A Review of Wearable Sensor Systems for Monitoring Body Movements of Neonates
Abstract
:1. Introduction
2. Methods
2.1. Literature Research Strategy
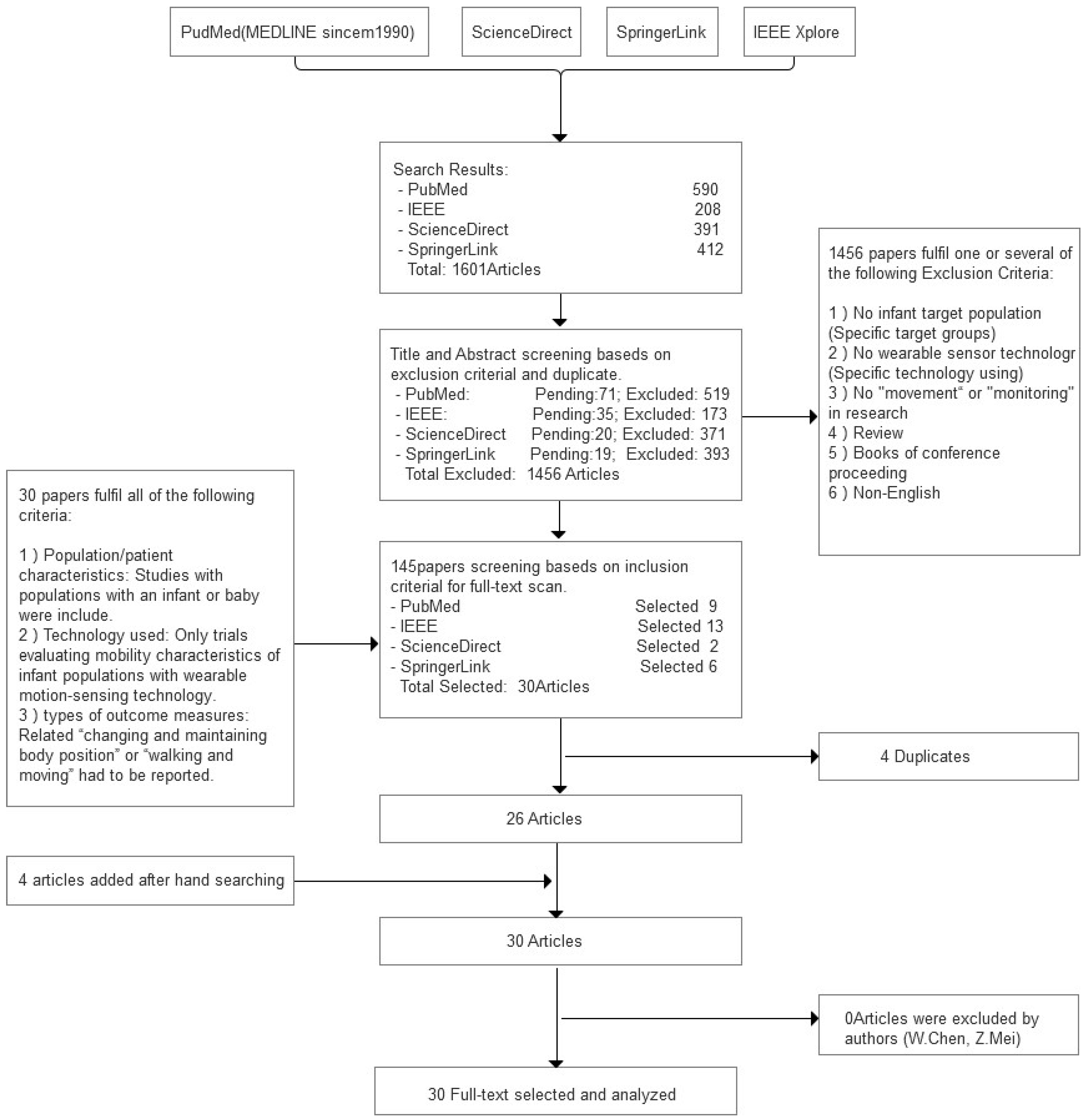
2.2. Study Selection Process
- No infant target population;
- No wearable sensor technology;
- No “movement” or “monitoring” in the research;
- Reviews;
- Books of conference proceeding;
- Language other than English.
- All studies with infants as subjects.
- Technology: wearable motion-sensing technology.
- Related “body movement” or “moving” or “motor pattern” had to be reported.
