An Ionic 1,4-Bis(styryl)benzene-Based Fluorescent Probe for Mercury(II) Detection in Water via Deprotection of the Thioacetal Group
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Mercury(II) Ion Detection Protocol
2.3. Synthesis
2.3.1. 4-Bis(ethylthio)Methylbenzaldehyde (5)
2.3.2. Synthesis of Neutral 1,4-Bis(Styryl)Benzene-Based Mercury Probe (M1)
2.3.3. Synthesis of Quaternized Ionic 1,4-Bis(Styryl)Benzene-Based Mercury (II) Probe (M1Q)
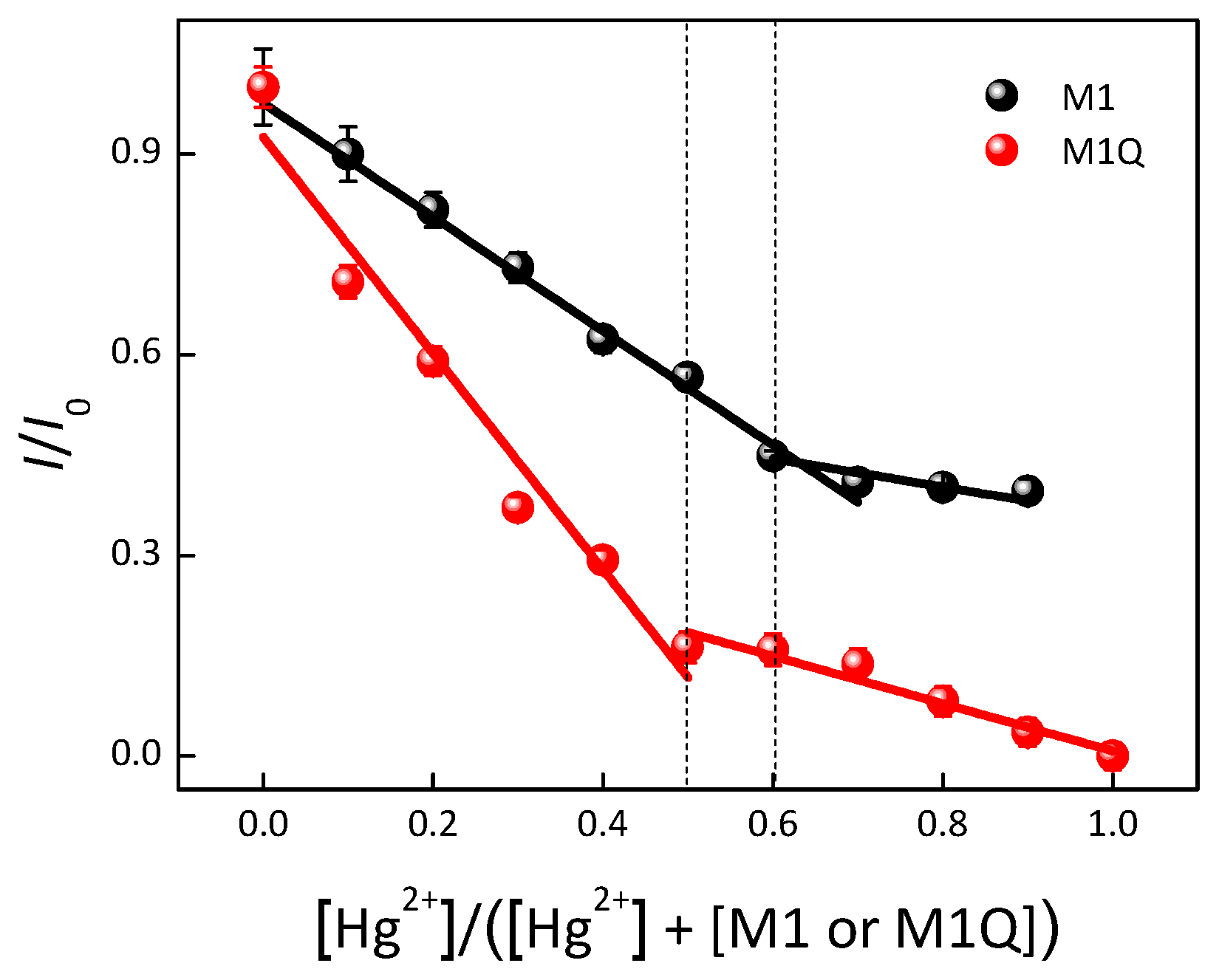
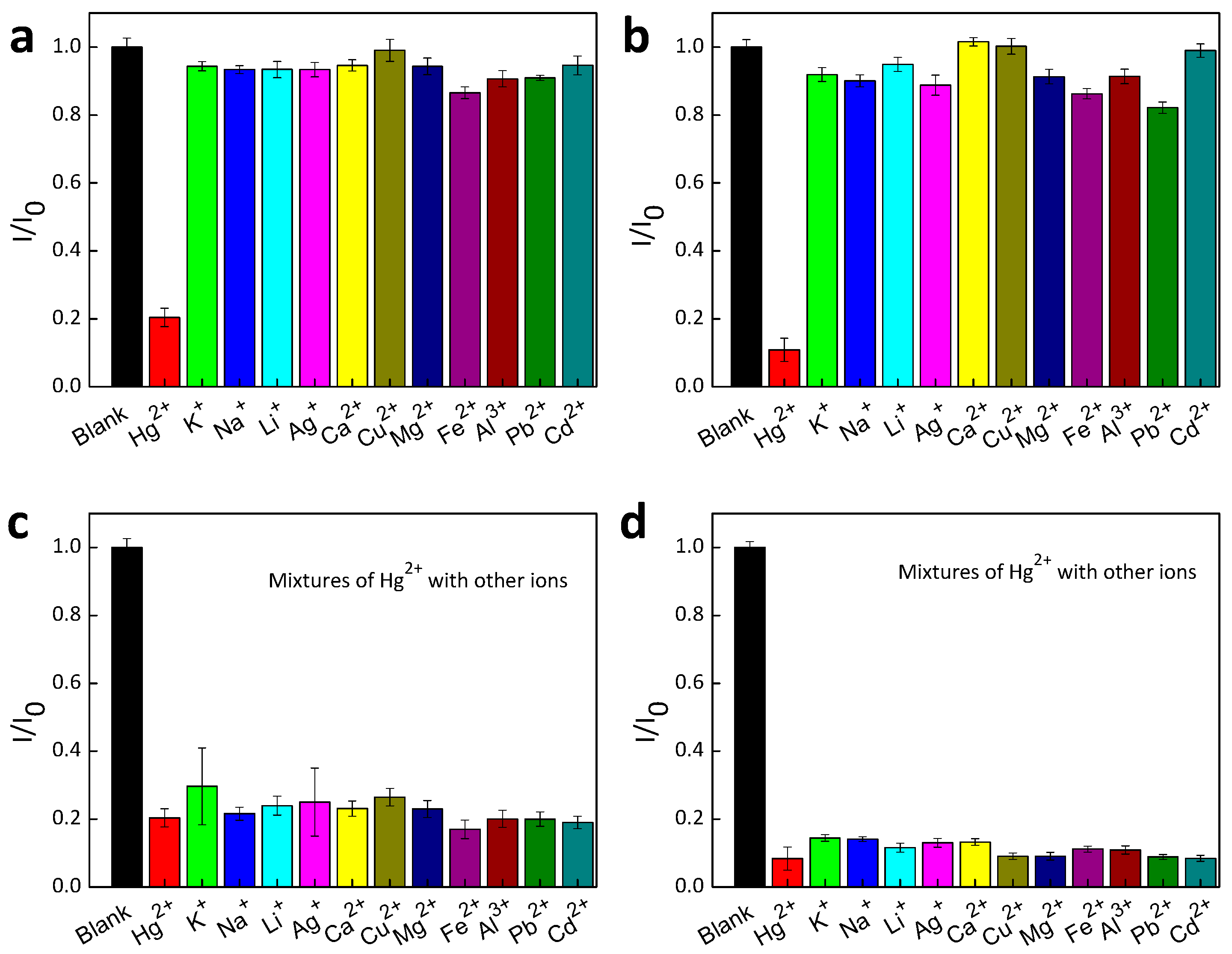
3. Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Shirinfar, B.; Ahmed, N.; Park, Y.S.; Cho, G.-S.; Youn, I.S.; Han, J.-K.; Nam, H.G.; Kim, K.S. Selective Fluorescent Detection of RNA in Living Cells by Using Imidazolium-Based Cyclophane. J. Am. Chem. Soc. 2013, 135, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Park, Y.J.; Jung, K.H.; Lee, K.-H. Ratiometric Detection of Nanomolar Concentrations of Heparin in Serum and Plasma Samples Using a Fluorescent Chemosensor Based on Peptides. Anal. Chem. 2014, 86, 6580–6586. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Liu, B.; Wang, Z.; Zhang, Z. Imprinting of Molecular Regcognition Sites on Nanostructures and Its Applications in Chemosensors. Sensors 2008, 8, 8291–8320. [Google Scholar] [CrossRef] [PubMed]
