Fluorescence Characterization of Gold Modified Liposomes with Antisense N-myc DNA Bound to the Magnetisable Particles with Encapsulated Anticancer Drugs (Doxorubicin, Ellipticine and Etoposide)
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Material
2.2. Preparation of Gold Nanoparticles (AuNPs)
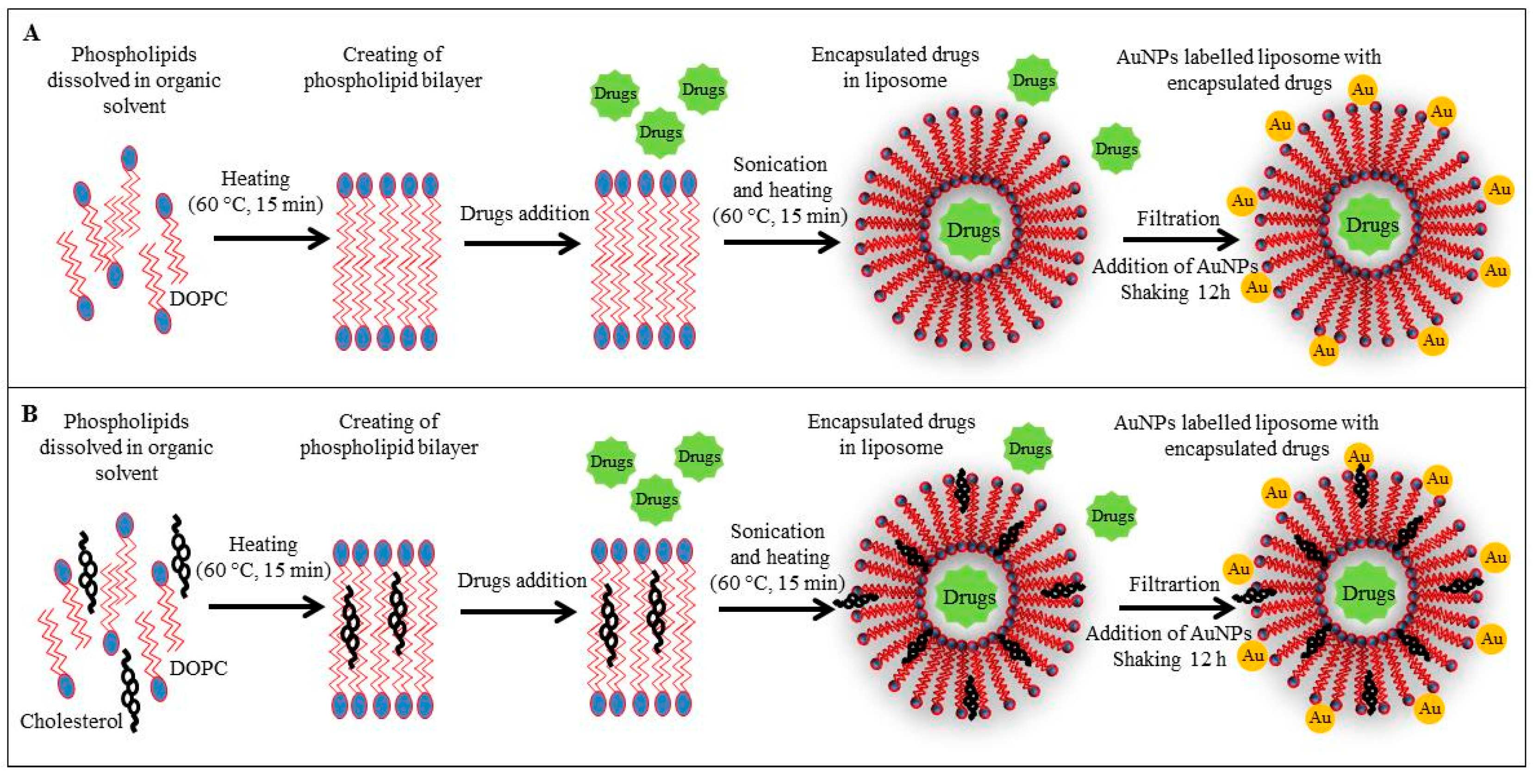
2.3. Preparation of Liposome Film and Liposome Encapsulating Doxorubicin, Ellipticine and/or Etoposide
2.4. Modification of Liposome Surface by AuNPs
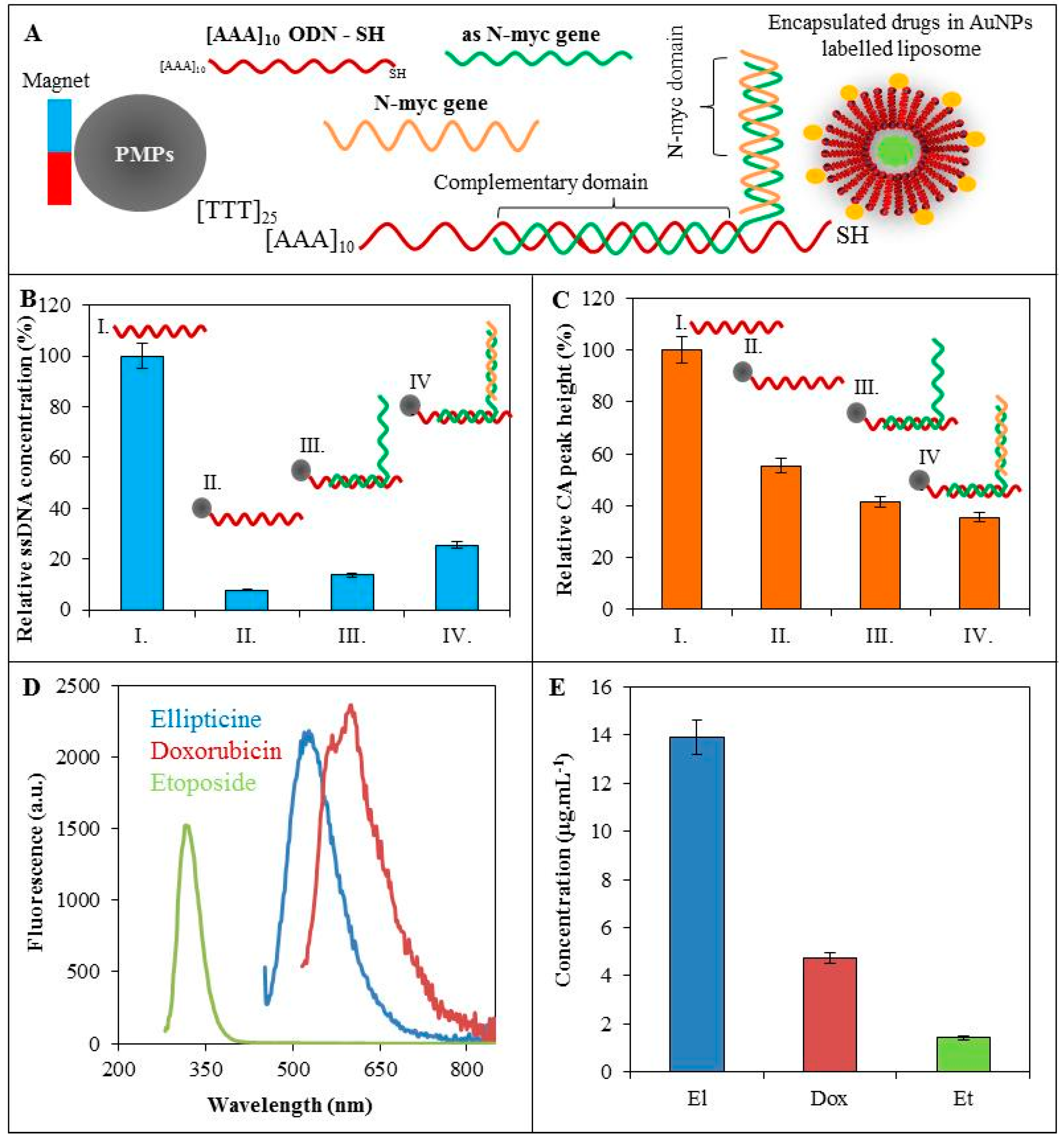
2.5. Amplification of Exon 2 of Human N-myc Gene
2.6. Isolation of AuNPs-Modified Liposomes Using Magnetic Microparticles
2.7. UV/VIS Spectroscopy
2.8. Determination of ODN-CA Peak
2.9. Descriptive Statistics
3. Results and Discussion
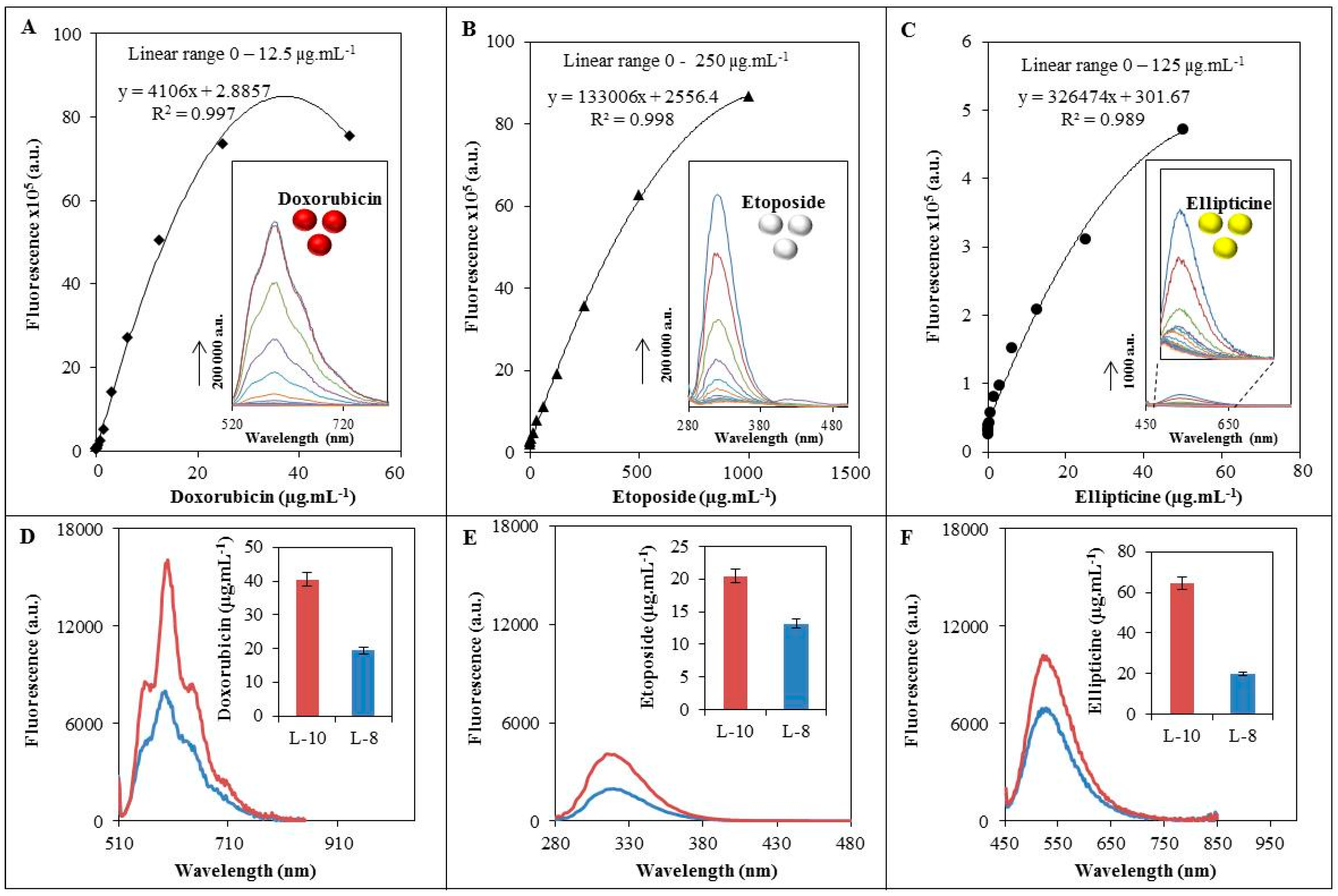
3.1. Characterization of Fluorescence Behaviour of Enclosed Drugs in Liposomes
3.2. Hybridization of ODN
3.3. Labelling of ODN
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.; Hobel, S.; Bakowsky, U.; Aigner, A. Liposome-polyethylenimine complexes for enhanced DNA and sirna delivery. Biomaterials 2010, 31, 6892–6900. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.A.; Wang, X.H.; Wang, Y.S.; Yang, L.; Hu, J.; Xiao, W.J.; Fu, A.; Cai, L.L.; Li, X.; Ye, X.; et al. Improved tumor-targeting drug delivery and therapeutic efficacy by cationic liposome modified with truncated bfgf peptide. J. Control. Release 2010, 145, 17–25. [Google Scholar] [PubMed]
- Villares, G.J.; Zigler, M.; Wang, H.; Melnikova, V.O.; Wu, H.; Friedman, R.; Leslie, M.C.; Vivas-Mejia, P.E.; Lopez-Berestein, G.; Sood, A.K.; et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering rna. Cancer Res. 2008, 68, 9078–9086. [Google Scholar] [PubMed]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery-liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Milla, P.; Dosio, F.; Cattel, L. Pegylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Bochot, A.; Fattal, E. Liposomes