Evaluating Soil Moisture Status Using an e-Nose
Abstract
:1. Introduction
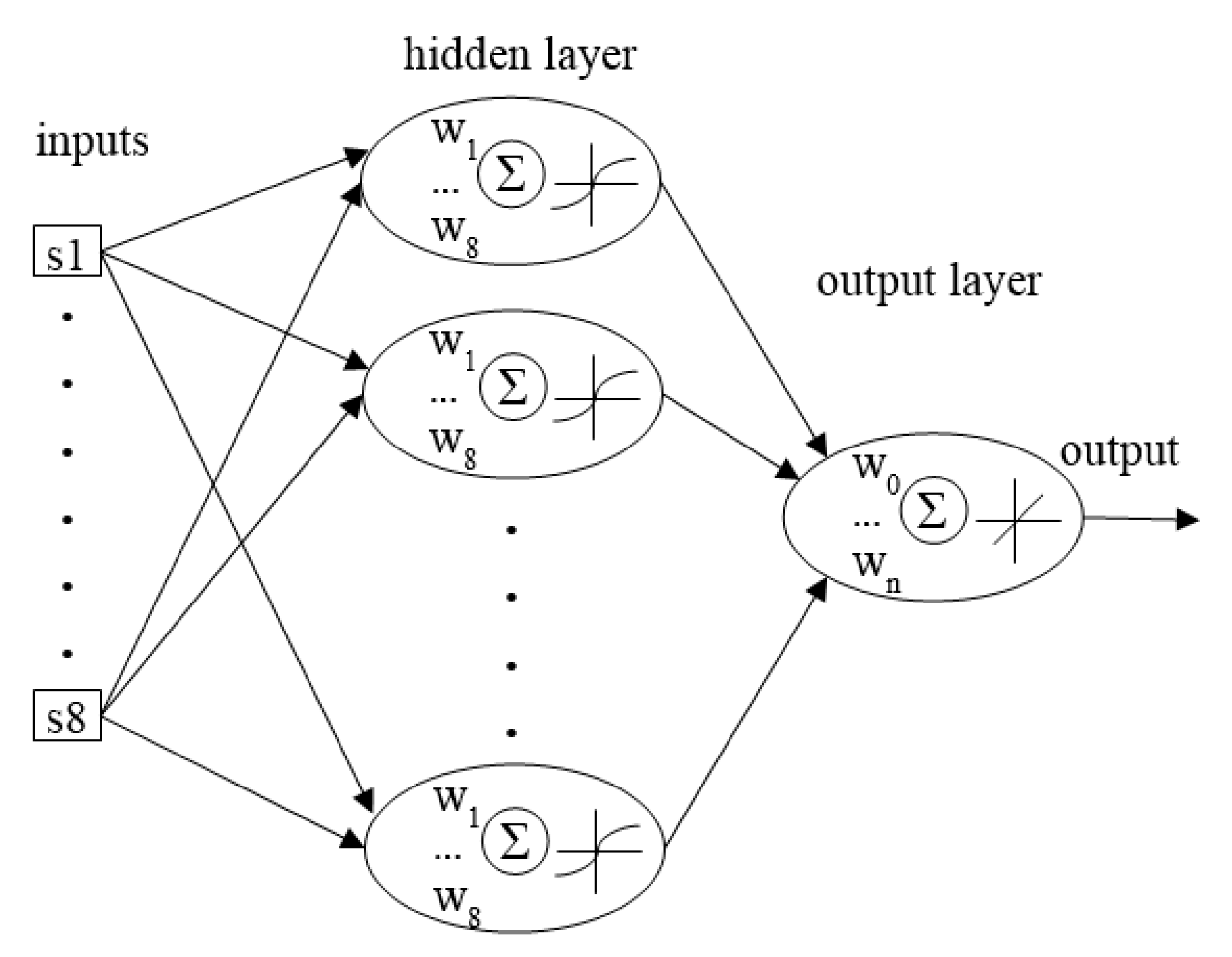
2. Materials and Methods
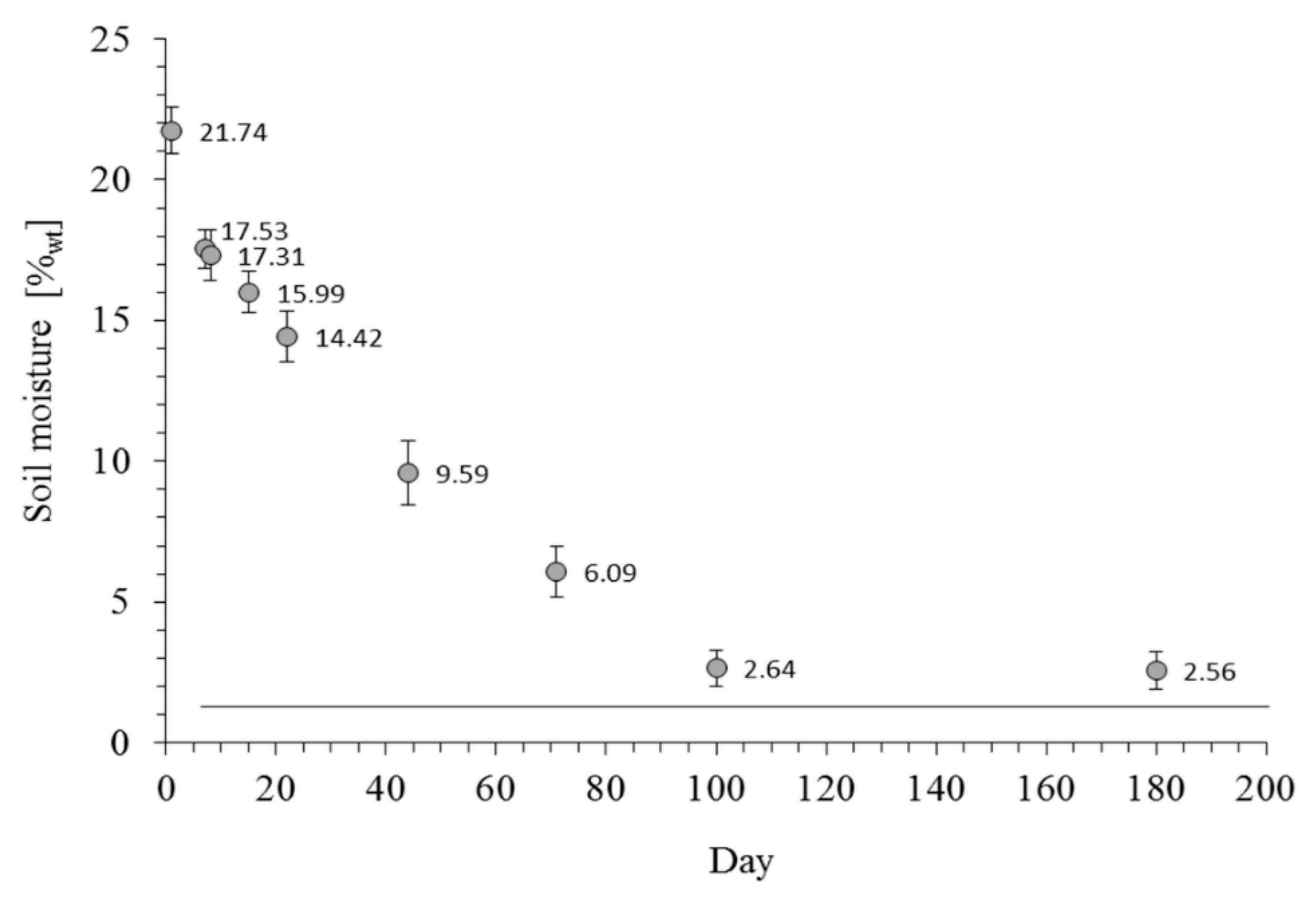
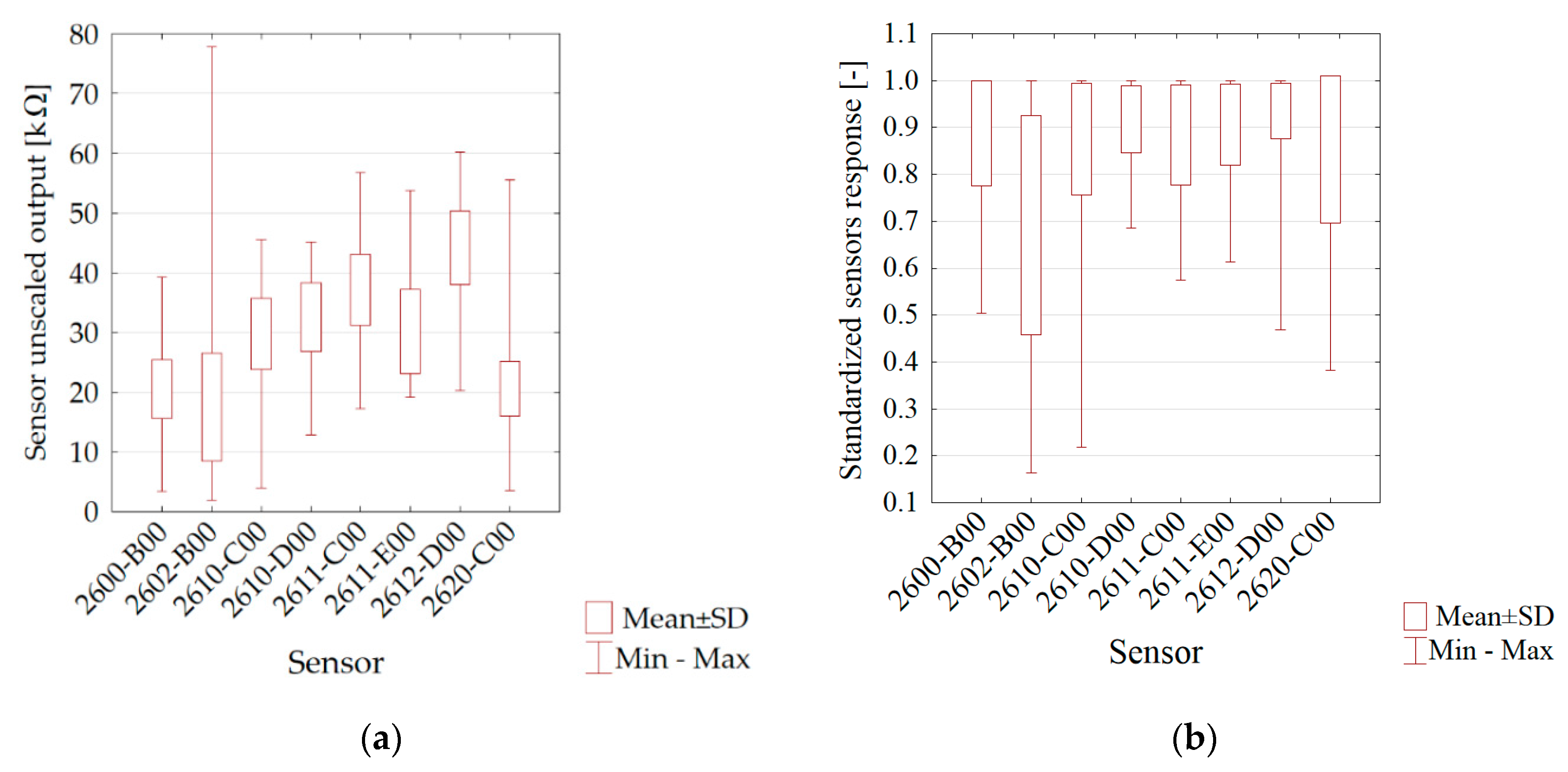
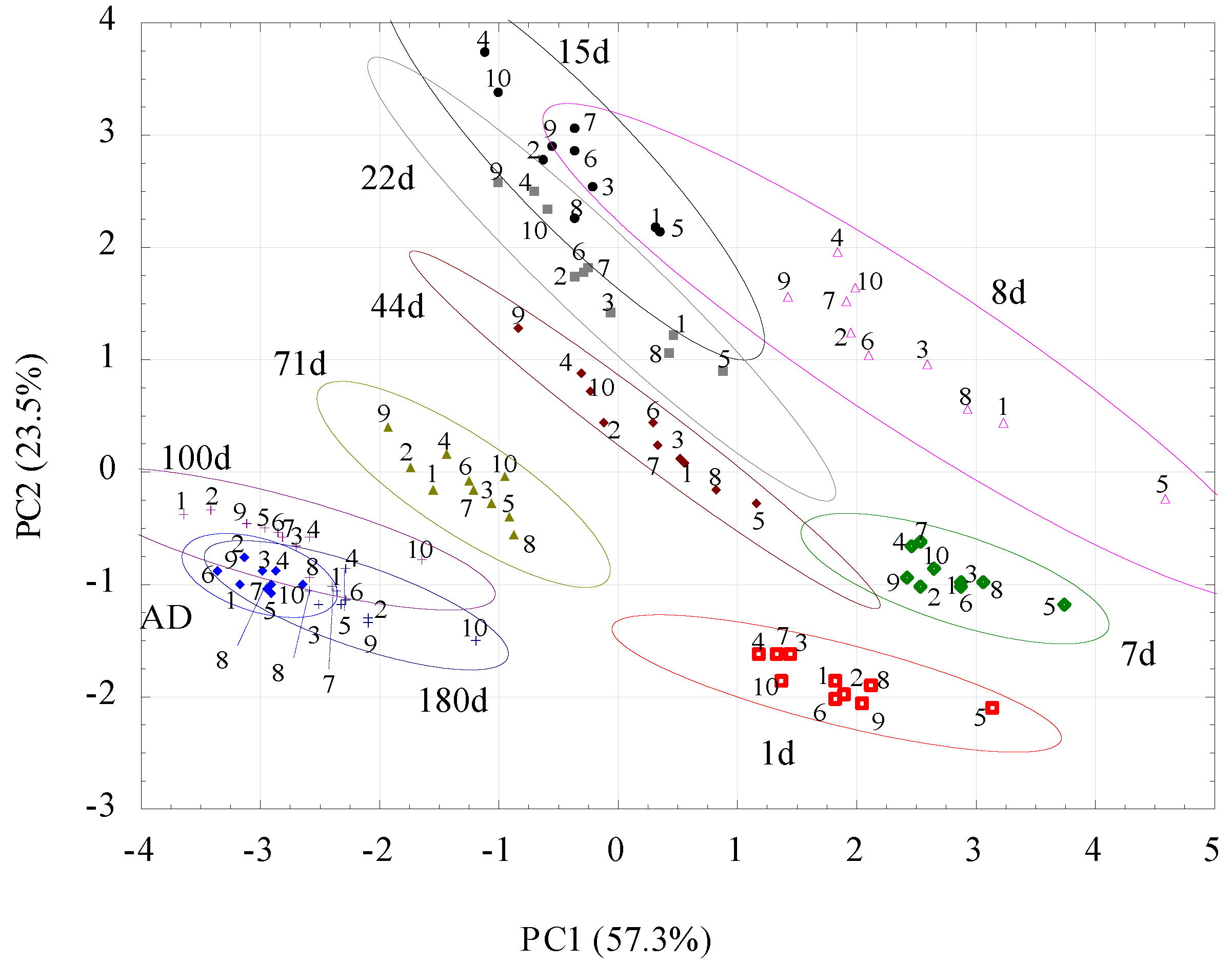
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Černý, R. Time-domain reflectometry method and its application for measuring moisture content in porous materials: A review. Measurement 2009, 42, 329–336. [Google Scholar] [CrossRef]
- Skierucha, W.; Wilczek, A.; Szyplowska, A. Dielectric spectroscopy in agrophysics. Int. Agrophys. 2012, 26, 187–197. [Google Scholar] [CrossRef]
- Pichler, V.; Homolák, M.; Skierucha, W.; Pichlerová, M.; Ramírez, D.; Gregor, J.; Jaloviar, P. Variability of moisture in coarse woody debris from several ecologically important tree species of the Temperate Zone of Europe. Ecohydrology 2012, 5, 424–434. [Google Scholar] [CrossRef]
- Skierucha, W.; Sławiński, C.; Szypłowska, A.; Lamorski, K.; Wilczek, A. A TDR-Based Soil Moisture Monitoring System with Simultaneous Measurement of Soil Temperature and Electrical Conductivity. Sensors 2012, 12, 13545–13566. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.B.; Wraith, J.M.; Or, D. Time domain reflectometry measurement principles and applications. Hydrol. Process. 2002, 16, 141–153. [Google Scholar] [CrossRef]