- Anbu, S.; Kamalraj, S.; Jayabaskaran, C.; Mukherjee, P.S. Naphthalene Carbohydrazone Based Dizinc(II) Chemosensor for a Pyrophosphate Ion and Its DNA Assessment Application in Polymerase Chain Reaction Products. Inorg. Chem. 2013, 52, 8294–8296. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury-Current Exposures and Clinical Manidestation. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Lash, L.H. Cystine Alters the Renal and Hepatic Disposition of Inorganic Mercury and Plasma Thiol Status. Toxicol. Appl. Pharmacol. 2006, 214, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M.; Zalups, R.K.; Fowler, B.A. Mercury. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Elsevier: New York, NY, USA, 2007; Chapter 33; pp. 675–729. [Google Scholar]
- Jӓrup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Chu, P.; Porcella, D.B. Mercury Stack Emissions from U.S. Electric Utility Power Plants. Water Air Soil Pollut. 1995, 80, 135–144. [Google Scholar] [CrossRef]
- Malm, O. Gold mining as a source of mercury exposure in the Brazillian Amazon. Environ. Res. Sect. A 1998, 77, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Boylan, H.M.; Cain, R.D.; Kingston, H.M. A new method to assess mercury emissions: A study of three coal-fired electric-generating power station configurations. J. Air Waste Manage. Assoc. 2003, 53, 1318–1325. [Google Scholar] [CrossRef]
- Restriction of Hazardous Substances in Electrical and Electronic Equipment. Available online: http://eur-lex.europa.eu/eli/dec/2009/443/oj (accessed on 27 September 2016).
