Love Acoustic Wave-Based Devices and Molecularly-Imprinted Polymers as Versatile Sensors for Electronic Nose or Tongue for Cancer Monitoring
Abstract
:1. Introduction
2. Background Review on Electronic Nose and Tongue for Fast Disease Diagnoses Issues
2.1. Gas Media
2.2. Liquid Media
3. Materials and Methods
3.1. Microsensor
3.1.1. Love Wave Transducer
3.1.2. Molecularly-Imprinted Polymeric Thin Film
- Piranha cleaning: The LW substrates were cleaned by piranha solution (1:1 (v/v) concentrated sulfuric acid/30% hydrogen peroxide) to suppress organic and metallic impurities and form an oxide layer at the sensor surface (caution: piranha solution is extremely corrosive and can react severely with organic compounds, gloves, etc., which makes it essential that personal protective equipment be used during this step). The substrates were then rinsed thoroughly with 18 Mohm·cm deionized water and dried under a stream of nitrogen [76].
- Surface activation: The sensors were rinsed with toluene and purged overnight in a silane/toluene (2%:1 (v/v)) mixture and then placed into a laboratory oven at 200 °C for 30 min. The silane promotes covalent attachment of the MIP layer to sensor surface [77].
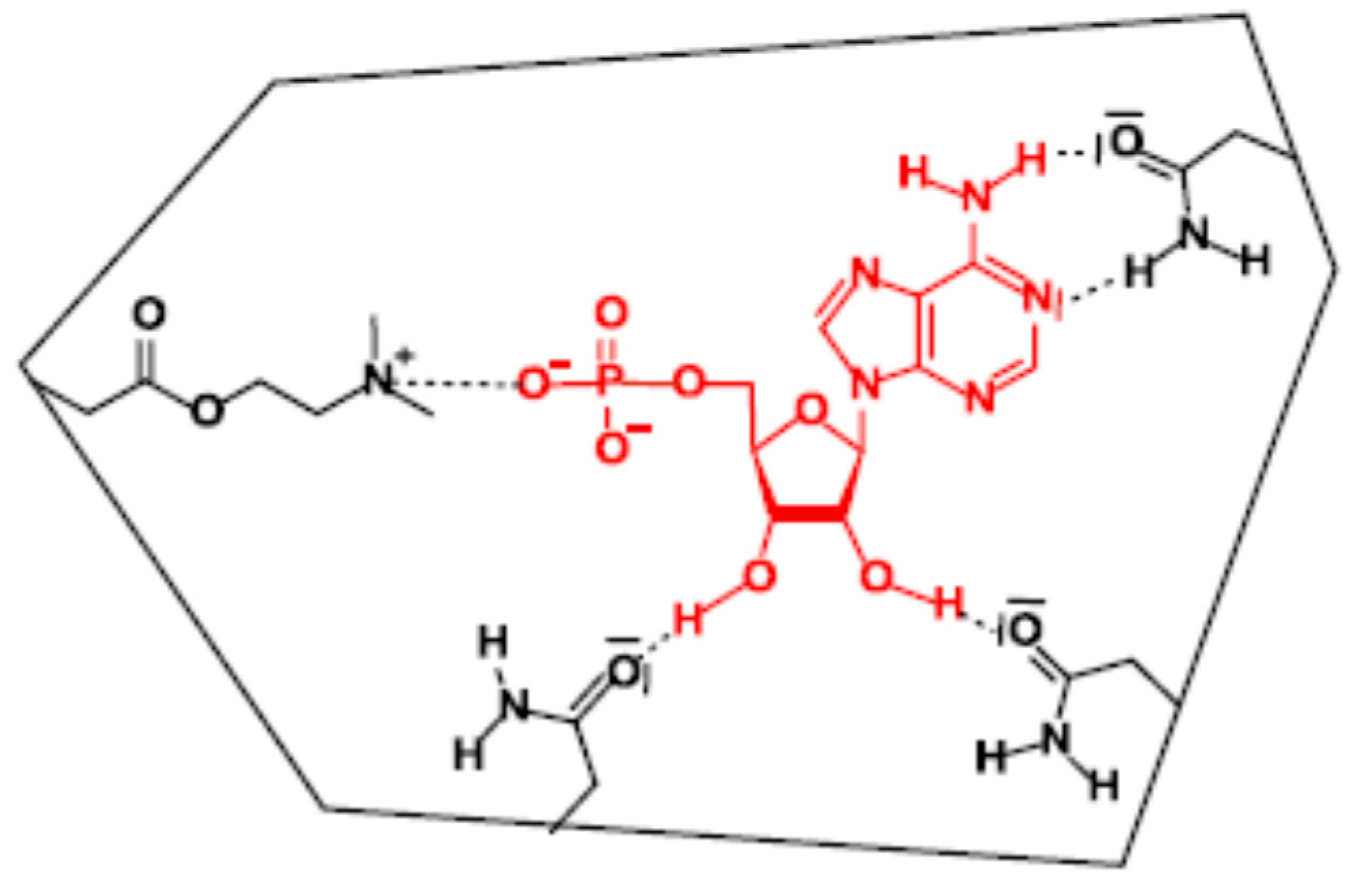
- Preparing of MIP solution: The AMP-MIP solution preparation process was adapted and optimized for surface coating. It consists of an ionic-noncovalent dual approach in which the best specific binding of AMP was obtained with one equivalent of 2-(dimethylamino)ethyl methacrylate for interaction with the negatively charged phosphate moiety, and ten equivalents of acrylamide to favor interactions with the nucleobase and the sugar moiety [75].
