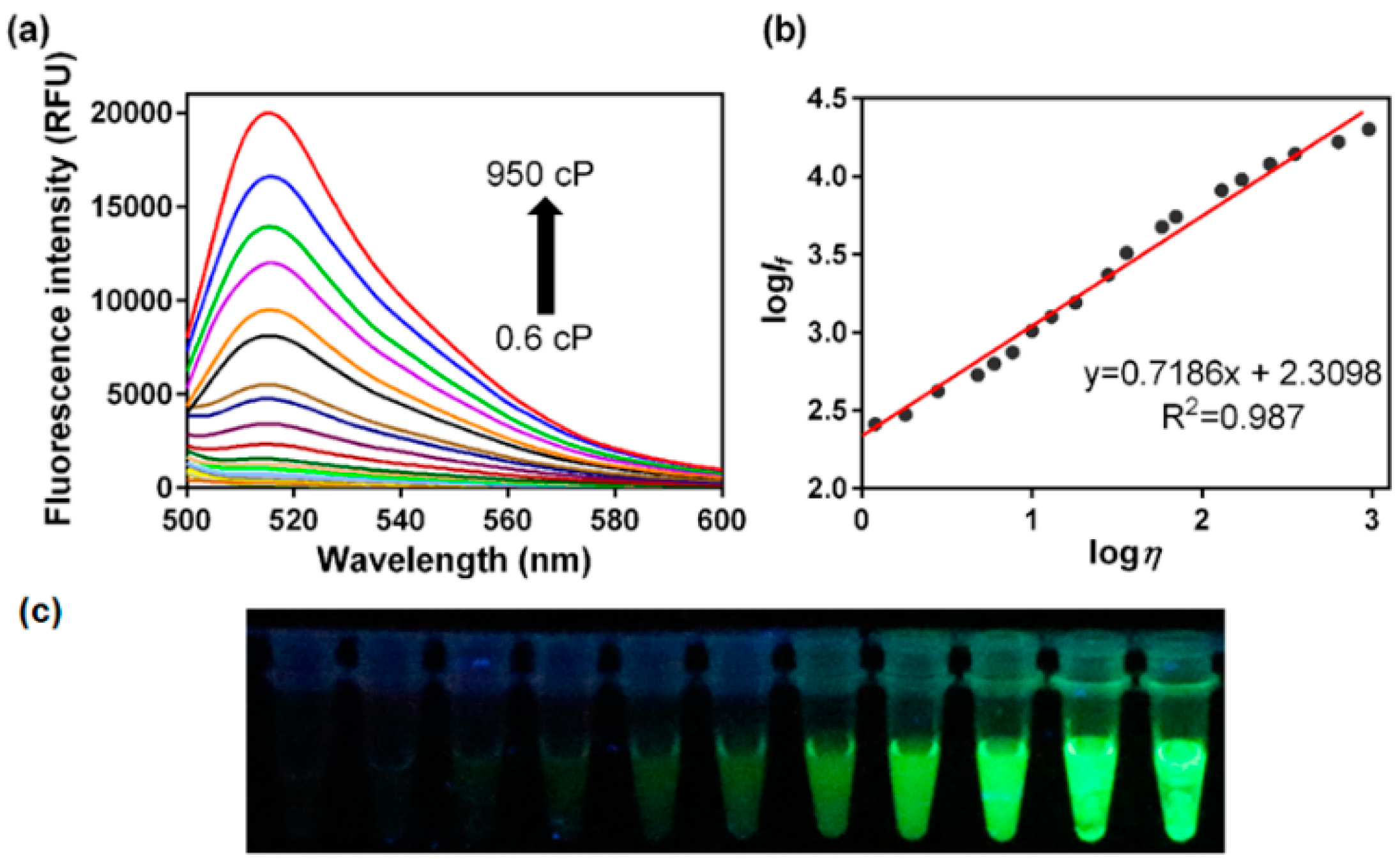
3.1. Fluorescence Response to Solvent Viscosity
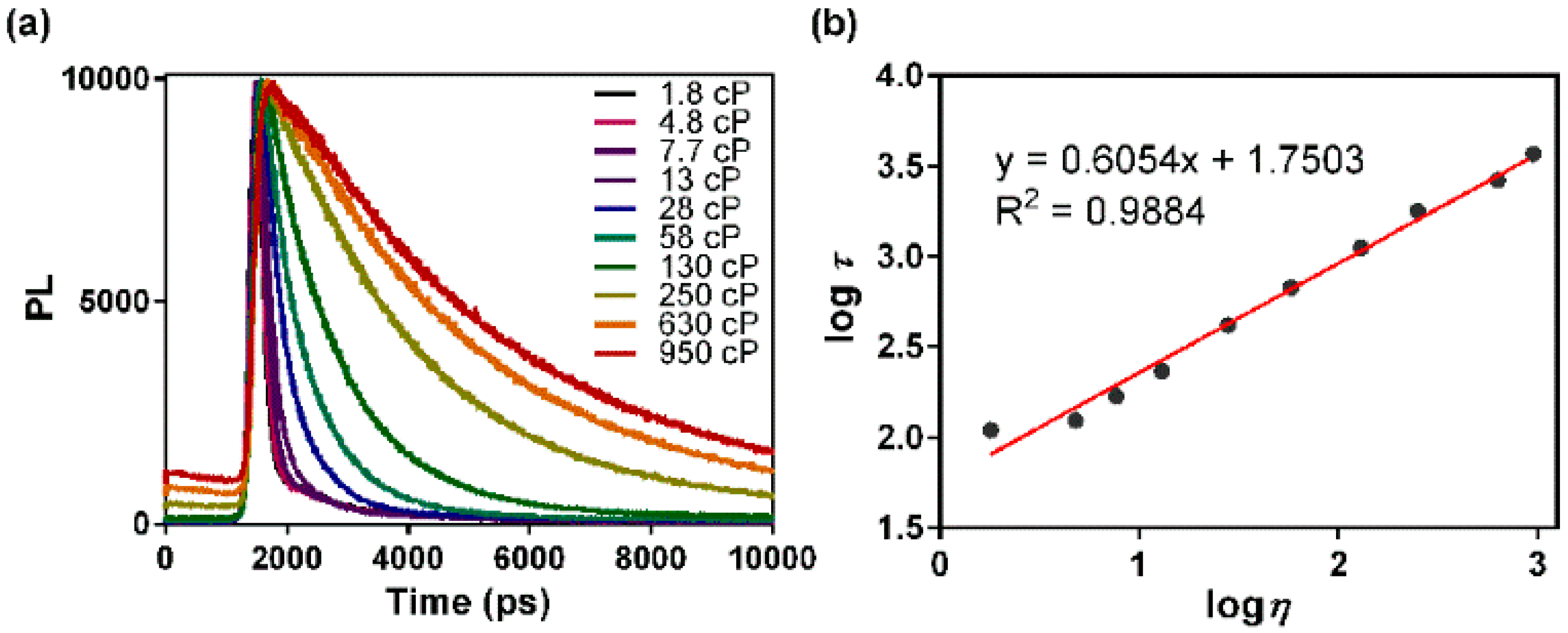
3.2. Fluorescence Lifetime Response to Solvent Viscosity
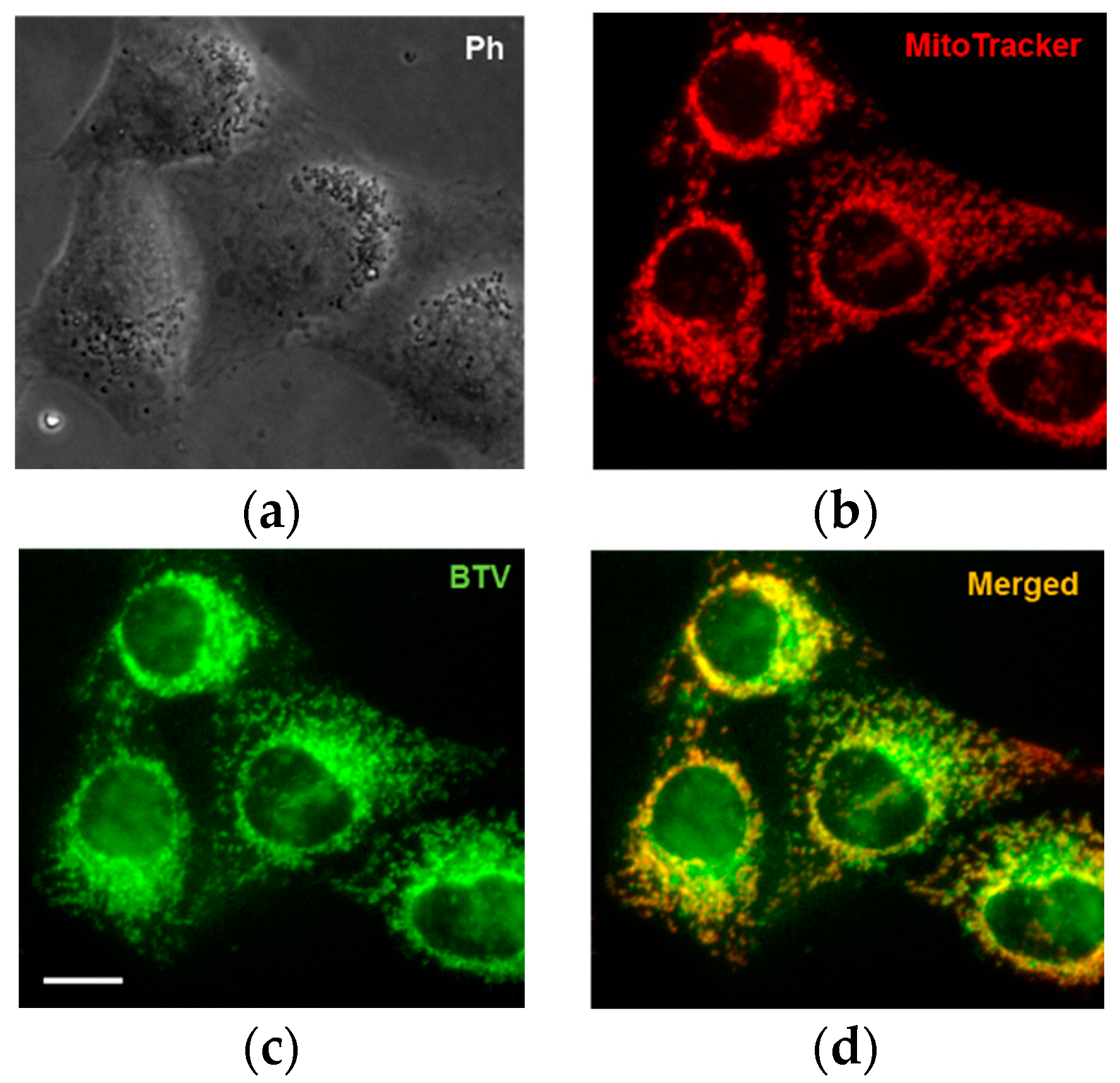
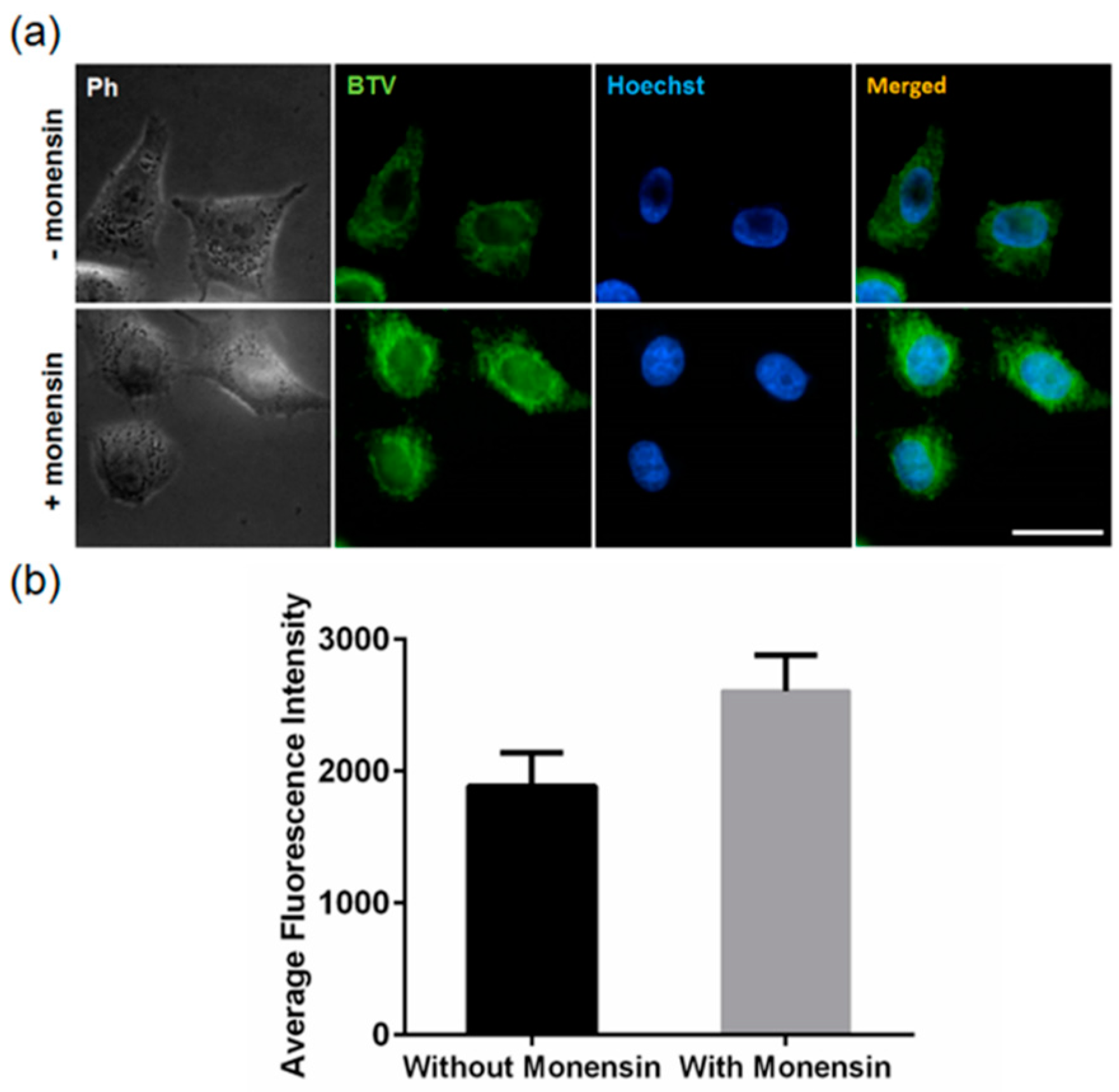
3.3. Application of BTV in Live Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Luby-Phelps, K. Physical properties of cytoplasm. Curr. Opin. Cell. Biol. 1994, 6, 3–9. [Google Scholar] [CrossRef]
- Luby-Phelps, K. Cytoarchitecture and physical properties of cytoplasm: Volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 2000, 192, 189–221. [Google Scholar] [PubMed]
- Kuimova, M.K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 2012, 14, 12671–12686. [Google Scholar] [CrossRef] [PubMed]
- Zubenko, G.S.; Kopp, U.; Seto, T.; Firestone, L.L. Platelet membrane fluidity individuals at risk for Alzheimer’s disease: A comparison of results from fluorescence spectroscopy and electron spin resonance spectroscopy. Psychopharmacology 1999, 145, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Deliconstantinos, G.; Villiotou, V.; Stavrides, J.C. Modulation of Particulate Nitric-Oxide Synthase Activity and Peroxynitrite Synthesis in Cholesterol-Enriched Endothelial-Cell Membranes. Biochem. Pharmacol. 1995, 49, 1589–1600. [Google Scholar] [CrossRef]