- United States Environmental Protection Agency. Available online: https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-water-contaminants (accessed on 27 September 2016).
- World Health Organization. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/mercuryfinal.pdf (accessed on 27 September 2016).
- Li, K.-B.; Wang, H.; Zang, Y.; He, X.-P.; Li, J.; Chen, G.-R.; Tian, H. One-Step Click Engineering Considerably Ameliorates the Practicality of an Unqualified Rhodamine Probe. ACS Appl. Mater. Interfaces 2014, 6, 19600–19605. [Google Scholar] [CrossRef] [PubMed]
- Neupane, L.N.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Selective and Sensitive Detection of Heavy Metal Ions in 100% Aqueous Solution and Cells with a Fluorescence Chemosensor Based on Peptide Using Aggregation-Induced Emission. Anal. Chem. 2016, 88, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Qu, W.J.; Gao, G.Y.; Shi, B.B.; Wu, G.Y.; Wei, T.B.; Lin, Q.; Yao, H. A Highly Selective Dual-Channel Chemosensor for Mercury Ions: Utilization of the Mechanism of Intramolecular Charge Transfer Blocking. New J. Chem. 2014, 38, 5075–5080. [Google Scholar] [CrossRef]
- Bhowmick, R.; Alam, R.; Mistri, T.; Bhattacharya, D.; Karmakar, P.; Ali, M. Morphology-Directing Synthesis of Rhodamine-Based Fluorophore Microstructures and Application toward Extra- and Intracellular Detection of Hg2+. ACS Appl. Mater. Interfaces 2015, 7, 7476–7485. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.-B.; Gao, G.-Y.; Qu, W.