2.3. Defination of Keywords in This Review
2.4. Screening Process
3. Results
4. Discussion
4.1. Wearable Sensor Technologies for Infant Movement Monitoring
4.2. Clinical Relevance of Movement Monitoring in Infant with Wearable Sensor System
4.2.1. Infant Movement and Motor Pattern
4.2.2. Assessment Function of Cerebral Nervous System
4.2.3. Other Clinical Relevance
4.2.4. Motion Artifacts Reduction
4.3. System Design
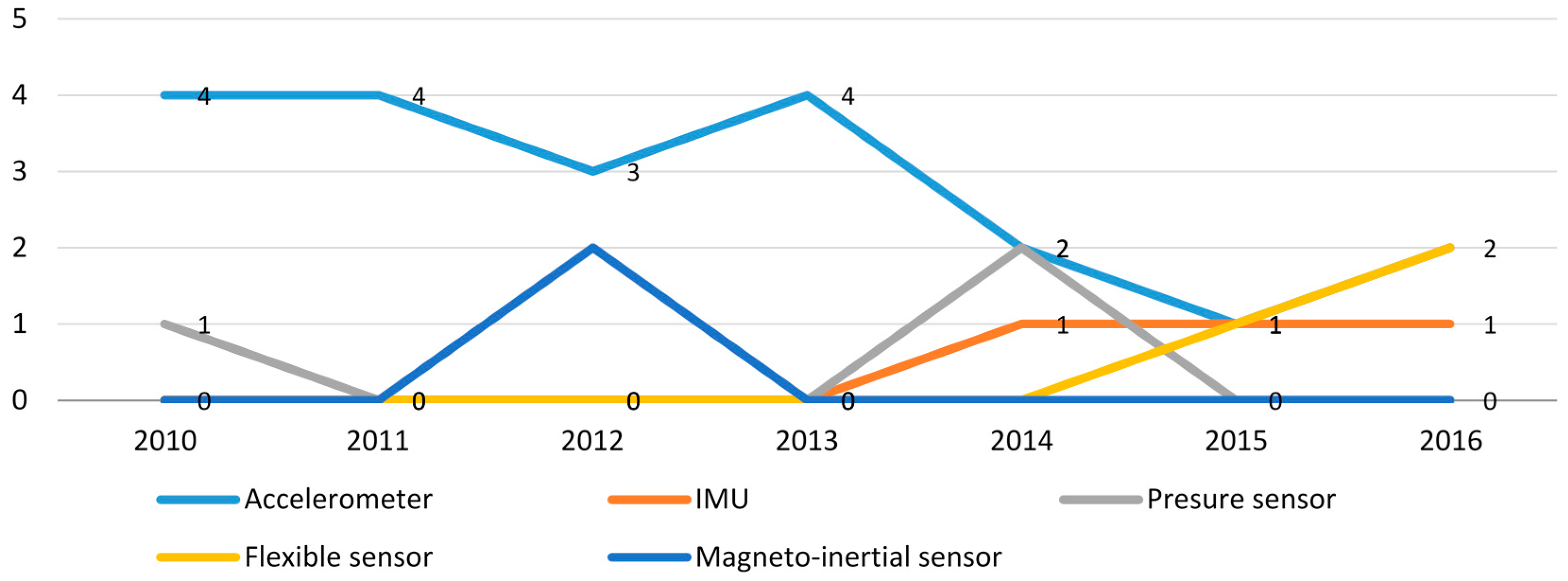
4.3.1. Tendency of Utilization of Wearable Sensors
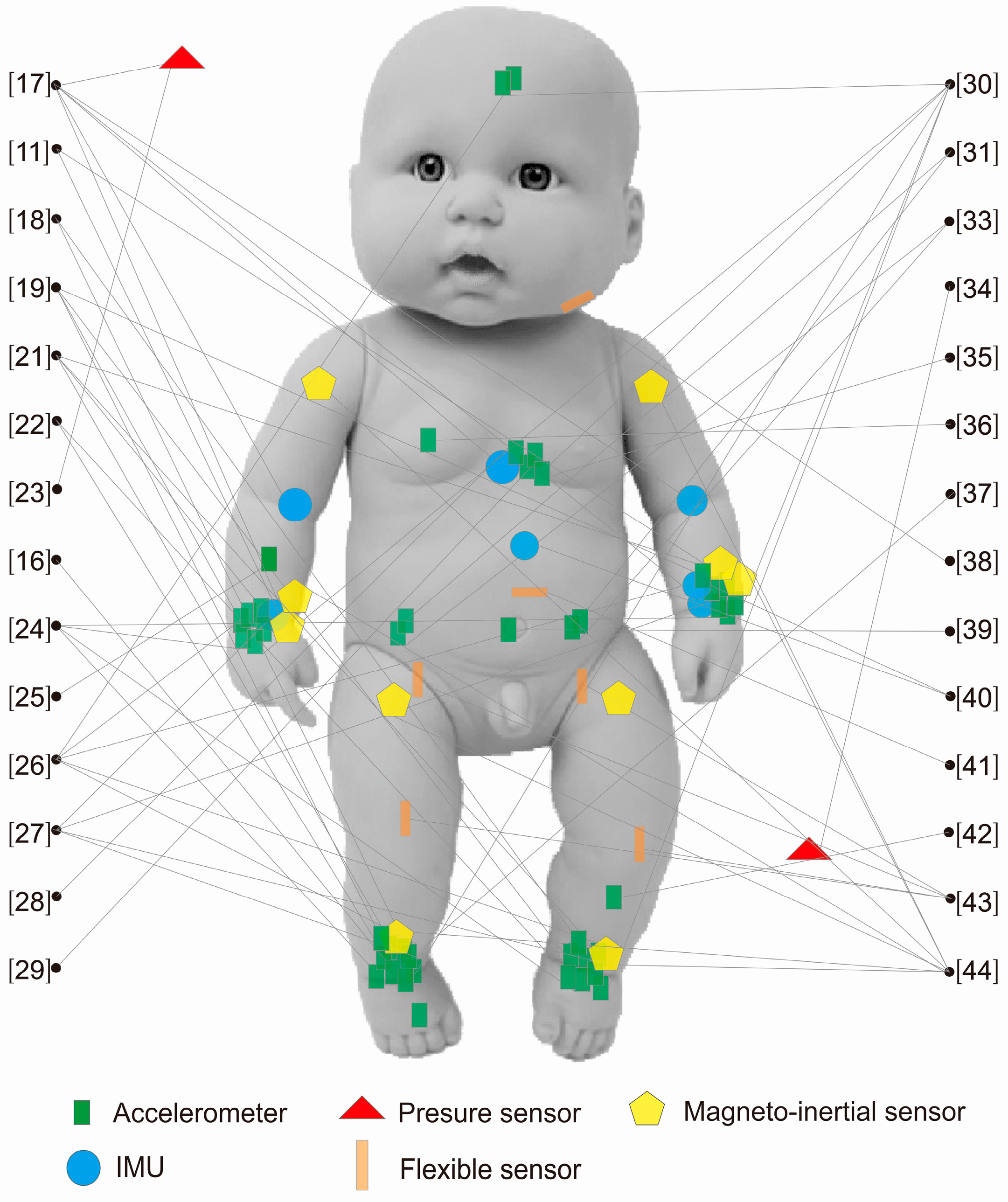
4.3.2. Exterior Structure
4.3.3. Design Criterion
- Be able to achieve continuous monitoring when the infant is inside an incubator or during Kangaroo mother care.
- Be non-intrusive and avoid disturbance of infants and avoid causes of stress or stimuli.
- Be safe to use in the NICU environment or at home.
- Provide appropriate feedback that is also interpretable for parents and doctors or related people on whether the system’s components are functioning correctly.
- Look friendly, playful, familiar, and attractive to gain a feeling of trust from parents and clinicians.
- Be scalable to include more monitoring functions, such as wireless communication and local signal processing.
- Be made of easy-to-remove non-washable parts.
5. Conclusions and Future Prospects
Conflicts of Interest
References
- Gruskin, A.; Williams, R.G.; McCabe, E.R.B.; Stein, F.; Strickler, J.; Chesney, R.W.; Mulvey, H.J.; Simon, J.L.; Alden, E.R. Final report of the FOPE II Pediatric Subspecialists of the Future Workgroup. Pediatrics 2000, 106, 1224–1244. [Google Scholar] [PubMed]