- Thus, 50 mg template AMP was added to 102.3 mg functional monomer acrylamide (AA) and stirred together with 24.2 µL 2-(dimethylamino) ethyl methacrylate (DMAEM) and 1.49 mL ethylene glycol dimethylacrylate (EGDMA), for 5 min. The mixture was dissolved in 1.1 mL dimethyl sulfoxide (DMSO) and stirred for 1 h. The mixture was then purged with nitrogen for 3 min to remove oxygen. Then 16 mg azo-bis isobutyronitrile (AIBN) was added to the solution and the flask was sealed with parafilm before mixing it for one hour. It should be noted that it was operated to obtain a change in the solution viscosity compared to the bulk solution so that this process became more suitable for thin film coating. The obtained solution was stored in a stained flask, as it is light and heat sensitive. A non-imprinted polymer (NIP) without the AMP particles was prepared similarly, for reference purpose.
- Thin Film MIP Coating: 10 μL of MIP solution was spin-coated on the sensors. The spin coating parameters are crucial for the control of the MIP film thickness and homogeneity, typical values of acceleration 4000 rpm/s and velocity 2000 rpm for 10 s were considered for 500 nm layer thickness. In order to localize the polymeric material onto the sensitive path between IDTs, adhesive tape (Kapton) was used as protection, which was removed immediately after spin-coating.
- Polymerization: The coated sensors were then polymerized at 365 nm UV light for 1 h in a polymerization box with continuous flow of nitrogen.
