Facile Fabrication of a Gold Nanocluster-Based Membrane for the Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentations
2.3. Synthesis of AuNCs
2.4. Toxicity Evaluation of AuNCs
2.5. Procedures for the Detection of Hydrogen Peroxide
3. Results and Discussion
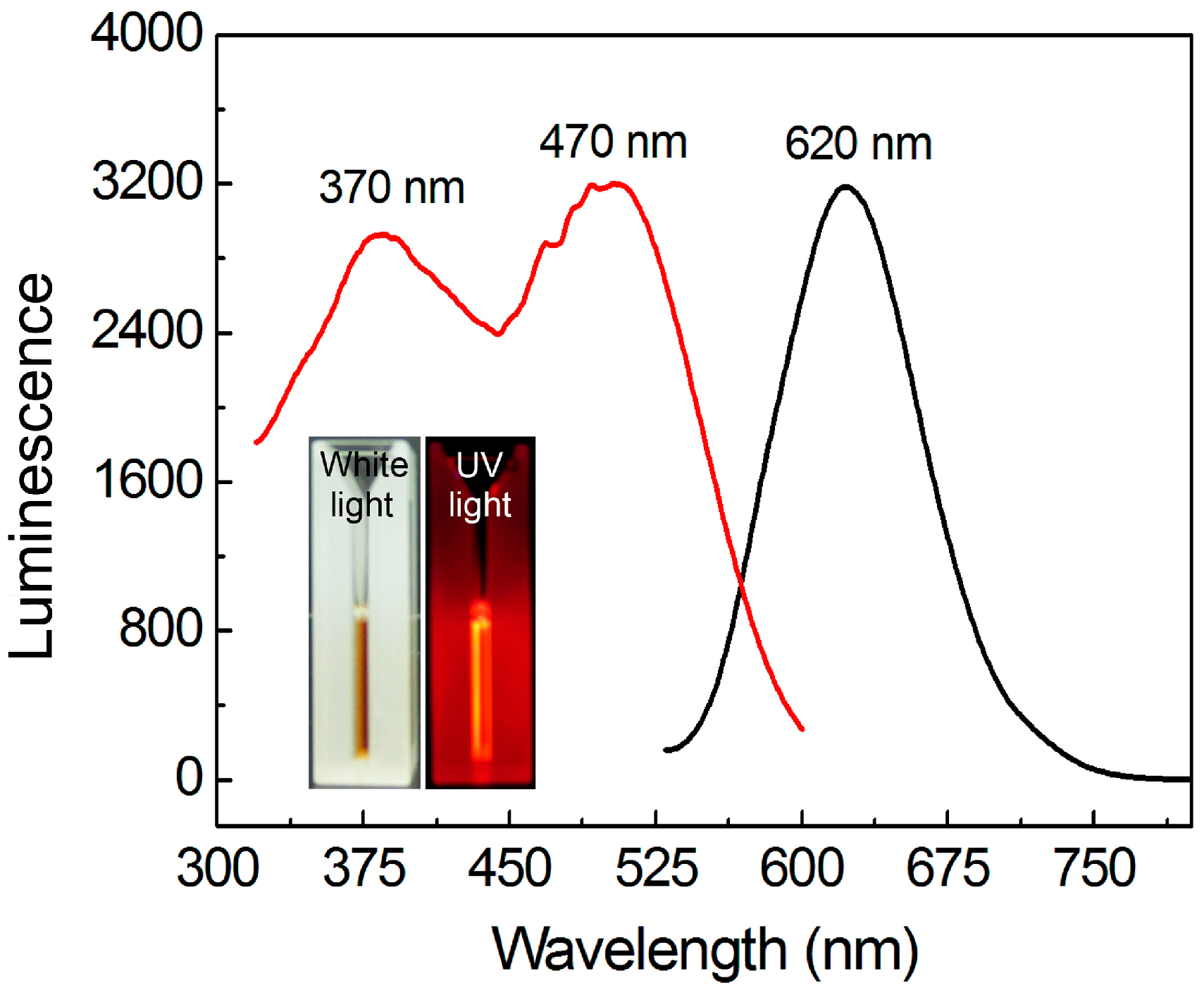
3.1. Synthesis and Optical Properties of AuNCs
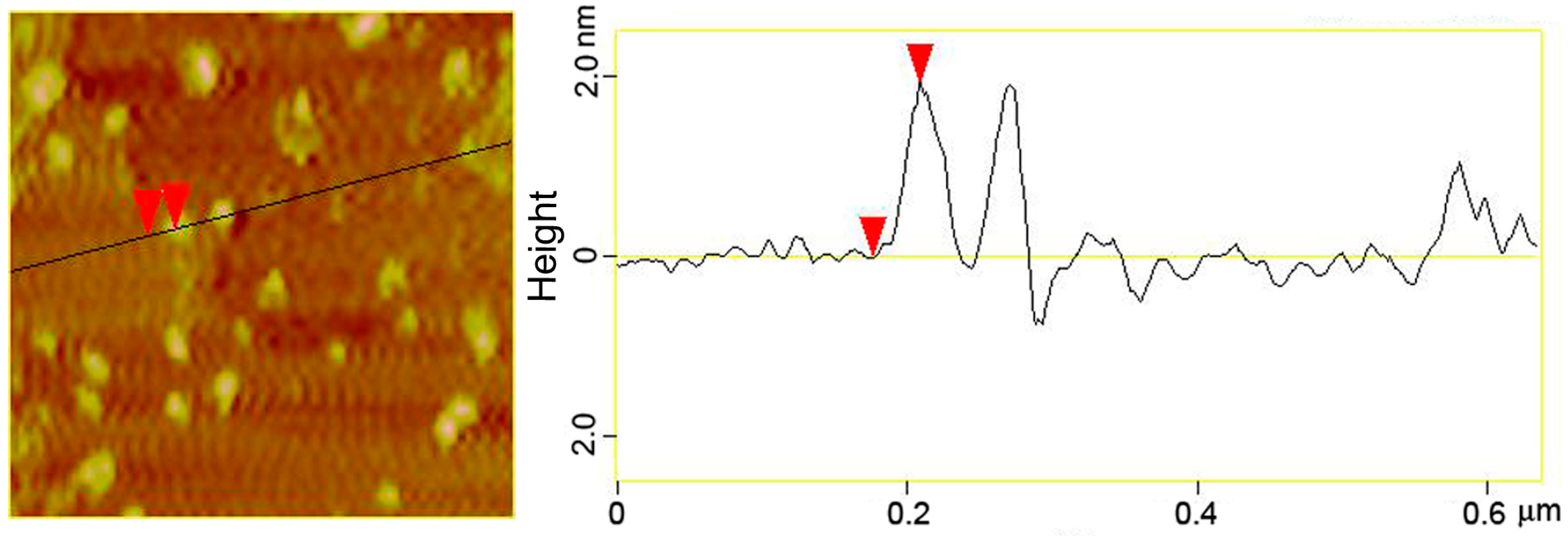
3.2. Structural Characterizations of the AuNCs
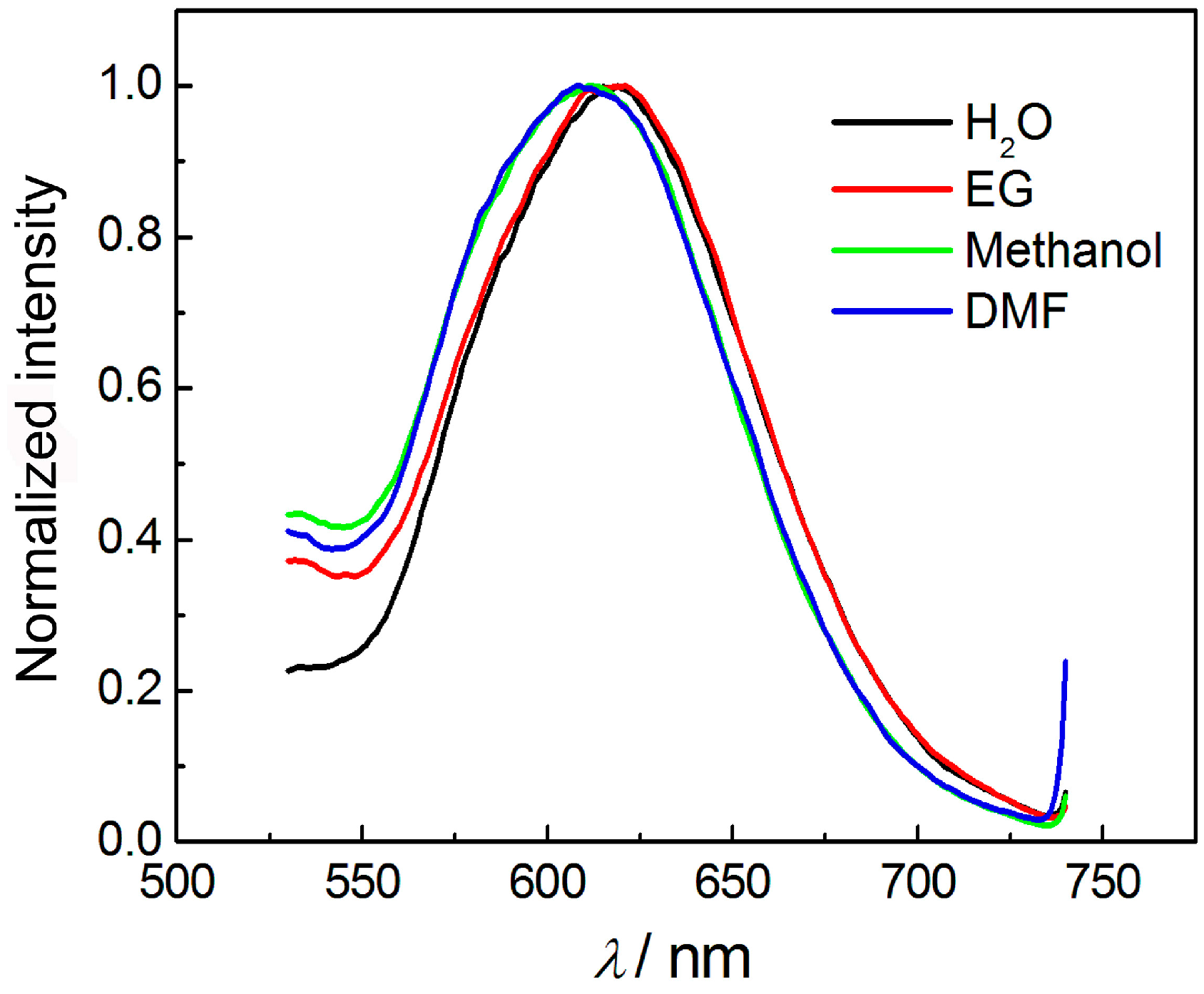
3.3. Effect of pH, Irradiation, and Solvents on the Luminescence of AuNCs
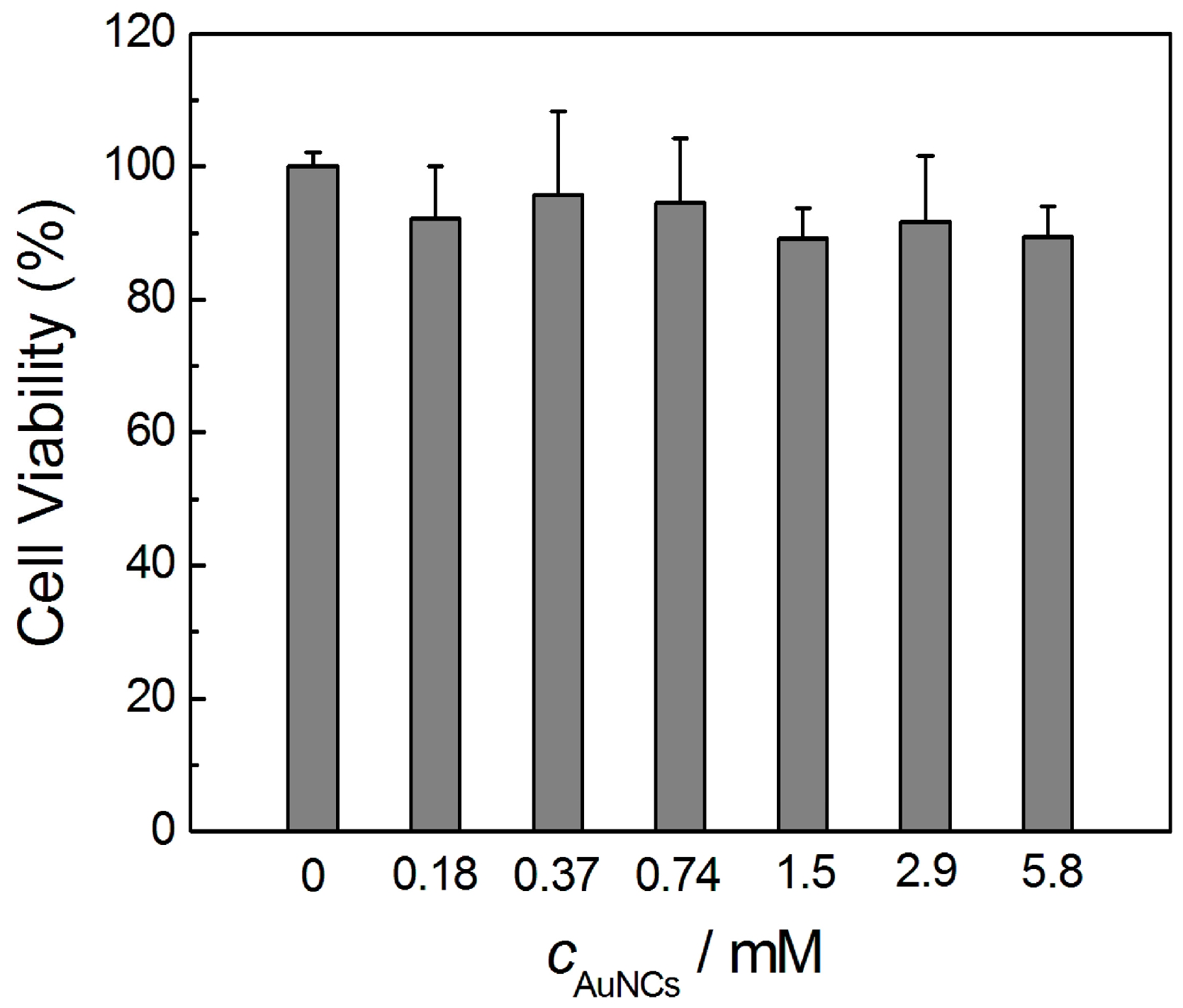
3.4. Toxicity Evaluation of AuNCs in Vitro
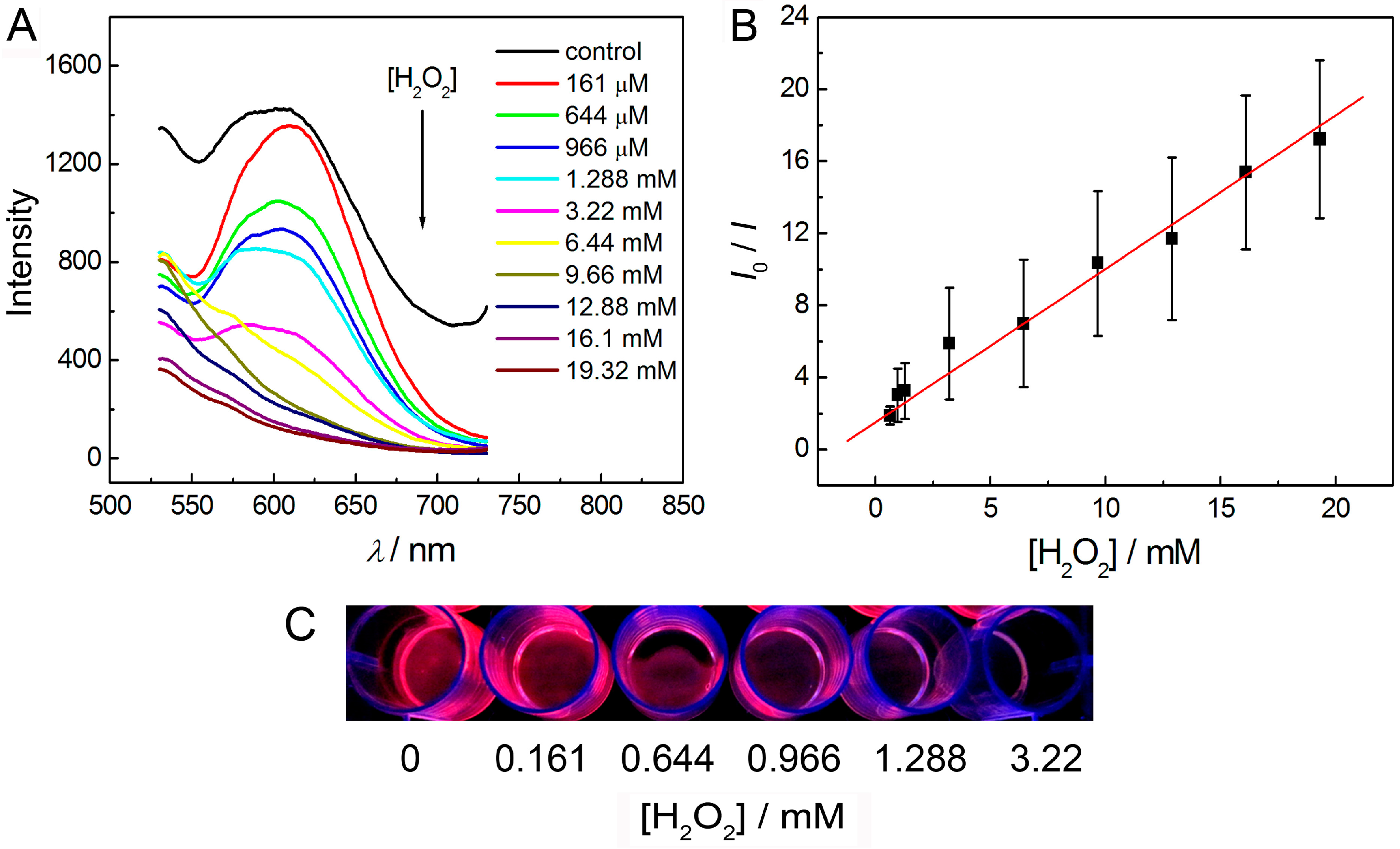
3.5. Detection of Hydrogen Peroxide in Solution Phase
3.6. Detection of Hydrogen Peroxide on Solid Membrane
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, Y.; Gao, X. Plasmonic fluorescent quantum dots. Nat. Nanotechnol. 2009, 4, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Ding, L.; Ju, H. Highly sensitive fluorescent analysis of dynamic glycan expression on living cells using glyconanoparticles and functionalized quantum dots. Anal. Chem. 2011, 83, 7006–7012. [Google Scholar] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.-F.; Chen, S. Amphiphilic egg-derived carbon dots: Rapid plasma fabrication, pyrolysis process, and multicolor printing patterns. Angew. Chem. Int. Ed. 2012, 51, 9297–9301. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhong, Y.; Peng, F.; Wei, X.; Su, Y.; Lu, Y.; Su, S.; Gu, W.; Liao, L.; Lee, S.