- Pickler, R.H.; McGrath, J.M.; Reyna, M.B.A.; McCain, N.; Lewis, M.M.; Cone, M.S.; Wetzel, P.; Best, A. A model of neurodevelopmental risk and protection for preterm infants. J. Perinat. Neonatal Nurs. 2010, 24, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Nzeh, D.A.; Erinle, S.A.; Saidu, S.A.; Pam, S.D. Transfontanelle Ultra-Sonography: An Invaluable Tool in the Assessment of the Infant Brain. Trop. Dr. 2004, 34, 226–227. [Google Scholar]
- Pfefferbaum, A.; Mathalon, D.H.; Sullivan, E.V.; Rawles, J.M.; Zipursky, R.B.; Lim, K.O. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994, 51, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hayakawa, F.; Okumura, A. Neonatal EEG: A powerful tool in the assessment of brain damage in preterm infants. Brain Dev. 1999, 21, 361–372. [Google Scholar] [CrossRef]
- Franceschini, M.A.; Thaker, S.; Themelis, G.; Krishnamoorthy, K.K.; Bortfeld, H.; Diamond, S.G.; Boas, D.A.; Arvin, K.; Grant, P.E. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatr. Res. 2007, 61, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M. Brain injury in the term infant. Semin. Perinatol. 2004, 28, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Rennie, J.M. Neonatal seizures. Eur. J. Pediatr. 1997, 156, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Levene, M. Neonatal seizures. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 78, F70–F75. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Heinz, F.R. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Taffoni, F.; Focaroli, V.; Formica, D.; Gugliemelli, E. Sensor-based technology in the study of motor skills in infants at risk for ASD. In Proceedings of the IEEE International Conference on Biomedical Robotics and Biomechatronic, Rome, Italy, 24–27 June 2012.