for intravitreal drug delivery: A state of the art. J. Control. Release 2012, 161, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. The carrier potential of liposomes in biology and medicine. N. Engl. J. Med. 1976, 295, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Long-circulating (sterically stabilized) liposomes for targeted drug-delivery. Trends Pharmacol. Sci. 1994, 15, 215–220. [Google Scholar] [CrossRef]
- Bangham, A.D. Review of lasic, liposomes: From physics to applications. Biophys. J. 1994, 67, 1358–1359. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Zhou, L.; Liu, Y.M.; Lu, Y. Safety and efficacy of paclitaxel liposome for elderly patients with advanced non-small cell lung cancer: A multi-center prospective study. Thorac. Cancer 2013, 4, 14–19. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Xu, H.Q.; Huang, X.E.; Qian, Y.D.; Xiang, J. Clinical comparison between paclitaxel liposome (lipusu®) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2591–2594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Feng, Q.; Dou, Z.P.; Yuan, W.; Sui, C.G.; Zhang, X.H.; Xia, G.M.; Sun, H.F.; Ma, J. Local targeted therapy of liver metastasis from colon cancer by galactosylated liposome encapsulated with doxorubicin. PLoS ONE 2013, 8, e73860. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin—Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.A.; Goh, S.L.; Feigenson, G.W.; Vogt, V.M. Hiv-1 gag protein can sense the cholesterol and acyl chain environment in model membranes. Proc. Natl. Acad. Sci. USA 2012, 109, 18761–18766. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, F.; Beke-Somfai, T.; Andreasson, J.; Norden, B. Interactions of a photochromic spiropyran with liposome model membranes. Langmuir 2013, 29, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Bhuvana, M.; Narayanan, J.S.; Dharuman, V.; Teng, W.; Hahn, J.H.; Jayakumar, K. Gold surface supported spherical liposome-gold nano-particle nano-composite for label free DNA sensing. Biosens. Bioelectron. 