- Removal of AMP template: The extraction of AMP was done by soaking the sensor in eluting solution of ammonium and methanol (aqueous NH3 100 mM/MeOH, 70:30 v/v) over one night (except other condition mentioned) in a flask covered with parafilm. The device was then rinsed with deionized water (four times) and methanol, before possible storage at 4 °C.
3.1.3. Buffer and Analog Nucleotides Solutions
3.2. Test Bench
3.2.1. Static and Dynamic Modes of Measurements
3.2.2. Microfluidic Set-up
4. Results
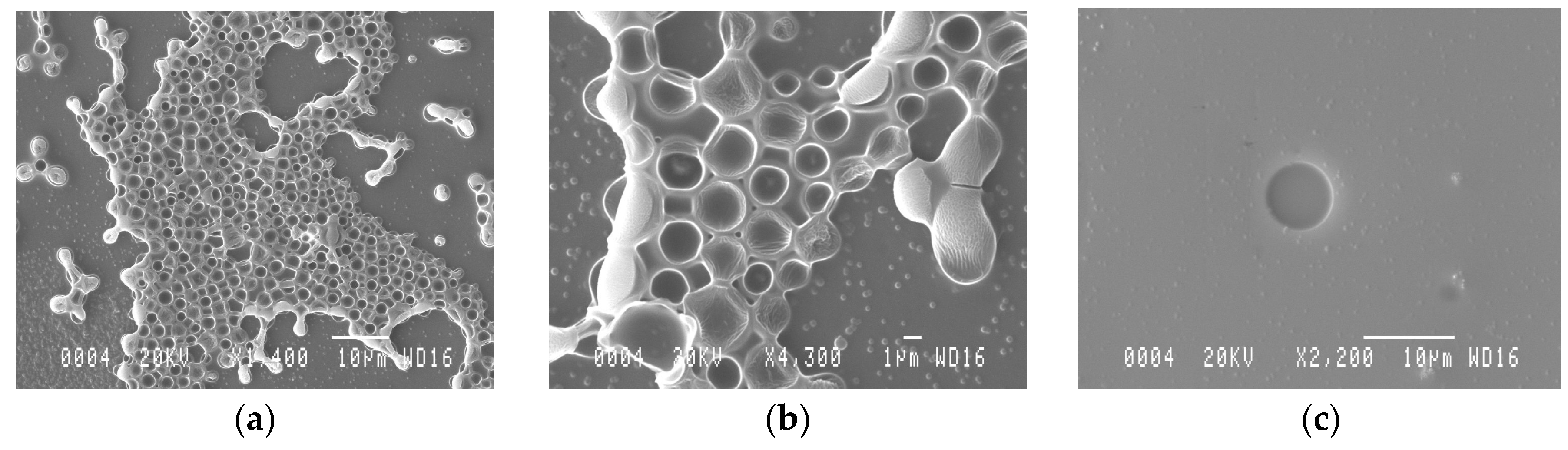
4.1. AMP-MIP Thin Film Coating
4.2. AMP-MIP Thin Film Functional Characterization Based on AMP Detection in Static Mode
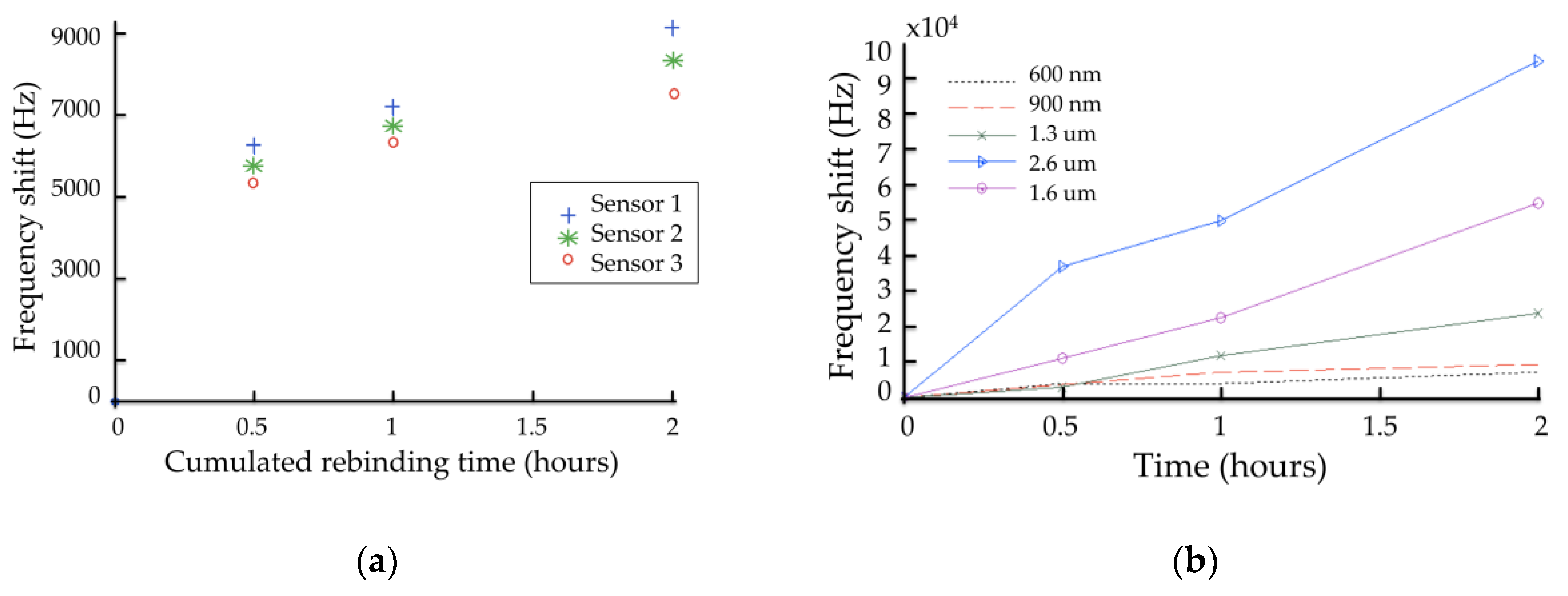
4.3. Real Time Measurements
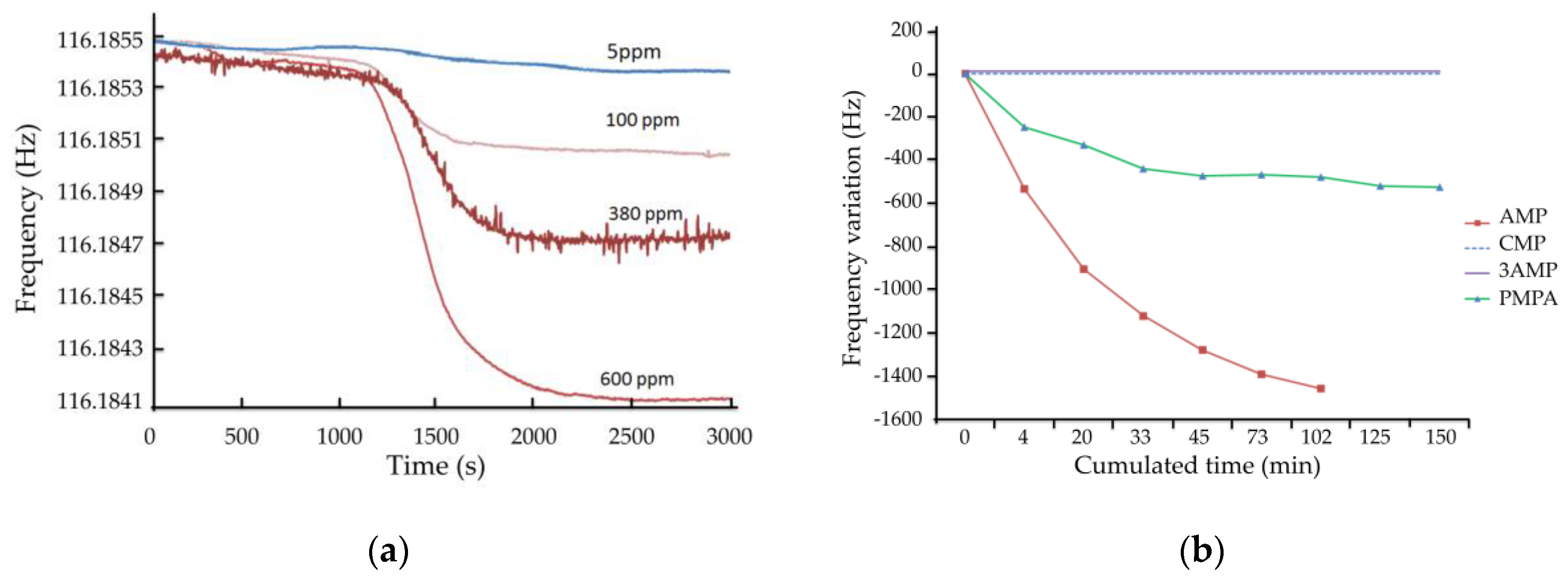
4.3.1. Detection of AMP
4.3.2. Specificity
5. Discussions
5.1. Results Analysis and Comparison with the State-of-the-Art
5.2. Future Use for Patient-Tailored Chronomodulated Therapy
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VOC | volatile organic compound |
| MIP, NIP | molecularly imprinted polymer, non-imprinted polymer |
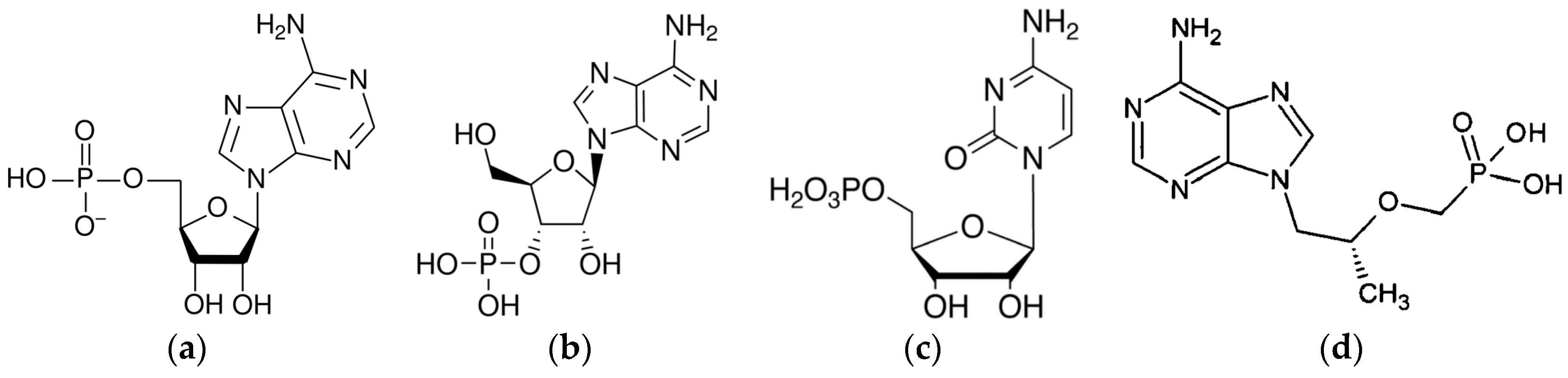
| AMP | adenosine-5′-monophosphate |
| 3AMP | adenosine-3′-monophosphate |
| CMP | cytidine-5′-monophosphate |
| PMPA | 2-phosphono methoxypropyl adenine |
| SEM | scanning electron microscopy |
| DALYs | disability-adjusted life years |
| IARC | International Agency for Research on Cancer |
| WHO | world health organization |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| DNA, tRNA | deoxyribonucleic acid, transfer ribonucleic acid |