- Szypłowska, A.; Wilczek, A.; Kafarski, M.; Skierucha, W. Soil Complex Dielectric Permittivity Spectra Determination Using Electrical Signal Reflections in Probes of Various Lengths. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef]
- Craven, M.A.; Gardner, J.W.; Bartlett, P.N. Electronic noses—Development and future prospects. TrAC Trends Anal. Chem. 1996, 15, 486–493. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, R.M.; Fenner, R.A.; Engin, G. Characterisation of Wastewater Using an Electronic Nose. Water Res. 1999, 33, 442–452. [Google Scholar] [CrossRef]
- Di Natale, C.; Martinelli, E.; Pennazza, G.; Orsini, A. Data analysis for chemical sensor arrays. In Advances in Sensing With Security Applications; Byrnes, J., Ed.; Prometheus Inc.: Newport, RI, USA, 2006; pp. 147–169. [Google Scholar]
- Fu, J.; Li, G.; Qin, Y.; Freeman, W.J. A Pattern Recognition Method for Electronic Noses Based on an Olfactory Neural Network. Sens. Actuators B Chem. 2007, 125, 489–497. [Google Scholar] [CrossRef]
- Smolarz, A.; Kotyra, A.; Wojcik, W.; Ballester, J. Advanced diagnostics of industrial pulverized coal burner using optical methods and artificial intelligence. Exp. Therm. Fluid Sci. 2012, 43, 82–89. [Google Scholar] [CrossRef]
- Gutierrez-Osuna, R. Pattern Analysis for Machine Olfaction: A Review. IEEE Sens. J. 2002, 2, 189–202. [Google Scholar] [CrossRef]