- Nadiv, O.; Shinitzky, M.; Manu, H.; Hecht, D.; Roberts, C.T.; Leroith, D.; Zick, Y. Elevated Protein-Tyrosine-Phosphatase Activity and Increased Membrane Viscosity Are Associated with Impaired Activation of the Insulin-Receptor Kinase in Old Rats. Biochem. J. 1994, 298, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, X.; Spring, D.R.; Qian, X.; Cui, J.; Xu, Z. Quantitatively mapping cellular viscosity with detailed organelle information via a designed PET fluorescent probe. Sci. Rep. 2014, 4, 5418. [Google Scholar] [CrossRef] [PubMed]
- Molin, M.D.; Verolet, Q.; Colom, A.; Letrun, R.; Derivery, E.; Gonzalez-Gaitan, M.; Vauthey, E.; Roux, A.; Sakai, N.; Matile, S. Fluorescent Flippers for Mechanosensitive Membrane Probes. J. Am. Chem. Soc. 2015, 137, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Hosny, N.A.; Mohamedi, G.; Rademeyer, P.; Owen, J.; Wu, Y.L.; Tang, M.X.; Eckersley, R.J.; Stride, E.; Kuimova, M.K. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proc. Natl. Acad. Sci. USA 2013, 110, 9225–9230. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Panettieri, S.; Abbandonato, G.; Jacchetti, E.; Cardarelli, F.; Signore, G.; Beltram, F.; Bizzarri, R. Imaging intracellular viscosity by a new molecular rotor suitable for phasor analysis of fluorescence lifetime. Anal. Bioanal. Chem. 2013, 405, 6223–6233. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Nucara, L.; Biver, T.; Battisti, A.; Signore, G.; Bizzarri, R. Organization of inner cellular components as reported by a viscosity-sensitive fluorescent Bodipy probe suitable for phasor approach to FLIM. Biophys. Chem. 2016, 208, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Theodorakis, E.A. Molecular rotors—Fluorescent