| SPE | solid phase extraction |
| HPLC | high-performance liquid chromatography |
| UV, IR | ultra-violet, infrared |
| CE | capillary electrophoresis |
| LC, GC, MS | liquid chromatography, gas chromatography, mass spectroscopy |
| FET | field-effect transistor |
| IDE | interdigitated electrode |
| FTIR, NDIR | Fourier transform infrared, non-dispersive infrared |
| SPR | surface plasmon resonance |
| QCM, SAW | quartz crystal microbalance, surface acoustic wave |
| LW, SH-SAW | Love wave, shear horizontal surface acoustic waves |
| PDMS | polydimethylsiloxane |
References
- WHO/Europe/Air Quality-Review of Evidence on Health Aspects of Air Pollution-REVIHAAP Project: Final Technical Report. Available online: http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2013/review-of-evidence-on-health-aspects-of-air-pollution-revihaap-project-final-technical-report (accessed on 27 March 2016).
- The European environment—State and outlook 2010: Synthesis, Chapter 5: Environment, health and quality of life—European Environment Agency. Available online: http://www.eea.europa.eu/soer/synthesis/synthesis/chapter5.xhtml (accessed on 27 March 2016).
- Mathers, C.; Stevens, G.; Mascarenhas, M. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- United Nations Environment Programme. Global Environment Outlook GEO 5: Environment for the Future We Want; United Nations Environment Program: Nairobi, Kenya, 2012. [Google Scholar]
- WHO|Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 27 March 2016).