- Wilson, A.D. Diverse Applications of Electronic-Nose Technologies in Agriculture and Forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, A.; Wojcik, W.; Gromaszek, K. Fuzzy modeling for optical sensor for diagnostics of pulverized coal burner. In Proceedings of the Procedia Engineering, 26th European Conference on Solid-State Transducers, Eurosensors 2012, Krakow, Poland, 9–12 September 2012; pp. 1029–1032.
- De Cesare, F.; Pantalei, S.; Zampetti, E.; Macagnano, A. Electronic nose and SPME techniques to monitor phenanthrene biodegradation in soil. Sens. Actuators B Chem. 2008, 131, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Kurup, P.U.; Issac, B.; Griffin, E.P. Electronic nose-membrane interface probe for geoenvironmental site characterization. J. Geotech. Geoenviron. Eng. 2006, 132, 1133–1142. [Google Scholar]
- Micone, P.G.; Guy, C. Odour Quantification by a Sensor Array: An Application to Landfill Gas Odours from Two Different Municipal Waste Treatment Works. Sens. Actuators B Chem. 2007, 120, 628–637. [Google Scholar] [CrossRef]
- Romain, A.-C.; Delva, J.; Nicolas, J. Complementary Approaches to Measure Environmental Odours Emitted by Landfill Areas. Sens. Actuators B Chem. 2008, 131, 18–23. [Google Scholar] [CrossRef]
- Wilson, A.D. Review of Electronic-Nose Technologies and Algorithms to Detect Hazardous Chemicals in the Environment. Procedia Technol. 2012, 1, 453–463. [Google Scholar] [CrossRef]
- Haas, T.; Lammers, P.S.; Diekmann, B.; Horner, G.; Boeker, P. A Method for Online Measurement of Odour with a Chemosensor System. Sens. Actuators B Chem. 2008, 132, 545–550. [Google Scholar] [CrossRef]