- Chan, M.; Estève, D.; Fourniols, J.-Y.; Escriba, C.; Campo, E. Smart wearable systems: Current status and future challenges. Artif. Intell. Med. 2012, 56, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, T.; Li, G.; Li, T.; Inoue, Y. Wearable Sensor Systems for Infants. Sensors 2015, 15, 3721–3749. [Google Scholar] [CrossRef] [PubMed]
- Marcroft, C. Movement Recognition Technology as a Method of Assessing Spontaneous General Movements in High Risk Infants. Front. Neurol. 2015, 5, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Wearable_technologyI (accessed on 28 January 2016).
- Lee, E. Baby by the numbers [Resources Tools]. IEEE. Spectr. 2015, 52, 24. [Google Scholar] [CrossRef]
- Rihar, A.; Mihelj, M.; Pasic, J.; Munih, M. Infant trunk posture and arm movement assessment using pressure mattress; inertial and magnetic measurement units (IMUs). J. Neuroeng. Rehabil. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Trujillo-Priego, I.A. Daily Quantity of Infant Leg Movement. Wearable Sensor Algorithm and Relationship to Walking Onset. Sensors 2015, 15, 19006–19020. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Patterson, D.J. Involuntary gesture recognition for predicting cerebral palsy in high-risk infants. In Proceedings of the International Symposium on Wearable Computers (ISWC), Seoul, Korea, 10–13 October 2010; pp. 1–8.
- Saadatian, E.; Iyer, S.P.; Lihui, C. Low cost infant monitoring and communication system. In Proceedings of the 2011 IEEE Colloquium on Humanities, Science and Engineering (CHUSER), Penang, Malaysia, 5–6 December 2011; pp. 503–508.
- Heinze, F.; Hesels, K.; Breitbach-Faller, N.; Schmitz-Rode, T.; Disselhorst-Klug, C. Movement analysis by accelerometry of newborns and infants for the early detection of movement disorders due to infantile cerebral palsy. Med. Biol. Eng. Comput. 2010, 48, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Gima, H.; Ohgi, S.; Morita, S.; Karasuno, H.; Fujiwara, T.; Abe, K. A dynamical system analysis of the development of spontaneous lower extremity movements in newborn and young infants. J. Physiol. Anthropol. 2011, 30, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Boughorbel, S.; Bruekers, F.; Breebaart, J. Baby-Posture Classification from Pressure-Sensor Data. In Proceedings of the 2010 20th International Conference on Pattern Recognition (ICPR), Istanbul, Turkey, 23–26 August 2010; pp. 556–559.
- Fan, M.; Gravem, D.; Cooper, D.M.; Patterson, D.J. Augmenting Gesture Recognition with Erlang-cox Models to Identify Neurological Disorders in Premature Babies. In Proceedings of the 2012 ACM Conference on Ubiquitous Computing, Pittsburgh, PA, USA, 5–8 September 2012; pp. 411–420.
- Waldmeier, S.; Grunt, S.; Delgado-Eckert, E.; Latzin, P.; Steinlin, M.; Fuhrer, K.; Frey, U. Correlation properties of spontaneous motor activity in healthy infants: A new computer-assisted method to evaluate neurological maturation. Exp. Brain Res. 2013, 226, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Gravem, D.; Singh, M.; Chen, C.; Rich, J.; Vaughan, J.; Goldberg, K.; Waffarn, F.; Chou, P.; Cooper, D.; Reinkensmeyer, D.; et al. Assessment of Infant Movement with a Compact Wireless Accelerometer System. J. Med. Devices 2012, 6, 021013. [Google Scholar] [CrossRef]
- Abney, D.H.; Warlaumont, A.S.; Hanussman, A.; Ross, J.M.; Wallot, S. Using nonlinear methods to quantify changes in infant limb movements and vocalizations. Front. Psychol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Brittelli, J.; Lehmann, C. Wireless Infant Monitoring Device for the prevention of sudden infant death syndrome. In Proceedings of the 2014 11th International Conference & Expo on IEEE Emerging Technologies for a Smarter World (CEWIT), Melville, NY, USA, 29–30 October 2014; pp. 1–4.
