Rapid Detection of Glycogen Synthase Kinase-3 Activity in Mouse Sperm Using Fluorescent Gel Shift Electrophoresis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
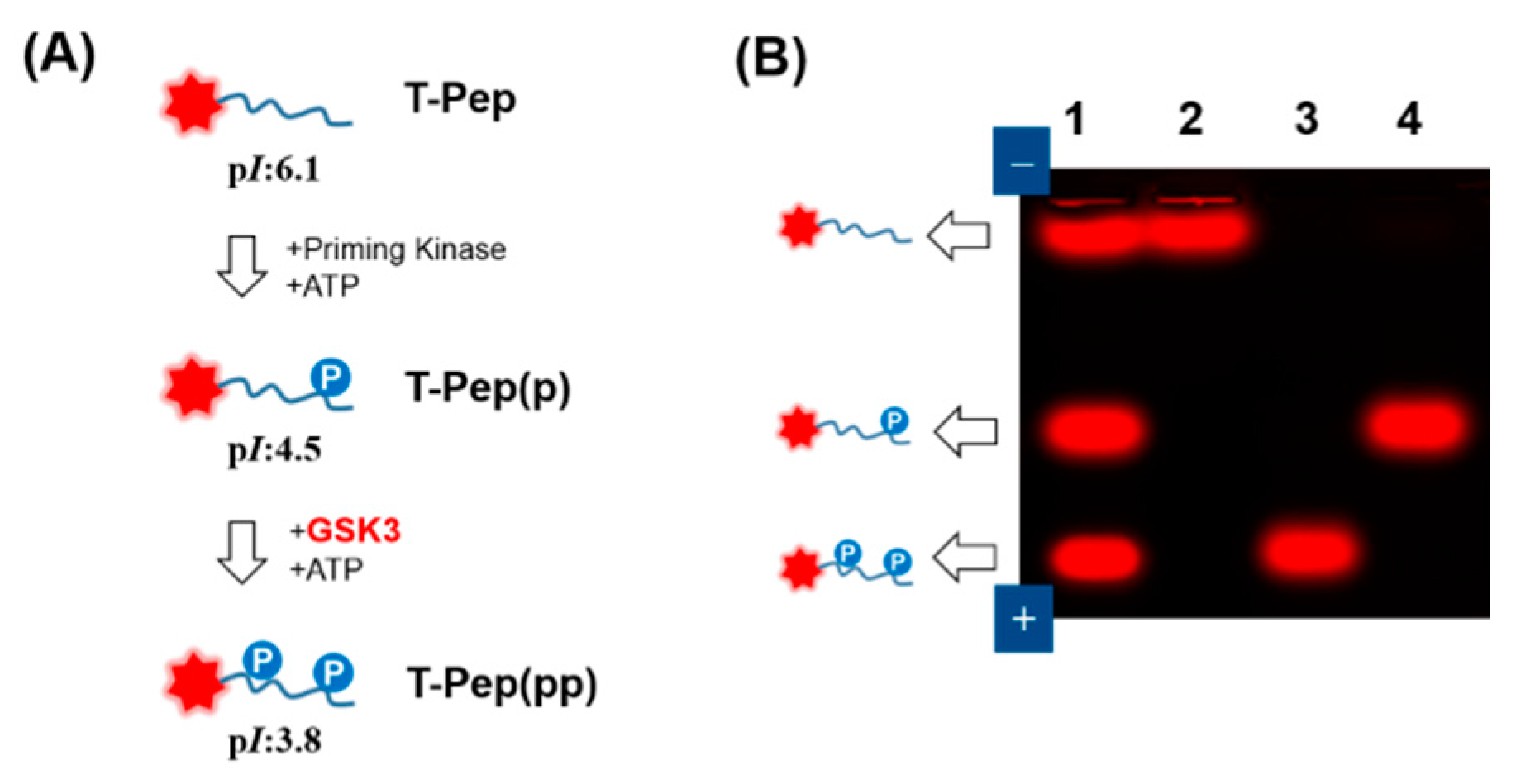
2.2. Fluorescent Gel Shift Assay on Agarose
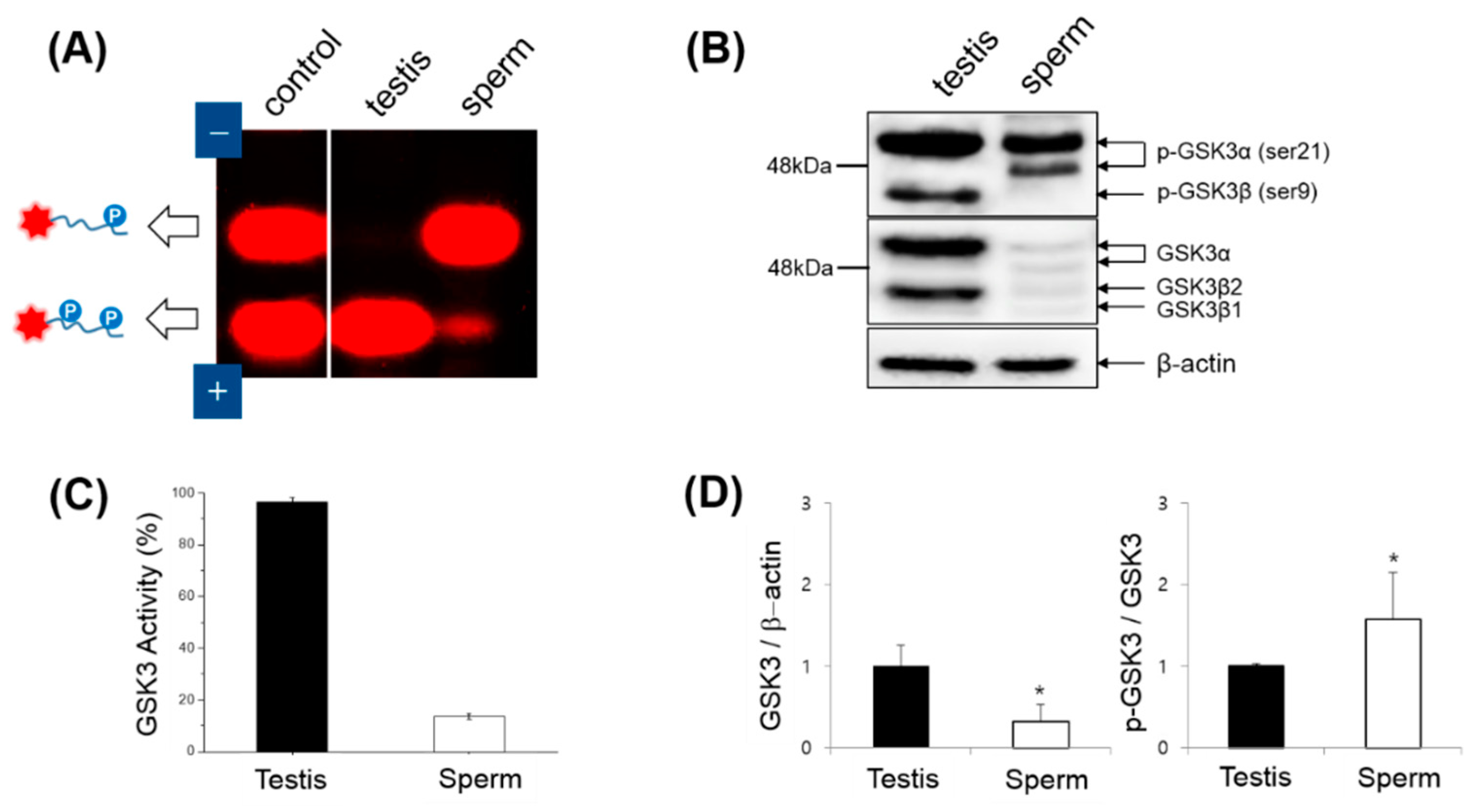
2.3. Determination of GSK3 Activity
2.4. Sperm Collection
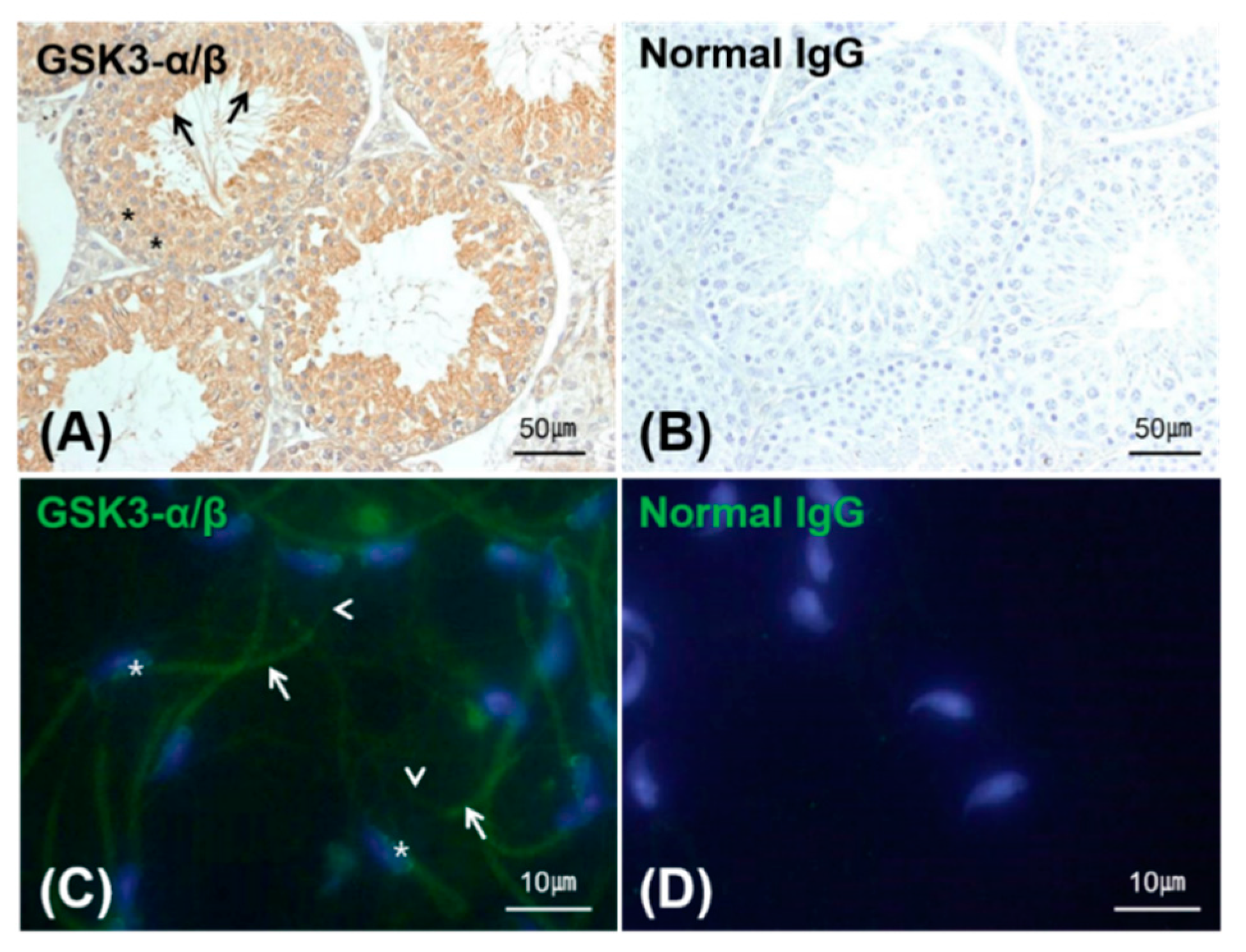
2.5. Localization of GSK3 in the Testis and Sperm
2.6. Western Blotting
3. Results and Discussion
3.1. GSK3 Activity Assay on Agarose
3.2. Expression, Kinase Activity and Phosphorylation of GSK3 in Sperm
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ali, A.; Hoeflich, K.P.; Woodgett, J.R. Glycogen synthase Kinase-3: Properties, functions, and regulation. Chem. Rev. 2001, 101, 2527–2540. [Google Scholar] [PubMed]
- Frame, S.; Cohen, P. Gsk3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001, 359, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. GSK3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Vadnais, M.L.; Aghajanian, H.K.; Lin, A.; Gerton, G.L. Signaling in sperm: Toward a molecular understanding of the acquisition of sperm motility in the mouse epididymis. Biol. Reprod. 2013, 89. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Goswami, S.; Popkie, A.; Phiel, C.; Kline, D.; Vijayaraghavan, S. GSK3α is essential for sperm function and male fertility. FASEB J. 2015, 29. [Google Scholar]