2013, 41, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Dilimon, V.S.; Rajalingam, S.; Delhalle, J.; Mekhalif, Z. Self-assembly mechanism of thiol, dithiol, dithiocarboxylic acid, disulfide and diselenide on gold: An electrochemical impedance study. Phys. Chem. Chem. Phys. 2013, 15, 16648–16656. [Google Scholar] [CrossRef] [PubMed]
- Nejdl, L.; Rodrigo, M.A.R.; Kudr, J.; Ruttkay-Nedecky, B.; Konecna, M.; Kopel, P.; Zitka, O.; Hubalek, J.; Kizek, R.; Adam, V. Liposomal nanotransporter for targeted binding based on nucleic acid anchor system. Electrophoresis 2014, 35, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Ahner, T.T.; Delissen, F.; Sokolov, S.; Emmerling, F.; Thunemann, A.F.; Kraehnert, R. Mechanism of gold nanoparticle formation in the classical citrate synthesis method derived from coupled in situ xanes and saxs evaluation. J. Am. Chem. Soc. 2010, 132, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Blauz, A.; Mockel, D.; Theek, B.; Kiessling, F.; Etrych, T.; Ulbrich, K.; van Bloois, L.; Storm, G.; Bartosz, G.; et al. Overcoming cellular multidrug resistance using classical nanomedicine formulations. Eur. J. Pharm. Sci. 2012, 45, 421–428. [Google Scholar] [PubMed]
- Huska, D.; Hubalek, J.; Adam, V.; Vajtr, D.; Horna, A.; Trnkova, L.; Havel, L.; Kizek, R. Automated nucleic acids isolation using paramagnetic microparticles coupled with electrochemical detection. Talanta 2009, 79, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Krejcova, L.; Dospivova, D.; Ryvolova, M.; Kopel, P.; Hynek, D.; Krizkova, S.; Hubalek, J.; Adam, V.; Kizek, R. Paramagnetic particles coupled with an automated flow injection analysis as a tool for influenza viral protein detection. Electrophoresis 2012, 33, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Jilkova, E.; Krejcova, L.; Cernei, N.; Hynek, D.; Ruttkay-Nedecky, B.; Sochor, J.; Kynicky, J.; Adam, V.; Kizek, R. Rapid superparamagnetic-beads-based