- Kaushik, A.; Singh, R. Infant Monitoring and Fall Avoidance System using Tri-Axial Accelerometer and ARM7 Microcontroller. Int. J. Comput. Appl. 2013, 78, 40–44. [Google Scholar] [CrossRef]
- Hayes, G.R.; Patterson, D.J.; Singh, M.; Gravem, D.; Rich, J. Supporting the transition from hospital to home for premature infants using integrated mobile computing and sensor support. Pers. Ubiquitous Comput. 2011, 15, 871–885. [Google Scholar] [CrossRef]
- Jourand, P.; De Clercq, H.; Puers, R. Robust monitoring of vital signs integrated in textile. Sens. Actuators A Phys. 2010, 161, 288–296. [Google Scholar] [CrossRef]
- López, G.; López, M.; Guerrero, L.A.; Nugent, C.; Coronato, A.; Bravo, J. An Augmented Object Prototype for Helping to Prevent the Sudden Infant Death Syndrome. In Proceedings of the International Workshop on Ambient Assisted Living, Carrillo, Costa Rica, 2–6 December 2013; Springer: Heidelberg, Germany, 2013; Volume 8277, pp. 132–135. [Google Scholar]
- De Clercq, H.; Jourand, P.; Puers, R. Textile Integrated Monitoring System for Breathing Rhythm of Infants. In Proceedings of the XII Mediterranean Conference on Medical and Biological Engineering and Computing, Chalkidiki, Greece, 27–30 May 2010; Volume 29, pp. 525–528.
- Donati, M.; Cecchi, F.; Bonaccorso, F.; Branciforte, M.; Dario, P.; Vitiello, N. A modular sensorized mat for monitoring infant posture. Sensors 2013, 14, 510–531. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.; Cabral, J.; Rocha, A.M. A smart wearable system for sudden infant death syndrome monitoring. In Proceedings of the 2016 IEEE International Conference on Industrial Technology (ICIT), Taipei, Taiwan, 14–17 March 2016; IEEE: New York, NY, USA, 2016. [Google Scholar]
- Bouwstra, S.; Chen, W.; Oetomo, S.B.; Feijs, L.M.G.; Cluitmans, P.J.M. Designing for reliable textile neonatal ECG monitoring using multi-sensor recordings. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: New York, NY, USA, 2011. [Google Scholar]
- Leier, M.; Jervan, G. Sleep apnea pre-screening on neonates and children with shoe integrated sensors. In Proceedings of the NORCHIP, Vilnius, Lithuania, 11–12 November 2013; IEEE: New York, NY, USA, 2013. [Google Scholar]
- Farooq, M.; Chandler-Laney, P.; Hernandez-Reif, M.; Sazonov, E. Monitoring of infant feeding behavior using a jaw motion sensor. J. Healthc. Eng. 2015, 6, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.; Eftestøl, T.; Engan, K.; Eilevstjønn, J.; Yarrot, L.B.; Linde, J.E.; Ersdal, H. Detection of Activities during Newborn Resuscitation Based on Short-Time Energy of Acceleration Signal. In Proceedings of the International Conference on Image and Signal Processing, Dubai, United Arab Emirates, 18–20 November 2016; Springer: Heidelberg, Germany, 2016. [Google Scholar]
- Rihar, A.; Sgandurra, G.; Beani, E.; Cecchi, F.; Pašič, J.; Cioni, G.; Dario, P.; Mihelj, M.; Munih, M. CareToy: Stimulation and Assessment of Preterm Infant’s Activity Using a Novel Sensorized System. Ann. Biomed. Eng. 2016, 44, 3593–3605. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Dietzel, A. Skin attachable flexible sensor array for respiratory monitoring. Sens. Actuators A Phys. 2016, 250, 138–144. [Google Scholar] [CrossRef]
- Galland, B.C.; Kennedy, G.J.; Mitchell, E.A.; Taylor, B.J. Algorithms for using an activity-based accelerometer for identification of infant sleep–wake states during nap studies. Sleep Med. 2012, 13, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.; Polygerinos, P.; Walsh, C.; Goldfield, E. Smart and Connected Actuated Mobile and Sensing Suit to Encourage Motion in Developmentally Delayed Infants. J. Med. Devices 2015, 9, 030914. [Google Scholar] [CrossRef]
- Karch, D.; Kang, K.S.; Wochner, K.; Philippi, H.; Hadders-Algra, M.; Pietz, J.; Dickhaus, H. Kinematic assessment of stereotypy in spontaneous movements in infants. Gait Posture 2012, 36, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, N.; Ayazi, F.; Najafi, K. Micromachined inertial sensors. Proc. IEEE 1998, 86, 1640–1659. [Google Scholar] [CrossRef]
- Seel, T.; Raisch, J.; Schauer, T. IMU-based joint angle measurement for gait analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Q. Inertial sensor-based methods in walking speed estimation: A systematic review. Sensors 2012, 12, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Asokanthan, S.F.; Wang, T. Instabilities in a MEMS gyroscope subjected to angular rate fluctuations. J. Vib. Control 2009, 15, 299–320. [Google Scholar] [CrossRef]