- Bhattacharjee, R.; Goswami, S.; Dudiki, T.; Popkie, A.P.; Phiel, C.J.; Kline, D.; Vijayaraghavan, S. Targeted disruption of glycogen synthase Kinase 3α (GSK3α) in mice affects sperm motility resulting in male infertility. Biol. Reprod. 2015, 92. [Google Scholar] [CrossRef] [PubMed]
- Somanath, P.R.; Jack, S.L.; Vijayaraghavan, S. Changes in sperm glycogen synthase Kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. J. Androl. 2004, 25, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Frame, S.; Cohen, P.; Biondi, R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 2001, 7, 1321–1327. [Google Scholar] [CrossRef]
- Dajani, R.; Fraser, E.; Roe, S.M.; Young, N.; Good, V.; Dale, T.C.; Pearl, L.H. Crystal structure of glycogen synthase kinase 3 beta: Structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 2001, 105, 721–732. [Google Scholar] [CrossRef]
- Hur, E.M.; Zhou, F.Q. Gsk3 signalling in neural development. Nat. Rev. Neurosci. 2010, 11, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, N.; Lim, B.; Kim, T.W.; Song, S.; Kim, Y.P. Sequential phosphorylation analysis using dye-tethered peptides and microfluidic isoelectric focusing electrophoresis. Biosens. Bioelectron. 2015, 73, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.G. Measurement of protein-DNA interaction parameters by electrophoresis mobility shift assay. Electrophoresis 1989, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Hellman, L.M.; Fried, M.G. Electrophoretic mobility shift assay (emsa) for detecting protein-nucleic acid interactions. Nat. Protoc. 2007, 2, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.V.; Miller, E.S. Rna-binding properties of in vitro expressed histidine-tagged RB69 RegA translational repressor protein. Anal. Biochem. 