-J.; Shi, B.-B.; Lin, Q.; Yao, H.; Zhang, Y.-M. Selective Fluorescent Sensor for Mercury(II) Ion based on an Easy to Prepare Double Naphthalene Schiff base. Sens. Actuators B Chem. 2014, 199, 142–147. [Google Scholar] [CrossRef]
- Maity, S.B.; Banerjee, S.; Sunwoo, K.; Kim, J.S.; Bharadwaj, P.K. A Fluorescent Chemosensor for Hg2+ and Cd2+ Ions in Aqueous Medium under Physiological pH and Its Applications in Imaging Living Cells. Inorg. Chem. 2015, 54, 3929–3936. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Gupta, R.K.; Shahid, M.; Maiti, B.; Misra, A.; Pandey, D.S. Synthesis and Characterization of Electroactive Ferrocene Derivatives: Ferrocenylimidazoquinazoline as a Multichannel Chemosensor Selectively for Hg2+ and Pb2+ Ions in an Aqueous Environment. Inorg. Chem. 2012, 51, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jang, Y.-C.; Den, W.; Kuo, P. Green Catalysts for Energy Transformation and Emission Control; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Chapter 3; pp. 49–69. [Google Scholar]
- Srivastava, P.; Razi, S.S.; Ali, R.; Gupta, R.C.; Yadav, S.S.; Narayan, G.; Misra, A. Selective Naked-Eye Detection of Hg2+ through an Efficient Turn-On Photoinduced Electron Transfer Fluorescent Probe and Its Real Applications. Anal. Chem. 2014, 86, 8693–8699. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liu, B. A cationic porphyrin-based self-assembled film for mercury ion detection. Tetrahedron Lett. 2008, 49, 2311–2315. [Google Scholar] [CrossRef]