- Casey, P.H.; Bradley, R.H.; Whiteside-Mansell, L.; Barrett, K.; Gossett, J.M.; Simpson, P.M. Effect of early intervention on 8-year growth status of low-birth-weight preterm infants. Arch. Pediatr. Adolesc. Med. 2009, 163, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.C.; Maehle, V.; Reid, J. The effects of an early physical therapy intervention for very preterm; very low birth weight infants: A randomized controlled clinical trial. Pediatr. Phys. Ther. 2005, 17, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.J.; Doyle, L.W.; Boyd, R.N. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev. Med. Child Neurol. 2008, 50, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Cioni, G.; Prechtl, H.F.; Ferrari, F.; Paolicelli, P.B.; Einspieler, C.; Roversi, M.F. Which better predicts later outcome in full-term infants: Quality of general movements or neurological examination. Early Hum. Dev. 1997, 50, 71–85. [Google Scholar] [CrossRef]
- Ferrari, F.; Cioni, G.; Prechtl, H.F.R. Qualitative changes of general movements in preterm infants with brain lesions. Early Hum. Dev. 1990, 23, 193–231. [Google Scholar] [CrossRef]
- Hadders-Algra, M. General movements: A window for early identification of children at high risk for developmental disorders. J. Pediatr. 2004, 145, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Bos, A.F. Differential effects of brain lesions and systemic disease on the quality of general movements: A preliminary report. Early Hum. Dev. 1993, 34, 39–45. [Google Scholar] [CrossRef]
- WIKI. 2015. Available online: https://en.wikipedia.org/wiki/Cerebral_palsy (accessed on 28 January 2016).
- Trapp, B.E. National Institute of Neurological Disorders and Stroke. J. Consum. Health Internet 2010, 14, 167–174. [Google Scholar] [CrossRef]
- Ferrari, F.; Cioni, G.; Einspieler, C.; Rocersi, M.F.; Bos, A.F.; Paolicelli, P.B.; Ranzi, A.; Oversi, H.F.R. CRamped synchronized general movements in preterm infants as an early marker for cerebral palsy. Arch. Pediatr. Adolesc. Med. 2002, 156, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Cui, X. The Firmware Development of a Portable Inertial Measurement Unit (IMU). 2014. Available online: https://opus.lib.uts.edu.au/handle/10453/35940 (accessed on 12 December 2016).
- Nordli, D.R.; Bazil, C.W.; Scheuer, M.L.; Pedley, T.A. Recognition and Classification of Seizures in Infants. Epilepsia 1997, 38, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Lockman, J.; Fisher, R.S.; Olson, D.M. Detection of seizure-like movements using a wrist accelerometer. Epilepsy Behav. 2011, 20, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Kamalizonouzi, B. Optimal Inertial Sensor Placement and Motion Detection for Epileptic Seizure Patient Monitoring. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2012. [Google Scholar]
- Van de Vel, A.; Cuppens, K.; Bonroy, B.; Milosevic, M.; Van Huffel, S.; Vanrumste, B.; Lagae, L.; Ceulemans, B. Long-term home monitoring of hypermotor seizures by patient-worn accelerometers. Epilepsy Behav. 2013, 26, 118–225. [Google Scholar] [CrossRef] [PubMed]
- Decaigny, A.S.; Cuppens, K.; Lagae, L.; Ceulemans, B.; Van Huffel, S.; Vanrumste, B. Accelerometers used for the detection of nocturnal frontal lobe seizures in pediatric patients. In Proceedings of the European Conference on the Use of Modern Information and Communication (ECUMICT), Ghent, Belgium, 25–26 March 2010; pp. 331–342.
- Lundqvist-Persson, C.; Lau, G.; Nordin, P.; Bona, E.; Sabel, K.G. Preterm infants’ early developmental status is associated with later developmental outcome. Acta Paediatr. 2012, 101, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Mayo, N.E. The Effect of Physical Therapy for Children with Motor Delay and Cerebral Palsy. Am. J. Phys. Med. Rehabil. 1991, 70, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Al-Dasoqi, N.; Mason, A.; Shaw, A.; Al-Shamma’a, A.I. Preventing cot death for infants in day care. In Proceedings of the 2010 IEEE Sensors Applications Symposium (SAS), Limerick, Ireland, 23–25 February 2010.
- Khan, I.M.; Jabeur, N.; Khan, M.Z.; Mokhtar, H. An overview of the impact of wireless sensor networks in medical health care. In Proceedings of the 1st International Conference on Computing and Information Technology (ICCT), Al-Madinah Al-Munawwarah, Saudi Arabia, 12–14 March 2012; pp. 576–580.