- Sironi, S.; Capelli, L.; Céntola, P.; Del Rosso, R. Development of a System for the Continuous Monitoring of Odours from a Composting Plant: Focus on Training, Data Processing and Results Validation Methods. Sens. Actuators B Chem. 2007, 124, 336–346. [Google Scholar] [CrossRef]
- Boholt, K.; Andreasen, K.; den Berg, F.; Hansen, T. A New Method for Measuring Emission of Odour from a Rendering Plant Using the Danish Odour Sensor System (DOSS) Artificial Nose. Sens. Actuators B Chem. 2005, 106, 170–176. [Google Scholar] [CrossRef]
- Bourgeois, W.; Burgess, J.E.; Stuetz, R.M. On-Line Monitoring of Wastewater Quality : A Review. J. Chem. Technol. Biotechnol. 2001, 76, 337–348. [Google Scholar] [CrossRef]
- Bourgeois, W.; Gardey, G.; Servieres, M.; Stuetz, R.M. A Chemical Sensor Array Based System for Protecting Wastewater Treatment Plants. Sens. Actuators B Chem. 2003, 91, 109–116. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Centola, P.; Del Rosso, R.; Grande, M.I. Electronic Noses for the Continuous Monitoring of Odours from a Wastewater Treatment Plant at Specific Receptors: Focus on Training Methods. Sens. Actuators B Chem. 2008, 131, 53–62. [Google Scholar] [CrossRef]
- Guz, Ł.; Łagód, G.; Jaromin-Gleń, K.; Suchorab, Z.; Sobczuk, H.; Bieganowski, A. Application of Gas Sensor Arrays in Assessment of Wastewater Purification Effects. Sensors 2015, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.; Bylinski, H.; Namiesnik, J. Measurement techniques for assessing the olfactory impact of municipal sewage treatment plants. Environ. Monit. Assess. 