-T. One-pot microwave synthesis of water-dispersible, ultraphoto- and pH-stable, and highly fluorescent silicon quantum dots. J. Am. Chem. Soc. 2011, 133, 14192–14195. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Xu, L.; Cheng, H.; Lin, Q.; Zhang, C. Protein-directed synthesis of pH-responsive red fluorescent copper nanoclusters and their applications in cellular imaging and catalysis. Nanoscale 2014, 6, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.-Y.; Huang, C.-C.; Chang, H.-T. Silver nanoclusters as fluorescent probes for selective and sensitive detection of copper ions. Chem. Commun. 2010, 46, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yuan, J.; Wang, E. Oligonucleotide-stabilized Ag nanoclusters as novel fluorescence probes for the highly selective and sensitive detection of the Hg2+ ion. Chem. Commun. 2009, 23, 3395–3397. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Lin, J.-Y.; Chen, C.-T.; Ciou, W.-R.; Chan, P.-H.; Luo, L.; Hsu, H.-Y.; Diau, E.W.-G.; Chen, Y.-C. Using gold nanoclusters as selective luminescent probes for phosphate-containing metabolites. Anal. Chem. 2012, 84, 5484–5488. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, G.U. Gold nanoclusters as novel optical probes for in vitro and in vivo fluorescence imaging. Biophys. Rev. 2012, 4, 313–322. [Google Scholar] [CrossRef]