biosensors for viscosity and flow. Org. Biomol. Chem. 2007, 5, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, J.L.; Li, M.; Cao, J.F.; Wang, J.Y.; Peng, X.J. A “Distorted-BODIPY”-Based Fluorescent Probe for Imaging of Cellular Viscosity in Live Cells. Chem. Eur. J. 2014, 20, 4691–4696. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, Y.; Tian, W.M.; Deng, L.Z. Activatable Rotor for Quantifying Lysosomal Viscosity in Living Cells. J. Am. Chem. Soc. 2013, 135, 2903–2906. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, Z.; Wang, J.; Fan, J.; He, Y.; Song, F.; Wang, B.; Sun, S.; Qu, J.; Qi, J.; Yan, M. Fluorescence ratiometry and fluorescence lifetime imaging: Using a single molecular sensor for dual mode imaging of cellular viscosity. J. Am. Chem. Soc. 2011, 133, 6626–6635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.K.; Shao, J.Y.; Yang, Y.B.; Zhao, J.Z.; Guo, H.M.; Li, X.L.; Ji, S.M.; Zhang, Z.Y. Molecular Rotors as Fluorescent Viscosity Sensors: Molecular Design, Polarity Sensitivity, Dipole Moments Changes, Screening Solvents, and Deactivation Channel of the Excited States. Eur. J. Org. Chem. 2011, 25, 4773–4787. [Google Scholar] [CrossRef]
- Sutharsan, J.; Lichlyter, D.; Wright, N.E.; Dakanali, M.; Haidekker, M.A.; Theodorakis, E.A. Molecular rotors: Synthesis and evaluation as viscosity sensors. Tetrahedron 2010, 66, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.A.; Kuimova, M.K.; Yahioglu, G.; Chung, P.H.; Suhling, K.; Phillips, D. Membrane-Bound Molecular Rotors Measure Viscosity in Live Cells via Fluorescence Lifetime Imaging. J. Phys. Chem. C 2009, 113, 11634–11642. [Google Scholar] [CrossRef]