2016, 188, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Alimelli, A.; Martinelli, E.; Di Natale, C.; D’Amico, A.; D’Egidio, M.G.; Aureli, G.; Ricelli, A.; Fanelli, C. Detection of Fungal Contamination of Cereal Grain Samples by an Electronic Nose. Sens. Actuators B Chem. 2006, 119, 425–430. [Google Scholar] [CrossRef]
- Kuske, M.; Padilla, M.; Romain, A.C.; Nicolas, J.; Rubio, R.; Marco, S. Detection of Diverse Mould Species Growing on Building Materials by Gas Sensor Arrays and Pattern Recognition. Sens. Actuators B Chem. 2006, 119, 33–40. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Rehman, A.; Najafi, B.; Dickert, F.L. Real-life application of a QCM-based e-nose: Quantitative characterization of different plant-degradation processes. Anal. Bioanal. Chem. 2008, 391, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yang, S.X. A New Intelligent Electronic Nose System for Measuring and Analysing Livestock and Poultry Farm Odours. Environ. Monit. Assess. 2007, 135, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lingshuang, C.; Koziel, J.A.; Hoff, S.J.; Schmidt, D.R.; Clanton, C.J.; Jacobson, L.D.; Parker, D.B.; Heber, A.J. Field Air Sampling and Simultaneous Chemical and Sensory Analysis of Livestock Odorants with Sorbent Tubes and GC–MS/olfactometry. Sens. Actuators B Chem. 2010, 146, 427–432. [Google Scholar] [CrossRef]