automated immunoseparation of zn-proteins from staphylococcus aureus with nanogram yield. Electrophoresis 2013, 34, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Ryvolova, M.; Hynek, D.; Eckschlager, T.; Hodek, P.; Masarik, M.; Adam, V.; Kizek, R. Immunoextraction of zinc proteins from human plasma using chicken yolk antibodies immobilized onto paramagnetic particles and their electrophoretic analysis. Electrophoresis 2012, 33, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Krizkova, S.; Krejcova, L.; Hynek, D.; Gumulec, J.; Masarik, M.; Sochor, J.; Adam, V.; Hubalek, J.; Trnkova, L.; et al. Microfluidic tool based on the antibody-modified paramagnetic particles for detection of 8-hydroxy-2′-deoxyguanosine in urine of prostate cancer patients. Electrophoresis 2011, 32, 3207–3220. [Google Scholar] [PubMed]
- Blazkova, I.; Nguyen, V.H.; Dostalova, S.; Kopel, P.; Stanisavljevic, M.; Vaculovicova, M.; Stiborova, M.; Eckschlager, T.; Kizek, R.; Adam, V. Apoferritin modified magnetic particles as doxorubicin carriers for anticancer drug delivery. Int. J. Mol. Sci. 2013, 14, 13391–13402. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, M.; Krystkowiak, E.; Steer, R.P. The kinetics of fast fluorescence quenching processes. J. Photochem. Photobiol. A Chem. 1998, 117, 1–16. [Google Scholar] [CrossRef]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Cagdas, F.M.; Ertugral, N.; Bucak, S.; Atay, N.Z. Effect of preparation method and cholesterol on drug encapsulation studies by phospholipid liposomes. Pharm. Dev. Technol. 2011, 16, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Kominkova, M.; Guran, R.; Rodrigo, M.A.R.; Kopel, P.; Blazkova, I.; Chudobova, D.; Nejdl, L.; Heger, Z.; Ruttkay-Nedecky, B.; Zitka, O.; et al. Study of functional qualities of different types of tailored liposomes with encapsulated doxorubicin using electrochemical and optical methods. Int. J. Electrochem. Sci. 2014, 9, 2993–3007. [Google Scholar]