- Stewart, B.; Wild, C.P. International Agency for Research on Cancer World Cancer Report 2014; IARC: Lyon, France, 2015. [Google Scholar]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Agrofoglio, L.A.; Krstulja, A.; De Schutter, C.; Favetta, P.; Delépée, R.; Roy, V.; Dejous, C.; Hallil, H.; Lachaud, J.-L.; Lebal, N.; et al. Detection of urinary modified nucleosides by a bulk acoustic wave MIP sensor-Results and future work. IRBM 2014, 35, 66–71. [Google Scholar] [CrossRef]
- Gronewold, T.M.A.; Baumgartner, A.; Quandt, E.; Famulok, M. Discrimination of Single Mutations in Cancer-Related Gene Fragments with a Surface Acoustic Wave Sensor. Anal. Chem. 2006, 78, 4865–4871. [Google Scholar] [CrossRef] [PubMed]
- Lebal, N.; Raimbault, V.; Hallil, H.; Plano, B.; Lachaud, J.-L.; Dejous, C.; Rebière, D.; Krstulja, A.; Delépée, R.; Agrofoglio, L.A. Love Wave-Based Acoustic Components as Versatile Sensors for Electronic Nose or Tongue. Application to Cancer Monitoring. In Proceedings of the 2014 IEEE SENSORS, Valencia, Spain, 2–5 November 2014; pp. 1380–1383.
- Watson, T. Environment: Breathing trouble. Nature 2014, 513, S14–S15. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Baietto, M. Advances in Electronic-Nose Technologies Developed for Biomedical Applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Khot, L.R.; Panigrahi, S. Biology and applications of olfactory sensing system: A review. Sens. Actuators B Chem. 2012, 171–172, 1–17. [Google Scholar] [CrossRef]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J. Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting cancer by breath volatile organic compound analysis: A review of array-based sensors. J. Breath Res. 2014, 8, 27112. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563. [Google Scholar] [CrossRef]
- Wang, C.; Mbi, A.; Shepherd, M. A Study on Breath Acetone in Diabetic Patients Using a Cavity Ringdown Breath Analyzer: Exploring Correlations of Breath Acetone with Blood Glucose and Glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Micali, G.; Donato, N. Design and Development of a Breath Acetone MOS Sensor for Ketogenic Diets Control. IEEE Sens. J. 2010, 10, 131–136. [Google Scholar] [CrossRef]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Xu, L.; Song, J.; Zhou, C.; Li, Q.; Liu, D.; Wei Song, H. Preparation and Gas Sensing Properties of In2O3/Au Nanorods for Detection of Volatile Organic Compounds in Exhaled Breath. Sci. Rep. 2015, 5, 10717. [Google Scholar] [CrossRef] [PubMed]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- DuBois, S.; Eng, S.; Bhattacharya, R.; Rulyak, S.; Hubbard, T.; Putnam, D.; Kearney, D.J. Breath Ammonia Testing for Diagnosis of Hepatic Encephalopathy. Dig. Dis. Sci. 2005, 50, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Gouma, P.; Kalyanasundaram, K.; Yun, X.; Stanacevic, M.; Wang, L. Nanosensor and Breath Analyzer for Ammonia Detection in Exhaled Human Breath. IEEE Sens. J. 2010, 10, 49–53. [Google Scholar] [CrossRef]
- Kuzmych, O.; Allen, B.L.; Star, A. Carbon nanotube sensors for exhaled breath components. Nanotechnology 2007, 18, 375502. [Google Scholar] [CrossRef]
- Namjou, K.; Roller, C.B.; Reich, T.E.; Jeffers, J.D.; McMillen, G.L.; McCann, P.J.; Camp, M.A. Determination of exhaled nitric oxide distributions in a diverse sample population using tunable diode laser absorption spectroscopy. Appl. Phys. B 2006, 85, 427–435. [Google Scholar]
- Smith, A.D.; Cowan, J.O.; Filsell, S.; McLachlan, C.; Monti-Sheehan, G.; Jackson, P.; Taylor, D.R. Diagnosing Asthma: Comparisons between Exhaled Nitric Oxide Measurements and Conventional Tests. Am. J. Respir. Crit. Care Med. 2004, 169, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Pantalei, S.; Zampetti, E.; Bearzotti, A.; De Cesare, F.; Macagnano, A. Improving sensing features of a nanocomposite PEDOT:PSS sensor for NO breath monitoring. Sens. Actuators B Chem. 2013, 179, 87–94. [Google Scholar] [CrossRef]
- Corradi, M.; Majori, M.; Cacciani, G.C.; Consigli, G.F.; de’Munari, E.; Pesci, A. Increased exhaled nitric oxide in patients with stable chronic obstructive pulmonary disease. Thorax 1999, 54, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Drangsholt, M.T. A New Causal Model of Dental Diseases Associated With Endocarditis. Ann. Periodontol. 1998, 3, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Okell, C.C.; Elliott, S.D. Bacteraemia and oral sepsis with special reference to the aetiology of subacute endocarditis. Lancet 1935, 226, 869–872. [Google Scholar] [CrossRef]
- Van den Velde, S.; van Steenberghe, D.; Van Hee, P.; Quirynen, M. Detection of Odorous Compounds in Breath. J. Dent. Res. 2009, 88, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Chen, Y.; Ma, J. Gas Sensing of SnO2 Nanocrystals Revisited: Developing Ultra-Sensitive Sensors for Detecting the H2S Leakage of Biogas. Sci. Rep. 2014, 4, 6028. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mahadevan, V.; Zieve, L. Volatile fatty acids in the breath of patients with cirrhosis of the liver. J. Lab. Clin. Med. 1970, 75, 622–627. [Google Scholar] [PubMed]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, Chemical Sensors, Electrochemical Sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Kim, K.-H.; Tang, K.-T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Singh, H.; Raj, V.B.; Kumar, J.; Mittal, U.; Mishra, M.; Nimal, A.T.; Sharma, M.U.; Gupta, V. Metal oxide SAW E-nose employing PCA and ANN for the identification of binary mixture of DMMP and methanol. Sens. Actuators B Chem. 2014, 200, 147–156. [Google Scholar] [CrossRef]
- Toshiba: Press Release (18 March 2014): Toshiba Develops Breath Analyzer for Medical Applications. Available online: http://www.toshiba.co.jp/about/press/2014_03/pr1801.htm (accessed on 27 March 2016).