- Oster, G.; Nishijima, Y. Fluorescence and Internal Rotation-Their Dependence on Viscosity of the Medium. J. Am. Chem. Soc. 1956, 78, 1581–1584. [Google Scholar] [CrossRef]
- Wong, K.S.; Wang, H.; Lanzani, G. Ultrafast excited-state planarization of the hexamethylsexithiophene oligomer studied by femtosecond time-resolved photoluminescence. Chem. Phys. Lett. 1998, 288, 59–64. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. A ratiometric fluorescent viscosity sensor. J. Am. Chem. Soc. 2006, 128, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, Y.; Lee, J.H.; Park, N.; Suh, M.; Chae, W.S.; Cao, J.; Peng, X.; Jung, H.; Kang, C.; Kim, J.S. A self-calibrating bipartite viscosity sensor for mitochondria. J. Am. Chem. Soc. 2013, 135, 9181–9185. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Keil, U.; Marques, C.A.; Bonert, A.; Frey, C.; Schussel, K.; Muller, W.E. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer’s disease. Biochem. Pharmacol. 2003, 66, 1627–1634. [Google Scholar] [CrossRef]
- Aleardi, A.M.; Benard, G.; Augereau, O.; Malgat, M.; Talbot, J.C.; Mazat, J.P.; Letellier, T.; Dachary-Prigent, J.; Solaini, G.C.; Rossignol, R. Gradual alteration of mitochondrial structure and function by beta-amyloids: Importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J. Bioenerg. Biomembr. 2005, 37, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Fan, J.L.; Zhang, S.; Wu, T.; Wang, J.Y.; Gao, P.; Qu, J.L.; Zhou, F.; Peng, X.J. Dual mode monitoring probe for mitochondrial viscosity in single cell. Sens. Actuators B Chem. 2014, 190, 685–693. [Google Scholar] [CrossRef]