- Zeise, W.C. Ueber das Mercaptan. Ann. Pharmacie 1834, 11, 1–10. [Google Scholar] [CrossRef]
- Jiang, T.; Ke, B.; Chen, H.; Wang, W.; Du, L.; Yang, K.; Li, M. Bioluminescent Probe for Detecting Mercury(II) in Living Mice. Anal. Chem. 2016, 88, 7462–7465. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Kim, D.; Wang, T.; Kim, K.H.; Hwang, S.; Ahn, K.H. Reaction-Based Two-Photon Probes for Mercury Ions: Fluorescence Imaging with Dual Optical Windows. Org. Lett. 2012, 14, 2598–2601. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, H.; Wang, C.; Yang, J.; Xie, Y.; Peng, Q.; Li, Q.; Li, Z. “Turn-On” Fluorescent Probe for Mercury(II): High Selectivity and Sensitivity and New Design Approach by the Adjustment of the π-Bridge. ACS Appl. Mater. Interfaces 2015, 7, 11369–11376. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, Q.; Li, C.; Qin, J.; Li, Z. Azobenzene-Based Colorimetric Chemosensors for Rapid Naked-Eye Detection of Mercury(II). Chem. Eur. J. 2011, 17, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, S.; Jia, H.; Zhong, A.; Zhong, C.; Feng, J.; Qin, J.; Li, Z. Fluorescent and Colorimetric Probes for Mercury (II): Tunable Structures of Electron Donor and π-Conjugated Bridge. Chem. Eur. J. 2012, 18, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, Q.; Qin, J.; Li, Z. A New approach to Design Ratiometric Fluorescent Probe for Mercury(II) based on the Hg2+- Promoted Deprotection of