- Detecting Cancer through Breath Analysis. Available online: http://medicalxpress.com/news/2015-02-cancer-analysis.html (accessed on 27 March 2016).
- Zheng, Y.-F.; Kong, H.-W.; Xiong, J.-H.; Lv, S.; Xu, G.-W. Clinical significance and prognostic value of urinary nucleosides in breast cancer patients. Clin. Biochem. 2005, 38, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Choi, M.H.; Lee, W.-Y.; Chung, B.C. Evaluation of urinary nucleosides in breast cancer patients before and after tumor removal. Clin. Biochem. 2009, 42, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Frickenschmidt, A.; Fröhlich, H.; Bullinger, D.; Zell, A.; Laufer, S.; Gleiter, C.H.; Liebich, H.; Kammerer, B. Metabonomics in cancer diagnosis: Mass spectrometry-based profiling of urinary nucleosides from breast cancer patients. Biomarkers 2008, 13, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Henneges, C.; Bullinger, D.; Fux, R.; Friese, N.; Seeger, H.; Neubauer, H.; Laufer, S.; Gleiter, C.H.; Schwab, M.; Zell, A.; et al. Prediction of breast cancer by profiling of urinary RNA metabolites using SVM-based feature selection. BMC Cancer 2009, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Rasmuson, T.; Björk, G.R.; Damber, L.; Jacobsson, L.; Jeppsson, A.; Stigbrand, T.; Westman, G. Tumor Markers in Mammary Carcinoma: An evaluation of carcinoembryonic antigen, placental alkaline phosphatase, pseudouridine and CA-50. Acta Oncol. 1987, 26, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nakao, T.; Schram, K.H.; Hammargren, W.M.; McClure, T.D.; Katz, M.; Petersen, E. Urinary excretion of modified nucleosides as biological marker of RNA turnover in patients with cancer and AIDS. Clin. Chim. Acta 1993, 218, 169–183. [Google Scholar] [CrossRef]
- Rasmuson, T.; Björk, G.R. Urinary Excretion of Pseudouridine and Prognosis of Patients with Malignant Lymphoma. Acta Oncol. 1995, 34, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jeng, L.-B.; Lo, W.-Y.; Hsu, W.-Y.; Lin, W.-D.; Lin, C.-T.; Lai, C.-C.; Tsai, F.-J. Analysis of urinary nucleosides as helper tumor markers in hepatocellular carcinoma diagnosis. Rapid Commun. Mass Spectrom. 2009, 23, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, G.; Kong, H.; Zheng, Y.; Pang, T.; Yang, Q. Artificial neural network classification based on high-performance liquid chromatography of urinary and serum nucleosides for the clinical diagnosis of cancer. J. Chromatogr. B 2002, 780, 27–33. [Google Scholar] [CrossRef]
- McEntire, J.E.; Kuo, K.C.; Smith, M.E.; Stalling, D.L.; Richens, J.W., Jr.; Zumwalt, R.W.; Gehrke, C.W.; Papermaster, B.W. Classification of Lung Cancer Patients and Controls by Chromatography of Modified Nucleosides in Serum. Cancer Res. 1989, 49, 1057–1062. [Google Scholar] [PubMed]
- Weissman, S.; Eisen, A.Z.; Lewis, M.; Karon, M. Pseudouridine metabolism. III. Studies with isotopically labeled pseudouridine. Chromatogr. Libr. 1962, 40–47. [Google Scholar]
- Borek, E.; Baliga, B.S.; Gehrke, C.W.; Kuo, C.W.; Belman, S.; Troll, W.; Waalkes, T.P. High Turnover Rate of Transfer RNA in Tumor Tissue. Cancer Res. 1977, 37, 3362. [Google Scholar] [PubMed]
- Sander, G.; Topp, H.; Wieland, J.; Heller-Schöch, G.; Schöch, G. Possible use of urinary modified RNA metabolites in the measurement of RNA turnover in the human body. Hum. Nutr. Clin. Nutr. 1986, 40, 103–118. [Google Scholar] [PubMed]
- Seidel, A.; Brunner, S.; Seidel, P.; Fritz, G.I.; Herbarth, O. Modified nucleosides: An accurate tumour marker for clinical diagnosis of cancer, early detection and therapy control. Br. J. Cancer 2006, 94, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Schöch, G.; Sander, G.; Topp, H.; Heller-Schoch, G. Chapter 13 Modified Nucleosides and Nucleobases in Urine and Serum as Selective Markers for The Whole-Body Turnover of tRNA, rRNA and mRNA-CAP-Future Prospects and Impact. In Journal of Chromatography Library; Elsevier: Amsterdam, the Netherlands, 1990; Volume 45, pp. C389–C441. [Google Scholar]
- Zhang, C.; Liu, Z.; Liu, X.; Wei, L.; Liu, Y.; Yu, J.; Sun, L. Targeted metabolic analysis of nucleotides and identification of biomarkers associated with cancer in cultured cell models. Acta Pharm. Sin. B 2013, 3, 254–262. [Google Scholar] [CrossRef]
- Schetinger, M.R.C.; Morsch, V.M.; Bonan, C.D.; Wyse, A.T.S. NTPDase and 5′-nucleotidase activities in physiological and disease conditions: New perspectives for human health. BioFactors Oxf. Engl. 2007, 31, 77–98. [Google Scholar] [CrossRef]