- Hu, L.; Han, S.; Parveen, S.; Yuan, Y.; Zhang, L.; Xu, G. Highly sensitive fluorescent detection of trypsin based on BSA-stabilized gold nanoclusters. Biosens. Bioelectron. 2012, 32, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Peng, M.; Shi, L.; Du, Y.; Cai, N.; He, Y.; Chang, H.-T.; Yeung, E.S. Disassembly mediated fluorescence recovery of gold nanodots for selective sulfide sensing. Nanoscale 2013, 5, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Guo, Y.; Hong, S.; Wang, Z.; Wang, K.; Chen, X.; Zhang, J.; Hu, J.; Pei, R. Label-free detection of Pb2+ based on aggregation induced emission enhancement of Au nanoclusters. RSC Adv. 2015, 5, 36582–36586. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chen, C.-T.; Shiang, Y.-C.; Lin, Z.-H.; Chang, H.-T. Synthesis of fluorescent carbohydrate-protected Au nanodots for detection of concanavalin A and Escherichia coli. Anal. Chem. 2009, 81, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Yang, Z.; Lee, K.-H.; Chang, H.-T. Synthesis of highly fluorescent gold nanoparticles for sensing mercury (II). Angew. Chem. Int. Ed. 2007, 46, 6824–6828. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Su, D.; Xia, Q.; Chai, F.; Wang, C.; Qu, F. Fluorescent detection of TNT and 4-nitrophenol by BSA Au nanoclusters. Dalton Trans. 2014, 43, 10057–10063. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, X.; Li, L.; Zhang, G.; Hussain, I.; Li, Z.; Tan, B. Photoreductive synthesis of water-soluble fluorescent metal nanoclusters. Chem. Commun. 2012, 48, 567–569. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Z.; Zhang, Q.; Zhang, X.; Zheng, Y.; Lee, J.Y.; Xie, J. Synthesis of highly fluorescent metal (Ag, Au, Pt, and Cu) nanoclusters by electrostatically induced reversible phase transfer. ACS Nano 2011, 5, 8800–8808. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Nie, S. Etching colloidal gold nanocrystals with hyperbranched and multivalent polymers: A new route to fluorescent and water-soluble atomic clusters. J. Am. Chem. Soc. 2007, 129, 2412–2413. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Xu, L.; Shi, X.; Li, X.; Xu, X.; Sun, H.; Yang, B.; Lin, Q. A galvanic replacement route to prepare strongly fluorescent and highly stable gold nanodots for cellular imaging. Small 2013, 9, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Zvyagin, A.V.; Plakhotnik, T. Synthesis and spectroscopic observation of dendrimer-encapsulated gold nanoclusters. Chem. Commun. 2006, 2400–2401. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.-Y.; Luo, Z.; Li, K.; Xie, J.; Liu, B. Energy transfer between conjugated-oligoelectrolyte-substituted POSS and gold nanocluster for multicolor intracellular detection of mercury ion. J. Phys. Chem. C 2011, 115, 13069–13075. [Google Scholar] [CrossRef]
- Kennedy, T.A.C.; Maclean, J.L.; Liu, J. Blue emitting gold nanoclusters templated by poly-cytosine DNA at low pH and poly-adenine DNA at neutral pH. Chem. Commun. 2012, 48, 6845–6847. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Cai, Y.; Zheng, B.; Yuan, H.; Guo, Y.; Xiao, D.; Choi, M.M.F. Microwave-assisted synthesis of BSA-stabilized and HSA-protected gold nanoclusters with red emission. J. Mater. Chem. 2012, 22, 1000–1005. [Google Scholar] [CrossRef]