Thioacetals. ACS Appl. Mater. Interfaces 2010, 2, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, S.; Zhong, A.; Qin, J.; Li, Z. New Fluorescent Probes for Mercury(II) with simple structure. Sens. Actuators B Chem. 2011, 157, 57–63. [Google Scholar] [CrossRef]
- Maqde, D.; Wong, R.; Seybold, P.G. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: Improved absolute standards for quantum yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, J.; Liu, L. Fluorescence Properties of Twenty Fluorescein Derivatives: Lifetime, Quantum Yield, Absorption and Emission Spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Nayak, R.R.; Lim, C.S.; Kim, I.H.; Kyhm, K.; Cho, B.R.; Woo, H.Y. Two-Photon Absorption Properties of Cationic 1,4-Bis(styryl)benzene Derivative and Its Inclusion Complexes with Cyclodextrins. J. Phys. Chem. B 2010, 114, 9684–9690. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, S.; Itoh, T.; Miyashita, I. Preparation and Polymerization of a New type of Stable Quinodimethanes with Captodative Substituents: 7,8-bis(ethylthio)-, 7,8-bis(phenylthio)-, and 7,8-bis(tert-butylthio)-7,8-dicyanoquinodimethanes. Macromolecules 1988, 21, 557–560. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Hu, J.; Wang, X.; Jie, J.; Guo, Q.; Chen, C.; Xia, A. Ultrafast Investigation of Intramolecular Charge Transfer and Solvation Dynamics of Tetrahydro[5]-helicene-Based Imide Derivatives. Sci. Rep. 2016, 6, 24313. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, B.; Elisei, F.; Mazzucato, U.; Spalletti, A. Unusual High Fluorescence of Two Nitro-distyrylbenzene-like Compounds Induced by CT Processes Affecting the Fluorescence/Intersystem-crossing Competition. Phys. Chem. Chem. Phys. 2015, 17, 14740–14749. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Arteaga, R.; Stephansen, A.B.; Guarin, C.A.; Solling, T.I.; Peon, J. The Influence of Push-Pull States on the Ultrafast Intersystem Crossing in Nitroaromatics. J. Phys. Chem. B 2013, 117, 9947–9955. [Google Scholar] [CrossRef] [PubMed]
- Kapinus, E.I.; Kucherova, I.Y.; Dilung, I.I. Influence of the solvent on the quenching of the fluorescence of anthracene by aromatic amines. Theor. Exp. Chem. 1985, 21, 355–358. [Google Scholar] [CrossRef]
- Wasielewski, M.R.; Johnson, D.G.; Niemczyk, M.P.; Galnes, G.L., III; O’Neil, M.P.; Svec, W.A. Chlorophyll-porphyrin heterodimers with orthogonal π systems: Solvent polarity dependent photophysics. J. Am. Chem. Soc. 1990, 112, 6482–6488. [Google Scholar] [CrossRef]
- Doroshenko, A.O.; Pivovarenko, V.G. Fluorescence quenching of the ketocyanine dyesin polar solvents: anti-TICT behavior. J. Photochem. Photobiol. 2003, 156, 55–64. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, X.; Su, H.; Xia, A. Photophysical Properties of Photoactive Molecules with Conjugated Push-Pull Structures. J. Phys. Chem. A 2007, 111, 5806–5812. [Google Scholar]