- Raut, S.; Kimball, J.; Fudala, R.; Doan, H.; Maliwal, B.; Sabnis, N.; Lacko, A.; Gryczynski, I.; Dzyuba, S.V.; Gryczynski, Z. A homodimeric BODIPY rotor as a fluorescent viscosity sensor for membrane-mimicking and cellular environments. Phys. Chem. Chem. Phys. 2014, 16, 27037–27042. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Han, J.H.; Lee, J.H.; Choi, H.G.; Kang, C.; Kim, J.S. Mitochondrial Thioredoxin-Responding Off-On Fluorescent Probe. J. Am. Chem. Soc. 2012, 134, 17314–17319. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Su, D.D.; Oh, J.; Lee, S.C.; Lim, J.M.; Sahu, S.; Yu, X.T.; Kim, D.; Chang, Y.T. Dark to light! A new strategy for large Stokes shift dyes: Coupling of a dark donor with tunable high quantum yield acceptors. Chem. Sci. 2014, 5, 4812–4818. [Google Scholar] [CrossRef]
- Su, D.D.; Teoh, C.L.; Sahu, S.; Das, R.K.; Chang, Y.T. Live cells imaging using a turn-on FRET-based BODIPY probe for biothiols. Biomaterials 2014, 35, 6078–6085. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.A.; Chung, P.H.; Kuimova, M.K.; Yahioglu, G.; Wang, Y.; Qu, J.; Suhling, K. Fluorescence anisotropy of molecular rotors. Chemphyschem 2011, 12, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular rotor measures viscosity of live cells via fluorescence lifetime imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Duarte, I.; Vu, T.T.; Izquierdo, M.A.; Bull, J.A.; Kuimova, M.K. A molecular rotor for measuring viscosity in plasma membranes of live cells. Chem. Commun. 