- De Cesare, F.; Di Mattia, E.; Pantalei, S.; Zampetti, E.; Vinciguerra, V.; Canganella, F.; Macagnano, A. Use of electronic nose technology to measure soil microbial activity through biogenic volatile organic compounds and gases release. Soil Biol. Biochem. 2011, 43, 2094–2107. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.C.; Magan, N. Soil volatile fingerprints: Use for discrimination between soil types under different environmental conditions. Sens. Actuators B Chem. 2007, 125, 556–562. [Google Scholar] [CrossRef]
- Szpeiner, A.; Martinez-Ghersa, A.M.; Ghersa, C.M. Wheat volatile emissions modified by top-soil chemical characteristics and herbivory alter the performance of neighbouring wheat plants. Agric. Ecosyst. Environ. 2009, 134, 99–107. [Google Scholar] [CrossRef]
- Bieganowski, A.; Witkowska-Walczak, B.; Gliński, J.; Sokołowska, Z.; Sławiński, C.; Brzezińska, M.; Włodarczyk, T. Database of Polish arable mineral soils: A review. Int. Agrophys. 2013, 27, 335–350. [Google Scholar] [CrossRef]
- International Organization for Standardization. Soil Quality—Sampling—Part 2: Guidance on Sampling Techniques; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Guz, Ł.; Sobczuk, H.; Suchorab, Z. Odor measurement using portable device with semiconductor gas sensors array (in Polish). Przemysł Chemiczny 2010, 89, 378–381. [Google Scholar]
- FIGARO USA, INC. Available online: http://www.figarosensor.com (assessed on 9 June 2016).