- Peng, L.; Minbo, H.; Fang, C.; Xi, L.; Chaocan, Z. The interaction between cholesterol and human serum albumin. Protein Pept. Lett. 2008, 15, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Smerkova, K.; Dostalova, S.; Skutkova, H.; Ryvolova, M.; Adam, V.; Provaznik, I.; Kizek, R. Isolation of xis gen fragment of lambda phage from agarose gel using magnetic particles for subsequent enzymatic DNA sequencing. Chromatographia 2013, 76, 329–334. [Google Scholar] [CrossRef]
- Kremplova, M.; Fialova, D.; Nejdl, L.; Hynek, D.; Trnkova, L.; Hubalek, J.; Kizek, R.; Adam, V. Influence of magnetic microparticles isolation on adenine homonucleotides structure. Materials 2014, 7, 1455–1472. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Merlos, M.A.R.; Krejcova, L.; Kopel, P.; Adam, V.; Kizek, R. Sequences of pandemic-causing viruses isolated and detected by paramagnetic particles coupled with microfluidic system and electrochemical detector. Int. J. Electrochem. Sci. 2013, 8, 12628–12642. [Google Scholar]
- Saiyed, Z.M.; Bochiwal, C.; Gorasia, H.; Telang, S.D.; Ramchand, C.N. Application of magnetic particles (Fe3O4) for isolation of genomic DNA from mammalian cells. Anal. Biochem. 2006, 356, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Gai, L.G.; Han, X.Y.; Hou, Y.H.; Chen, J.; Jiang, H.H.; Chen, X.C. Surfactant-free synthesis of Fe3O4@pani and Fe3O4@ppy microspheres as adsorbents for isolation of pcr-ready DNA. Dalton Trans. 2013, 42, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Mao, Q.X.; Liu, J.W.; Wang, J.H. Isolation/separation of plasmid DNA using hemoglobin modified magnetic nanocomposites as solid-phase adsorbent. Talanta 2012, 100, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Prodelalova, J.; Rittich, B.; Spanova, A.; Petrova, K.; Benes, M.J. Isolation of genomic DNA using magnetic cobalt ferrite and silica particles. J. Chromatogr. A 2004, 1056, 43–48. [Google Scholar] [CrossRef]