1999, 269, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.E.; Yu, W.P.; Yu, H.H.; Yau, K.W. Identification of a domain on the beta-subunit of the rod cgmp-gated cation channel that mediates inhibition by calcium-calmodulin. J. Biol. Chem. 1998, 273, 9148–9157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Kim, E.J.; Lim, B.; Kim, T.W.; Kim, Y.P. Rapid detection of protein phosphatase activity using Zn(ii)-coordinated gold nanosensors based on his-tagged phosphopeptides. Anal. Chem. 2015, 87, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Park, J.I.; Lee, K.J.; Lee, J.W.; Kim, T.W.; Kim, Y.P. Zn(ii)-coordinated quantum dot-fret nanosensors for the detection of protein kinase activity. Sensors 2015, 15, 17977–17989. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.M.; Gil, M.C.; Garcia-Herreros, M.; Pena, F.J.; Garcia-Marin, L.J. Inhibition of phosphatidylinositol 3-Kinase modifies boar sperm motion parameters. Reproduction 2005, 129, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.Y.; Soncin, F.; Price, B.D.; Stevenson, M.A.; Calderwood, S.K. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase Kinase 3 represses transcriptional activation by heat shock factor-1. J. Biol. Chem. 1996, 271, 30847–30857. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Meng, Y.H.; Mivechi, N.F. Glycogen synthase kinase 3beta and extracellular signal-regulated kinase inactivate heat shock transcription factor 1 by facilitating the disappearance of transcriptionally active granules after heat shock. Mol. Cell. Biol. 1998, 18, 6624–6633. [Google Scholar] [CrossRef] [PubMed]