- Krzanowski, W.J. Principles of Multivariate Analysis: A User’s Perspective, 2nd ed.; Oxford University Press, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Gardner, M.W.; Dorling, S.R. Artificial Neural Networks (the Multilayer Perceptron)—A Review of Applications in the Atmospheric Sciences. Atmos. Environ. 1998, 32, 2627–2636. [Google Scholar] [CrossRef]
- Schaller, E.; Bosset, J.O.; Escher, F. Electronic Noses’ and Their Application to Food. LWTFood Sci. Technol. 1998, 31, 305–316. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. A 21st Century Technique for Food Control: Electronic Noses. Anal. Chim. Acta. 2009, 638, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-W.; Tang, K.-T. Towards a Chemiresistive Sensor-Integrated Electronic Nose: A Review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-H.; Hsieh, H.-Y.; Tang, K.-T. An Analog Multilayer Perceptron Neural Network for a Portable Electronic Nose. Sensors 2013, 13, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Onkal-Engin, G.; Demir, I.; Engin, S.N. Determination of the relationship between sewage odour and BOD by neural networks. Environ. Modell. Softw. 2005, 20, 843–850. [Google Scholar] [CrossRef]
- Hill, T.; Lewicki, P. General Regression Models (GRM). In STATISTICS: Methods and Applications, 1st ed.; StatSoft, Inc.: Tulsa, OK, USA, 2005; pp. 349–365. [Google Scholar]
- Electronic Statistics Textbook. Tulsa, OK: StatSoft. Available online: http://www.statsoft.com/textbook/ (accessed on 8 March 2016).
- Włodarczyk, T.; Szarlip, P.; Kozieł, W.; Nosalewicz, M.; Brzezińska, M.; Pazur, M.; Urbanek, E. Effect of long storage and soil type on the actual denitrification and denitrification capacity to N2O formation. Int. Agrophys. 2014, 28, 371–381. [Google Scholar] [CrossRef]
- Frąc, M.; Jezierska-Tys, S. Agricultural utilisation of dairy sewage sludge: Its effect on enzymatic activity and microorganisms of the soil environment. Afr. J. Microbiol. Res. 2011, 5, 1755–1762. [Google Scholar]
- Walkiewicz, A.; Bulak, P.; Brzezińska, M.; Włodarczyk, T.; Polakowski, C. Kinetics of methane oxidation in selected mineral soils. Int. Agrophys. 2012, 26, 401–406. [Google Scholar] [CrossRef]
- Oszust, K.; Frąc, M.; Gryta, A.; Bilińska, N. The influence of ecological and conventional plant production systems on soil microbial quality under hops (Humulus lupulus). Int. J. Mol. Sci. 2014, 15, 9907–9923. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, A.; Rekosz-Burlaga, H.; Goryluk-Salmonowicz, A.; Błaszczyk, M.; Stępniewska, Z. Bacterial abundance and dehydrogenase activity in selected agricultural soils from Lublin region. Pol. J. Environ. Stud. 2015, 24, 2677–2682. [Google Scholar] [CrossRef]
- Dörner, J.; Sandoval, P.; Dec, D. The role of soil structure on the pore functionality of an Ultisol. J. Soil Sci. Plant Nutr. 2010, 10, 495–508. [Google Scholar] [CrossRef]
- Wolińska, A.; Stepniewska, Z.; Szafranek-Nakonieczna, A. Effect of Selected Physical Parameters on Respiration Activities in Common Polish Mineral Soils. Pol. J. Environ. Stud. 2011, 20, 1075–1082. [Google Scholar]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]

| No. | WRB Soil Group | Particle Size Group | Corg (%) * |
|---|---|---|---|
| 1 | Brunic Arenosol | Sand | 0.86 |
| 2 | Stagnic Luvisol | Sandy loam | 1.19 |
| 3 | Haplic Cambisol | Sandy loam | 0.57 |
| 4 | Leptic Cambisol | Silt loam | 1.08 |
| 5 | Mollic Stagnic Fluvisol | Silt loam | 1.14 |