- Schoeman, J.C.; Loots, D.T. Improved disease characterisation and diagnostics using metabolomics: A review. J. Cell Tissue Res. 2011, 11, 2673. [Google Scholar]
- Kurada, S.; Alkhouri, N.; Fiocchi, C.; Dweik, R.; Rieder, F. Review article: Breath analysis in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2015, 41, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.-P.; Kutner, W. Molecularly imprinted polymers as recognition materials for electronic tongues. Biosens. Bioelectron. 2015, 74, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Josse, F.; Cernosek, R.W. Resonant Piezoelectric Devices as Physical and Biochemical Sensors. In Smart Sensors and MEMS; Yurish, S.Y., Gomes, M.T.S.R., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 181, pp. 91–123. [Google Scholar]
- Lebal, N.; Hallil, H.; Dejous, C.; Plano, B.; Krstulja, A.; Delepee, R.; Rebiere, D.; Agrofoglio, L.A. Association of a Love wave sensor to thin film molecularly imprinted polymers for nucleosides analogs detection. In Proceedings of the 2013 IEEE NEMS, Suzhou, China, 7–10 April 2013; pp. 550–553.
- Raimbault, V.; Rebiére, D.; Dejous, C.; Guirardel, M.; Lachaud, J.L. Molecular weight influence study of aqueous poly(ethylene glycol) solutions with a microfluidic Love wave sensor. Sens. Actuators B Chem. 2010, 144, 318–322. [Google Scholar] [CrossRef]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, X.; Ma, X.; Ou, G.; Gao, Z. Rapid and multiple detections of staphylococcal enterotoxins by two-dimensional molecularly imprinted film-coated QCM sensor. Sens. Actuators B Chem. 2014, 191, 326–331. [Google Scholar] [CrossRef]
- Schirhagl, R. Bioapplications for Molecularly Imprinted Polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Ersöz, A.; Diltemiz, S.E.; Özcan, A.A.; Denizli, A.; Say, R. Synergie between molecular imprinted polymer based on solid-phase extraction and quartz crystal microbalance technique for 8-OHdG sensing. Biosens. Bioelectron. 2008, 24, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Guo, Z.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Dzyadevych, S.; Săndulescu, R.; Jaffrezic-Renault, N. Anticancer drug detection using a highly sensitive molecularly imprinted electrochemical sensor based on an electropolymerized microporous metal organic framework. Talanta 2015, 138, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, A.; Kohara, K. Biomimetic sensor for cAMP using an ion-sensitive field-effect transistor. Mater. Sci. Eng. C 2009, 29, 959–962. [Google Scholar] [CrossRef]
- Zayats, M.; Lahav, M.; Kharitonov, A.B.; Willner, I. Imprinting of specific molecular recognition sites in inorganic and organic thin layer membranes associated with ion-sensitive field-effect transistors. Tetrahedron 2002, 58, 815–824. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Karimian, N. Fabrication of a highly selective and sensitive voltammetric ganciclovir sensor based on electropolymerized molecularly imprinted polymer and gold nanoparticles on multiwall carbon nanotubes/glassy carbon electrode. Sens. Actuators B Chem. 2015, 215, 471–479. [Google Scholar] [CrossRef]
- El Gohary, N.A.; Madbouly, A.; El Nashar, R.M.; Mizaikoff, B. Synthesis and application of a molecularly imprinted polymer for the voltammetric determination of famciclovir. Biosens. Bioelectron. 2015, 65, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Turkewitsch, P.; Wandelt, B.; Darling, G.D.; Powell, W.S. Fluorescent Functional Recognition Sites through Molecular Imprinting. A Polymer-Based Fluorescent Chemosensor for Aqueous cAMP. Anal. Chem. 1998, 70, 2025–2030. [Google Scholar] [CrossRef]
- Breton, F.; Delépée, R.; Agrofoglio, L.A. Molecular imprinting of AMP by an ionic-noncovalent dual approach. J. Sep. Sci. 2009, 32, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Buvailo, A.; Xing, Y.; Hines, J.; Borguet, E. Thin polymer film based rapid surface acoustic wave humidity sensors. Sens. Actuators B Chem. 2011, 156, 444–449. [Google Scholar] [CrossRef]
- He, Q.; Sévérac, F.; Hajjoul, H.; Viero, Y.; Bancaud, A. Directed Assembly of Nanoparticles along Predictable Large-Scale Patterns Using Micromolded Hydrogels. Langmuir 2011, 27, 6598–6605. [Google Scholar] [CrossRef] [PubMed]
- Delépée, R.; Breton, F.; Jegourel, D.; Agrofoglio, L.A. Molecularly imprinted polymer as biomimetic sensor of nucleoside phosphorylation mediated by a kinase. In Proceedings of the 2010 Biosensors, Glasgow, UK, 25–28 May 2010.