- Dai, X.W.; Yue, Z.L.; Eccleston, M.E.; Swartling, J.; Slater, N.K.H.; Kaminski, C.F. Fluorescence intensity and lifetime imaging of free and micellar-encapsulated doxorubicin in living cells. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 49–56. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalickova, S.; Nejdl, L.; Kudr, J.; Ruttkay-Nedecky, B.; Jimenez Jimenez, A.M.; Kopel, P.; Kremplova, M.; Masarik, M.; Stiborova, M.; Eckschlager, T.; et al. Fluorescence Characterization of Gold Modified Liposomes with Antisense N-myc DNA Bound to the Magnetisable Particles with Encapsulated Anticancer Drugs (Doxorubicin, Ellipticine and Etoposide). Sensors 2016, 16, 290. https://doi.org/10.3390/s16030290
Skalickova S, Nejdl L, Kudr J, Ruttkay-Nedecky B, Jimenez Jimenez AM, Kopel P, Kremplova M, Masarik M, Stiborova M, Eckschlager T, et al. Fluorescence Characterization of Gold Modified Liposomes with Antisense N-myc DNA Bound to the Magnetisable Particles with Encapsulated Anticancer Drugs (Doxorubicin, Ellipticine and Etoposide). Sensors. 2016; 16(3):290. https://doi.org/10.3390/s16030290
Chicago/Turabian StyleSkalickova, Sylvie, Lukas Nejdl, Jiri Kudr, Branislav Ruttkay-Nedecky, Ana Maria Jimenez Jimenez, Pavel Kopel, Monika Kremplova, Michal Masarik, Marie Stiborova, Tomas Eckschlager, and et al. 2016. "Fluorescence Characterization of Gold Modified Liposomes with Antisense N-myc DNA Bound to the Magnetisable Particles with Encapsulated Anticancer Drugs (Doxorubicin, Ellipticine and Etoposide)" Sensors 16, no. 3: 290. https://doi.org/10.3390/s16030290
APA StyleSkalickova, S., Nejdl, L., Kudr, J., Ruttkay-Nedecky, B., Jimenez Jimenez, A. M., Kopel, P., Kremplova, M., Masarik, M., Stiborova, M., Eckschlager, T., Adam, V., & Kizek, R. (2016). Fluorescence Characterization of Gold Modified Liposomes with Antisense N-myc DNA Bound to the Magnetisable Particles with Encapsulated Anticancer Drugs (Doxorubicin, Ellipticine and Etoposide). Sensors, 16(3), 290. https://doi.org/10.3390/s16030290