- De Jonge, G.A.; Engelberts, A.C.; Koomen-Liefting, A.J.; Kostense, P.J. Cot death and prone sleeping position in The Netherlands. Br. Med. J. 1989, 298, 722. [Google Scholar] [CrossRef]
- Dwyer, T.; Ponsonby, A.-L. Sudden Infant Death Syndrome and Prone Sleeping Position. Ann. Epidemiol. 2009, 19, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.; Engelberts, A.C.; Bettelheim, K.A.; Smith, H.; Goldwater, P.N.; Morris, J.A.; Murrell, T.G.C.; Sweet, C.; Weaver, S.A. Sleeping position and cot deaths. Lancet 1991, 338, 192. [Google Scholar] [CrossRef]
- American SIDS Institute Website. 2010. Available online: http://www.sids.org (accessed on 12 December 2016).
- Sudharsanan, S.; Karthikeyan, B. The design of a real-time accelerometer-based baby sleeping position monitoring and correction system. Int. J. Biomed. Eng. Technol. 2013, 12, 189–198. [Google Scholar] [CrossRef]
- Hung, P.D.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Yen, P.T.N. Estimation of respiratory waveform using an accelerometer. In Proceedings of the 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 14–17 May 2008; pp. 1493–1496.
- Clifford, G.D.; Long, W.J.; Moody, G.B.; Szolovits, P. Robust parameter extraction for decision support using multimodal intensive care data. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2009, 367, 411–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourbakis, N.G. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans. Syst. Man Cybern. C 2010, 40, 1–12. [Google Scholar]
- Bouwstra, S.; Chen, W.; Feijs, L.; Oetomo, S.B. Smart Jacket Design for Neonatal Monitoring with Wearable Sensors. In Proceedings of the Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 162–167.
- Yang, C.C.; Hsu, Y.L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef] [PubMed]
| Infant | Infant OR Baby OR Neonatal OR Newborn |
|---|---|
| AND | |
| Movement | “Seizure activity” OR Convulsion OR “Motor behavior” OR Movement OR Position OR Motion OR Moving |
| AND | |
| Monitoring | Monitoring OR Feedback |
| AND | |
| Wearable | Wearable OR Mobile OR Ambulatory OR garment OR soft suit OR exosuit |
| NEAR | |
| Sensor | Accelerometer OR “Motion sensing” OR “Activity sensing” OR Gyroscope OR MEMS OR IMUs OR bend sensor OR flexible sensor |
| Research Work | Year | Sensor | Placement | Form | Evaluation | Purpose |
|---|---|---|---|---|---|---|
| Rihar et al. [17] | 2014 | 6 Wireless IMUs, 2 pressure mattresses | Trunk and arm | Silicone bracelets | Technical experiment (test baby doll) technical report user test | Infant motor pattern assessment |
| Taffoni et al. [11] | 2012 | 2 Wired magneto-inertial sensor | Wrist | N/A | Technical experiment | Study motor skill at risk for autism spectrum (ASD) |
| Smith et al. [18] | 2015 | 2 Inertial movement sensor(Opals, APDM) IMUs | Leg | Placed sensor on each leg using knee socks | Clinical test (n = 12) | Quantification of daily infant leg movements |
| Singh et al. [19] | 2010 | 4 Custom Accelerometer (Eco) | Wrist and ankle | N/A | Clinical test (n = 10) | Predict CP |
| Saadatian et al. [20] | 2011 | 1 Accelerometer | N/A | Wearable hardware gadget | Technical experiment | Baby care |
| Heinze et al. [21] | 2010 | 4 Accelerometer | Extremities | N/A | Clinical test (n = 23) | Predict CP |
| Gima et al. [22] | 2011 | 2 Accelerometer | Ankle | N/A | Clinical test (n = 8) | Infant motor pattern assessment |
| Boughorbel et al. [23] | 2010 | 4 Pressure sensitive sensor | N/A | Mat | Technical experiment, Usability Evaluation (n = 1) | Infant care/SIDS |
| Lee, E. [16] | 2015 | 1 Accelerometer | Ankle | Ankle band | Commercial product | Baby safety |
| Fan et al. [24] | 2012 | 4 Accelerometer | Wrists and ankles | Clothes bands | Clinical validation (n = 10) | Infant motor pattern assessment/predict CP |
| Waldmeier et al. [25] | 2013 | 1 Accelerometer | Hand | Fixed to the infant with a tape | Preclinical test, Usability Evaluation (n = 22) | Infant motor pattern assessment |
| Gravem et al. [26] | 2012 | 5 Accelerometer | Ankle, wrists and forehead | Cloth bands | Clinical test (n = 10) Comparison Experiment | Infant motor pattern assessment/diagnosis CP |