- Fiol, C.J.; Mahrenholz, A.M.; Wang, Y.; Roeske, R.W.; Roach, P.J. Formation of protein-kinase recognition sites by covalent modification of the substrate—Molecular mechanism for the synergistic action of casein kinase-ii and glycogen-synthase Kinase 3. J. Biol. Chem. 1987, 262, 14042–14048. [Google Scholar] [PubMed]
- Eldar-Finkelman, H. Glycogen synthase Kinase 3: An emerging therapeutic target. Trends Mol. Med. 2002, 8, 126–132. [Google Scholar] [CrossRef]
- Ryves, W.J.; Harwood, A.J. Lithium inhibits glycogen synthase Kinase 3 by competition for magnesium. Biochem. Biophys. Res. Commun. 2001, 280, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Woodgett, J.R. Molecular-cloning and expression of glycogen-synthase Kinase-3 factor-a. EMBO J. 1990, 9, 2431–2438. [Google Scholar] [PubMed]
- Mukai, F.; Ishiguro, K.; Sano, Y.; Fujita, S.C. Alternative splicing isoform of tau protein Kinase i/glycogen synthase Kinase 3 beta. J. Neurochem. 2002, 81, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Acebron, S.P.; Herbst, J.; Hatiboglu, G.; Niehrs, C. Post-transcriptional wnt signaling governs epididymal sperm maturation. Cell 2015, 163, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Olive, D.M. Quantitative methods for the analysis of protein phosphorylation in drug development. Expert Rev. Proteom. 2004, 1, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.J.; McLauchlan, H.J.; Cohen, P. Assay of protein kinases using radiolabeled ATP: A protocol. Nat. Protoc. 