| 6 | Stagnic Phaeozem (Siltic) | Silt | 1.97 |
| 7 | Haplic Chernozem (Siltic) | Silt loam | 1.11 |
| 8 | Haplic Luvisol (Siltic) | Silt | 1.06 |
| 9 | Leptic Skeletic Dystric Cambisol | Silt loam | 0.90 |
| 10 | Haplic Fluvisol (Clayic) | Silt | 1.86 |
| Sensor Type | Description | Detection Range | Sensitivity |
|---|---|---|---|
| TGS2600-B00 | general air contaminants, hydrogen, ethanol, etc. | 1–30 ppm of hydrogen | 0.3–0.6 for |
| TGS2602-B00 | air contaminants, toluene, VOCs, ammonia, hydrogen disulfide | 1–30 ppm of ethanol | 0.08–0.5 for |
| TGS2610-C00 | butane, LP gas | 500–10,000 ppm | 0.56 ± 0.06 for |
| TGS2610-D00 | butane, LP gas (carbon filter) | 500–10,000 ppm | 0.56 ± 0.06 for |
| TGS2611-C00 | methane, natural gas | 500–10,000 ppm | 0.6 ± 0.06 for |
| TGS2611-E00 | methane, natural gas (carbon filter) | 500–10,000 ppm | 0.6 ± 0.06 for |
| TGS2612-D00 | methane, propane, iso-butane, solvent vapors | 1%–25% LEL * | 0.5–0.65 for |
| TGS2620-C00 | alcohol, solvent vapors, carbon oxide, hydrogen | 50–5000 ppm | 0.3–0.5 for |
| Variable | Mean (kΩ) | Min (kΩ) | Max (kΩ) | SD | SD/Variability |
|---|---|---|---|---|---|
| 2600-B00 | 21.54036 | 20.39690 | 22.25086 | 0.453896 | 0.01267 |
| 2602-B00 | 44.42514 | 38.94425 | 47.85411 | 2.232958 | 0.02946 |
| 2610-C00 | 33.41887 | 31.82044 | 34.55444 | 0.654846 | 0.01558 |
| 2610-D00 | 40.69828 | 38.78311 | 41.76918 | 0.566877 | 0.01756 |
| 2611-C00 | 46.59227 | 44.62462 | 51.32284 | 1.440915 | 0.03627 |
| 2611-E00 | 41.10873 | 39.58662 | 42.15165 | 0.441327 | 0.01276 |
| 2612-D00 | 55.10230 | 53.02001 | 56.51318 | 0.664912 | 0.01664 |
| 2620-C00 | 22.67450 | 21.48789 | 23.51278 | 0.493596 | 0.00950 |
| Variable | 2600-B00 | 2602-B00 | 2610-C00 | 2610-D00 | 2611-C00 | 2611-E00 | 2612-D00 | 2620-C00 |
|---|---|---|---|---|---|---|---|---|
| PC1 | 0.007752 | 0.202078 | 0.143791 | 0.060383 | 0.117228 | 0.19586 | 0.197858 | 0.075051 |
| PC2 | 0.004066 | 0.004421 | 0.190849 | 0.362531 | 0.011935 | 0.058742 | 0.043401 | 0.324054 |
| Net ID | Training | Validation | Testing | |||
|---|---|---|---|---|---|---|
| MSE | R | MSE | R | MSE | R | |
| 1 | 0.04122 | 0.9995 | 0.06046 | 0.9993 | 0.03647 | 0.9960 |
| 2 | 0.00889 | 0.9999 | 0.01185 | 0.9998 | 0.05011 | 0.9994 |
| 2 | 0.02807 | 0.9997 | 0.05587 | 0.9994 | 0.07800 | 0.9991 |
| 4 | 0.05226 | 0.9994 | 0.06437 | 0.9993 | 0.06812 | 0.9926 |
| 5 | 0.05044 | 0.9994 | 0.17490 | 0.9981 | 0.05910 | 0.9993 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieganowski, A.; Jaromin-Glen, K.; Guz, Ł.; Łagód, G.; Jozefaciuk, G.; Franus, W.; Suchorab, Z.; Sobczuk, H. Evaluating Soil Moisture Status Using an e-Nose. Sensors 2016, 16, 886. https://doi.org/10.3390/s16060886
Bieganowski A, Jaromin-Glen K, Guz Ł, Łagód G, Jozefaciuk G, Franus W, Suchorab Z, Sobczuk H. Evaluating Soil Moisture Status Using an e-Nose. Sensors. 2016; 16(6):886. https://doi.org/10.3390/s16060886
Chicago/Turabian StyleBieganowski, Andrzej, Katarzyna Jaromin-Glen, Łukasz Guz, Grzegorz Łagód, Grzegorz Jozefaciuk, Wojciech Franus, Zbigniew Suchorab, and Henryk Sobczuk. 2016. "Evaluating Soil Moisture Status Using an e-Nose" Sensors 16, no. 6: 886. https://doi.org/10.3390/s16060886
APA StyleBieganowski, A., Jaromin-Glen, K., Guz, Ł., Łagód, G., Jozefaciuk, G., Franus, W., Suchorab, Z., & Sobczuk, H. (2016). Evaluating Soil Moisture Status Using an e-Nose. Sensors, 16(6), 886. https://doi.org/10.3390/s16060886