| Abney et al. [27] | 2014 | 4 Accelerometer | Wrist and ankle | N/A | Preclinical test, Usability Evaluation (n = 2) | Characterizations of infant behavioral development |
| Lin et al. [28] | 2014 | 1 Accelerometer | Chest | Soft belt | Technical experiment | Prevent SIDS |
| Kaushik et al. [29] | 2013 | 1 Accelerometer | Chest | Jacket | Technical experiment | Fall protection |
| Hayes et al. [30] | 2011 | 5 Custom Accelerometer (Eco) | Ankle, wrists and forehead | Cloth bands | Preclinical test, Usability Evaluation (n = 10) | Infant motor pattern assessment/Predict CP |
| Jourand et al. [31] | 2010 | 2 Accelerometer | Abdomen | N/A | Technical experiment | Monitor SIDS |
| López et al. [32] | 2013 | 1 Accelerometer | N/A | Bear gadget | N/A | Prevent SIDS |
| Clercq et al. [33] | 2010 | 2 Accelerometer | Abdomen | N/A | Technical experiment | Infant care/SIDS |
| Donati et al. [34] | 2014 | 768 Pressure Sensor | N/A | Mat | Preclinical test, Usability Evaluation (n = 1) | Infant motor pattern |
| Fernandes [35] | 2016 | 1 Accelerometer | Chest | Belt | Technical experiment | Monitor SIDS |
| Bouwstra, S et al. [36] | 2011 | 1 Accelerometer | Right chest | Smark Jacket | Technical experiment | Motion artifacts reduction |
| Leier et al. [37] | 2013 | 1 Accelerometer | Foot | Shoe | N/A | Baby safety |
| Farooq et al. [38] | 2015 | 1 Jew Motion Sensor/Flexible sensor | Jaw | N/A | Clinical validation (n = 10) | Feeding Behavior |
| Huyen et al. [39] | 2016 | 1 Accelerometer | Abdomen | Belt | Technical experiment | Baby safety |
| Rihar et al. [40] | 2016 | 2IMU | Trunk and wrist | Bracelets and chest strap | Technical experiment | Infant motor development assessment/early intervention treatment |
| Koch et al. [41] | 2016 | Flexible 6 × 6 sensor | Abdomen | N/A | Technical experiment | Respiratory monitoring |
| Galland et al. [42] | 2012 | 1 Accelerometer | Shin | N/A | Clinical validation (n = 33) | Sleep state monitoring |
| Rogers et al. [43] | 2015 | 4 Joint angle sensors/Flexible sensor | Knees and hips | Sensing suit | Preclinical test, Usability Evaluation (n = 1) | Early intervention treatment |
| Karch et al. [44] | 2012 | Electromagnetic tracking system | upper and lower limb | N/A | Preclinical test (n = 75) | Predict CP |
| Category | Discussed by Papers |
|---|---|
| IMU | [17,18,40] |
| Accelerometer | [16,19,20,21,22,24,25,26,27,28,29,30,31,32,33,35,36,37,39,42] |
| Magneto-inertial | [11,44] |
| Pressure sensor | [17,23,34] |
| Flexible sensor | [38,41,43] |
| Purpose | Discussed by Papers |
|---|---|
| Movement and motor pattern development | [17,18,21,22,23,25,26,27,30,38,40,42,43] |
| Cerebral palsy | [19,24,44] |
| Sleep safe/breathing rhythm/Sudden infant death syndrome (SIDS)/Prevent falls of infants/Autism spectrum disorders (ASD) | [11,16,20,28,29,31,32,33,34,35,37,39,41] |
| GM Type | Period of Presence in Weeks’ PMA | Description |
|---|---|---|
| Preterm GMs | From ± 28 weeks to 36–38 weeks | Great variation over time, more proximal than that in earlier days and are characterized by small to moderate amplitude and slow to moderate speed |
| Writhing GMs | From 36–38 weeks to 46–52 weeks | Seem to be somewhat slower and to show less participation of the pelvis and trunk. |
| Fidgety GMs | From 46–52 weeks to 54–58 weeks | Consists of a continuous flow of small and elegant movements, occur irregularly all over the body, head, trunk, and limbs participate to a similar extent |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Xue, M.; Mei, Z.; Bambang Oetomo, S.; Chen, W. A Review of Wearable Sensor Systems for Monitoring Body Movements of Neonates. Sensors 2016, 16, 2134. https://doi.org/10.3390/s16122134
Chen H, Xue M, Mei Z, Bambang Oetomo S, Chen W. A Review of Wearable Sensor Systems for Monitoring Body Movements of Neonates. Sensors. 2016; 16(12):2134. https://doi.org/10.3390/s16122134
Chicago/Turabian StyleChen, Hongyu, Mengru Xue, Zhenning Mei, Sidarto Bambang Oetomo, and Wei Chen. 2016. "A Review of Wearable Sensor Systems for Monitoring Body Movements of Neonates" Sensors 16, no. 12: 2134. https://doi.org/10.3390/s16122134