- Zheng, J.; Petty, J.T.; Dickson, R.M. High quantum yield blue emission from water-soluble Au8 nanodots. J. Am. Chem. Soc. 2003, 125, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, Z.; Chevrier, D.M.; Leong, D.T.; Zhang, P.; Jiang, D.; Xie, J. Identification of a highly luminescent Au22(SG)18 nanocluster. J. Am. Chem. Soc. 2014, 136, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B.; Nelson, K.J. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr. Opin. Chem. Biol. 2008, 12, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008, 130, 9638–9639. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.L.; Stevenson, K.J. Picomolar peroxide detection using a chemically activated redox mediator and square wave voltammetry. Anal. Chem. 2006, 78, 8518–8525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, X.X.; Wang, Y.; Zhao, N.W.; Xiong, Z.H.; Huang, C.Z. Rapid synthesis of highly luminescent and stable Au20 nanoclusters for active tumor-targeted imaging in vitro and in vivo. Nanoscale 2014, 6, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Retnakumar, A.; Setua, S.; Menon, D.; Ravindran, P.; Muhammed, H.; Pradeep, T.; Nair, S.; Koyakutty, M. Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology 2010, 21, 055103. [Google Scholar] [CrossRef] [PubMed]
- Díez, I.; Pusa, M.; Kulmala, S.; Jiang, H.; Walther, A.; Goldmann, A.S.; Müller, A.H.E.; Ikkala, O.; Ras, R.H.A. Color tunability and electrochemiluminescence of silver nanoclusters. Angew. Chem. Int. Ed. 2009, 48, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, D.M.; Chatt, A.; Zhang, P. Properties and applications of protein-stabilized fluorescent gold nanoclusters: Short review. J. Nanophotonics 2012, 6, 064504. [Google Scholar] [CrossRef]
- Jin, L.; Shang, L.; Guo, S.; Fang, Y.; Wen, D.; Wang, L.; Yin, J.; Dong, S. Biomolecule-stabilized Au nanoclusters as a fluorescence probe for sensitive detection of glucose. Biosens. Bioelectron. 2011, 26, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.-H.; Wu, G.-W.; He, D.; Peng, H.-P.; Liu, A.-L.; Xia, X.-H.; Chen, W. Fenton reaction-mediated fluorescence quenching of N-acetyl-l-cysteine-protected gold nanoclusters: analytical applications of hydrogen peroxide, glucose, and catalase detection. Analyst 2015, 140, 7650–7656. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yang, X.; Li, J.; Li, D.; Wang, E. Stable Cu nanoclusters: From an aggregationinduced emission mechanism to biosensing and catalytic applications. Chem. Commun. 2014, 50, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Qu, F.; Li, N.B.; Luo, H.Q. Polyethyleneimine-capped silver nanoclusters as a fluorescence probe for sensitive detection of hydrogen peroxide and glucose. Anal. Chim. Acta 2012, 749, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, J.; Zhou, T.; Rong, M.; Jiang, Y.; Chen, X. A novel solid-state electrochemiluminescence sensor for the determination of hydrogen peroxide based on an Au nanocluster–silica nanoparticle nanocomposite. Analyst 2013, 138, 5563–5565. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Feng, J.; Liu, S.; Zhang, T. Preparation of reduced graphene oxide decorated with high density Ag nanorods for non-enzymatic hydrogen peroxide detection. RSC Adv. 2013, 3, 14303–14307. [Google Scholar] [CrossRef]
- Hou, C.; Xu, Q.; Yin, L.; Hu, X. Metal–organic framework templated synthesis of Co3O4 nanoparticles for direct glucose and H2O2 detection. Analyst 2012, 137, 5803–5808. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, N.A.; Kolontaeva, O.A.; Duerkop, A. New nanomaterials and luminescent optical sensors for detection of hydrogen peroxide. Chemosensors 2015, 3, 253–273. [Google Scholar] [CrossRef]
- Wen, F.; Dong, Y.; Feng, L.; Wang, S.; Zhang, S.; Zhang, X. Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing. Anal. Chem. 2011, 83, 1193–1196. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, Y.; Yin, Y. Facile Fabrication of a Gold Nanocluster-Based Membrane for the Detection of Hydrogen Peroxide. Sensors 2016, 16, 1124. https://doi.org/10.3390/s16071124
Zhang P, Wang Y, Yin Y. Facile Fabrication of a Gold Nanocluster-Based Membrane for the Detection of Hydrogen Peroxide. Sensors. 2016; 16(7):1124. https://doi.org/10.3390/s16071124
Chicago/Turabian StyleZhang, Pu, Yi Wang, and Yibing Yin. 2016. "Facile Fabrication of a Gold Nanocluster-Based Membrane for the Detection of Hydrogen Peroxide" Sensors 16, no. 7: 1124. https://doi.org/10.3390/s16071124