2014, 50, 5282–5284. [Google Scholar] [CrossRef] [PubMed]
- Förster, T.; Hoffmann, G.Z. Viscosity dependence of fluorescent quantum yields of some dye systems. Phys. Chem. 1971, 75, 63–76. [Google Scholar]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, H.H.; Morre, D.J.; Rowe, L.D. Alteration of Intracellular Traffic by Monensin-Mechanism, Specificity and Relationship to Toxicity. Biochim. Biophys. Acta 1990, 1031, 225–246. [Google Scholar] [CrossRef]
- Soltoff, S.P.; Mandel, L.J. Potassium transport in the rabbit renal proximal tubule: Effects of barium, ouabain, valinomycin, and other ionophores. J. Membr. Biol. 1986, 94, 153–161. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Teoh, C.L.; Gao, N.; Xu, Q.-H.; Chang, Y.-T. A Simple BODIPY-Based Viscosity Probe for Imaging of Cellular Viscosity in Live Cells. Sensors 2016, 16, 1397. https://doi.org/10.3390/s16091397
Su D, Teoh CL, Gao N, Xu Q-H, Chang Y-T. A Simple BODIPY-Based Viscosity Probe for Imaging of Cellular Viscosity in Live Cells. Sensors. 2016; 16(9):1397. https://doi.org/10.3390/s16091397
Chicago/Turabian StyleSu, Dongdong, Chai Lean Teoh, Nengyue Gao, Qing-Hua Xu, and Young-Tae Chang. 2016. "A Simple BODIPY-Based Viscosity Probe for Imaging of Cellular Viscosity in Live Cells" Sensors 16, no. 9: 1397. https://doi.org/10.3390/s16091397