2006, 1, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Boiocchi, M.; Bonizzoni, M.; Moletti, A.; Pasini, D.; Taglietti, A. Linear recognition of dicarboxylates by ditopic macrocyclic complexes. New. J. Chem. 2007, 31, 352–356. [Google Scholar] [CrossRef]
- Moletti, A.; Coluccini, C.; Pasini, D.; Taglietti, A. A chiral probe for the detection of Cu(ii) by UV, CD and emission spectroscopies. Dalton Trans. 2007, 16, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Litvinchuk, S.; Tanaka, H.; Miyatake, T.; Pasini, D.; Tanaka, T.; Bollot, G.; Mareda, J.; Matile, S. Synthetic pores with reactive signal amplifiers as artificial tongues. Nat. Mater. 2007, 6, 576–580. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Choi, B.; Seo, J.T.; Lee, K.J.; Gye, M.C.; Kim, Y.-P. Rapid Detection of Glycogen Synthase Kinase-3 Activity in Mouse Sperm Using Fluorescent Gel Shift Electrophoresis. Sensors 2016, 16, 551. https://doi.org/10.3390/s16040551
Choi H, Choi B, Seo JT, Lee KJ, Gye MC, Kim Y-P. Rapid Detection of Glycogen Synthase Kinase-3 Activity in Mouse Sperm Using Fluorescent Gel Shift Electrophoresis. Sensors. 2016; 16(4):551. https://doi.org/10.3390/s16040551
Chicago/Turabian StyleChoi, Hoseok, Bomi Choi, Ju Tae Seo, Kyung Jin Lee, Myung Chan Gye, and Young-Pil Kim. 2016. "Rapid Detection of Glycogen Synthase Kinase-3 Activity in Mouse Sperm Using Fluorescent Gel Shift Electrophoresis" Sensors 16, no. 4: 551. https://doi.org/10.3390/s16040551