- Lebal, N.; Rebière, D.; Delepée, R.; Agrofoglio, L.; Plano, B.; Hallil, H.; Krstulja, A.; Dejous, C. Nucleosides analogues recognition by molecularly imprinted polymer-coated Love wave sensor. Micro Amp Nano Lett. 2013, 8, 563–566. [Google Scholar] [CrossRef]
- Raimbault, V.; Rebière, D.; Dejous, C. A microfluidic surface acoustic wave sensor platform: Application to high viscosity measurements. Mater. Sci. Eng. C 2008, 28, 759–764. [Google Scholar] [CrossRef]
- Aouled, N.O.; Hallil, H.; Plano, B.; Rebiere, D.; Dejous, C.; Delepee, R.; Agrofoglio, L. Love wave sensor based on thin film molecularly imprinted polymer: MIP layer morphology and nucleosides analogs detection. In Proceedings of the 2013 IEEE Sensors, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4.
- Lin, T.-Y.; Hu, C.-H.; Chou, T.-C. Determination of albumin concentration by MIP-QCM sensor. Biosens. Bioelectron. 2004, 20, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Tigli, O.; Bivona, L.; Berg, P.; Zaghloul, M.E. Fabrication and Characterization of a Surface-Acoustic-Wave Biosensor in CMOS Technology for Cancer Biomarker Detection. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Srivastava, S.; Tiwari, K.; Sharma, P.S. Development of Uracil and 5-Fluorouracil Sensors Based on Molecularly Imprinted Polymer-Modified Hanging Mercury Drop Electrode. Sens. Mater. 2009, 21, 291–306. [Google Scholar]
- Prasad, B.B.; Kumar, A. Development of molecularly imprinted polymer nanoarrays of N-acryloyl-2-mercaptobenzamide on a silver electrode for ultratrace sensing of uracil and 5-fluorouracil. J. Mater. Chem. B 2015, 3, 5864–5876. [Google Scholar] [CrossRef]
- Alnaim, L. Therapeutic drug monitoring of cancer chemotherapy. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2007, 13, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Hrushesky, W.J.; Bjarnason, G.A. Circadian cancer therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 1403–1417. [Google Scholar]
- Rosbash, M.; Takahashi, J.S. Circadian rhythms: The cancer connection. Nature 2002, 420, 373–374. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejous, C.; Hallil, H.; Raimbault, V.; Lachaud, J.-L.; Plano, B.; Delépée, R.; Favetta, P.; Agrofoglio, L.; Rebière, D. Love Acoustic Wave-Based Devices and Molecularly-Imprinted Polymers as Versatile Sensors for Electronic Nose or Tongue for Cancer Monitoring. Sensors 2016, 16, 915. https://doi.org/10.3390/s16060915
Dejous C, Hallil H, Raimbault V, Lachaud J-L, Plano B, Delépée R, Favetta P, Agrofoglio L, Rebière D. Love Acoustic Wave-Based Devices and Molecularly-Imprinted Polymers as Versatile Sensors for Electronic Nose or Tongue for Cancer Monitoring. Sensors. 2016; 16(6):915. https://doi.org/10.3390/s16060915
Chicago/Turabian StyleDejous, Corinne, Hamida Hallil, Vincent Raimbault, Jean-Luc Lachaud, Bernard Plano, Raphaël Delépée, Patrick Favetta, Luigi Agrofoglio, and Dominique Rebière. 2016. "Love Acoustic Wave-Based Devices and Molecularly-Imprinted Polymers as Versatile Sensors for Electronic Nose or Tongue for Cancer Monitoring" Sensors 16, no. 6: 915. https://doi.org/10.3390/s16060915