- Porchetta, A.; Vallée-Bélisle, A.; Plaxco, K.W.; Ricci, F. Using Distal-Site Mutations and Allosteric Inhibition to Tune, Extend, and Narrow the Useful Dynamic Range of Aptamer-Based Sensors. J. Am. Chem. Soc. 2012, 134, 20601–20604. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chim. Appl. 1928, 9, 113–203. [Google Scholar]

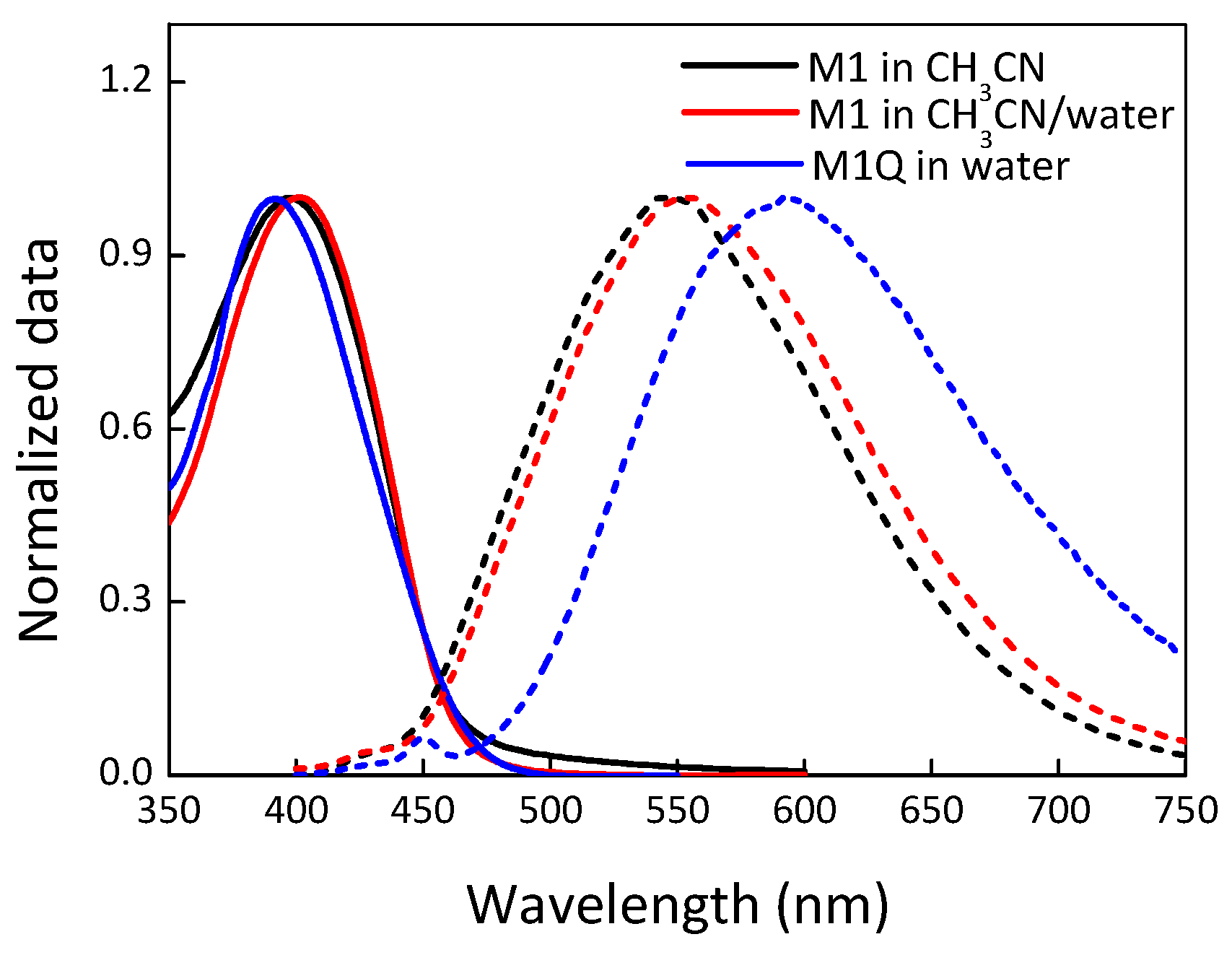
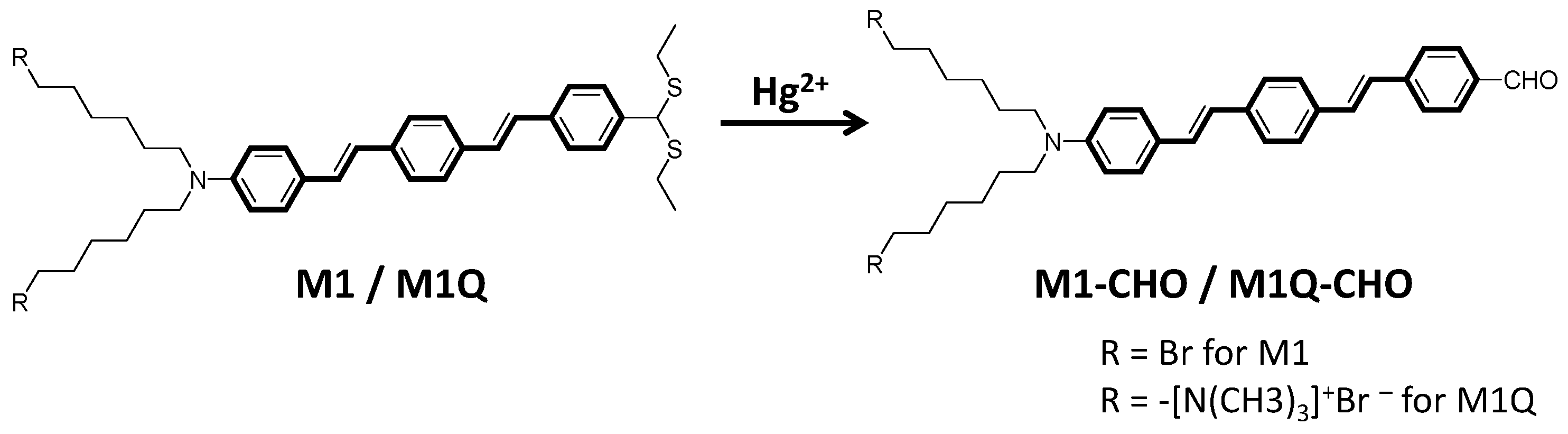
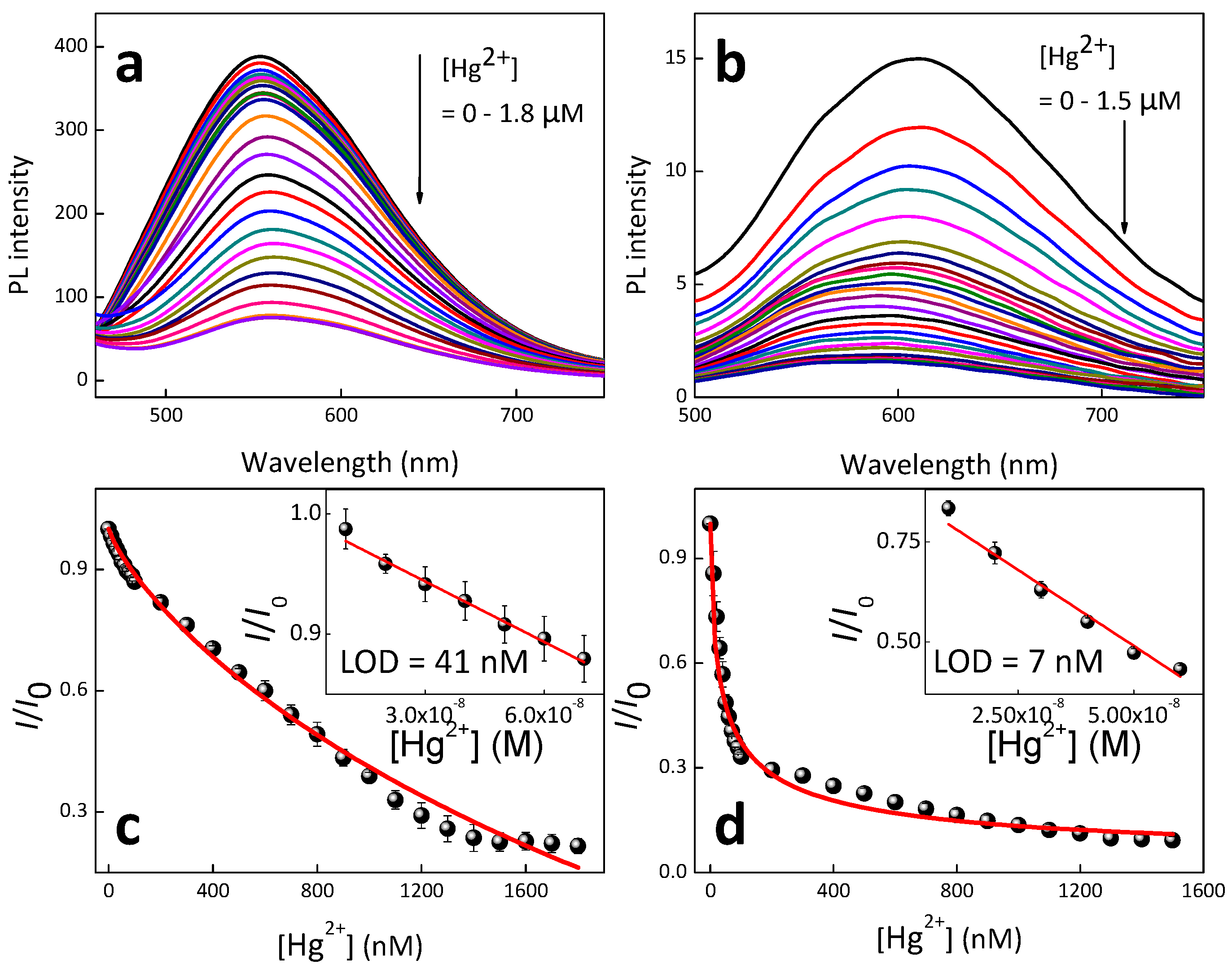
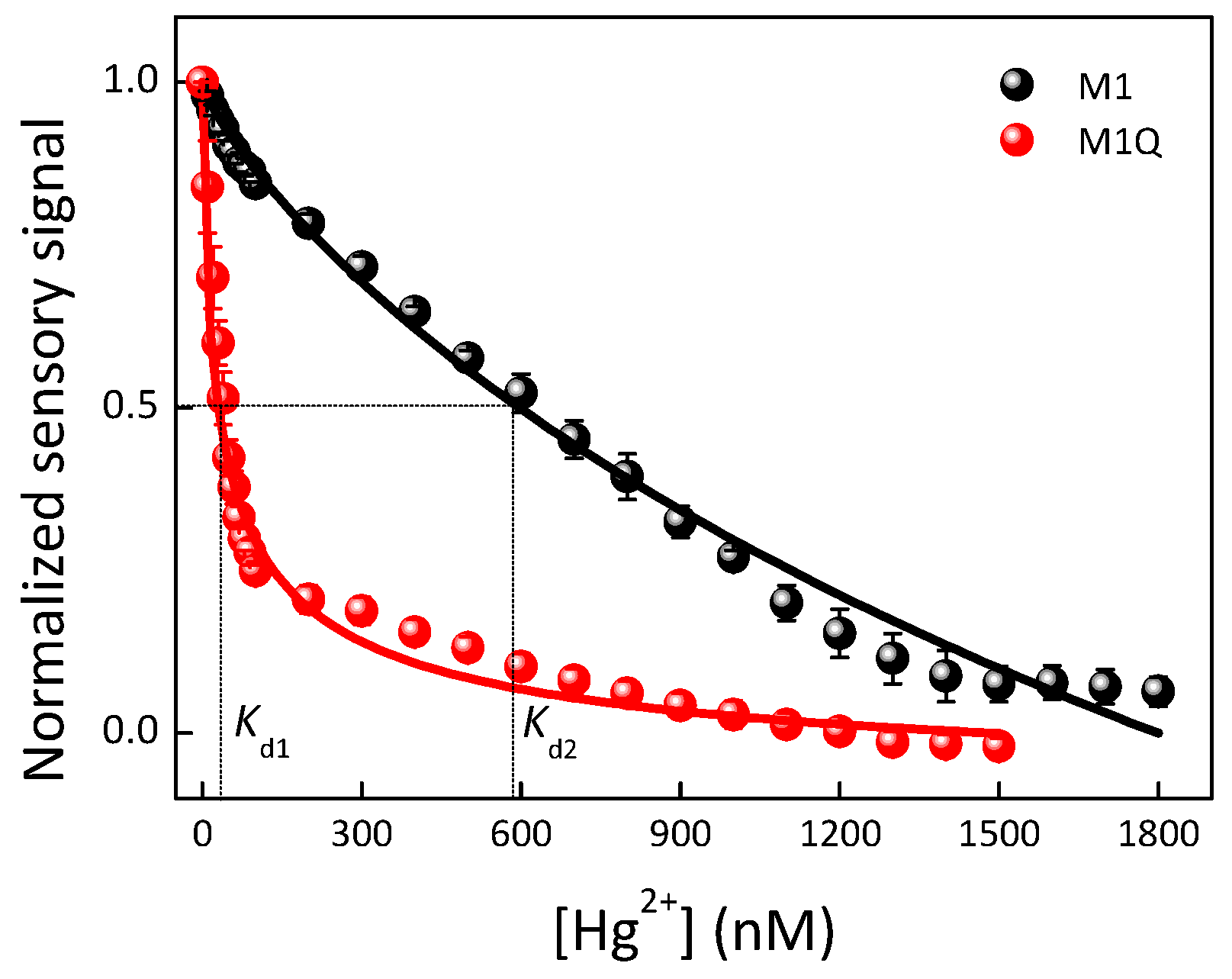
| Materials | Solvent | λabs (nm) | λPL (nm) | ΦPL (%) | εmax (M−1·cm−1) |
|---|---|---|---|---|---|
| M1 | CH3CN | 397 | 545 | 58.7 | 5.35 × 104 |
| CH3CN/water | 402 | 554 | 56.4 | 5.52 × 104 | |
| M1-CHO | CH3CN/water | 410 | 560 | 22.1 | 5.37 × 104 |
| M1Q | Water | 392 | 592 | 11.6 | 3.85 × 104 |
| M1Q-CHO | Water | 409 | 584 | 0.78 | 1.33 × 104 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, V.S.; Jeong, J.-E.; Huynh, H.T.; Lee, J.; Woo, H.Y. An Ionic 1,4-Bis(styryl)benzene-Based Fluorescent Probe for Mercury(II) Detection in Water via Deprotection of the Thioacetal Group. Sensors 2016, 16, 2082. https://doi.org/10.3390/s16122082
Le VS, Jeong J-E, Huynh HT, Lee J, Woo HY. An Ionic 1,4-Bis(styryl)benzene-Based Fluorescent Probe for Mercury(II) Detection in Water via Deprotection of the Thioacetal Group. Sensors. 2016; 16(12):2082. https://doi.org/10.3390/s16122082
Chicago/Turabian StyleLe, Van Sang, Ji-Eun Jeong, Huy Tuan Huynh, Jiae Lee, and Han Young Woo. 2016. "An Ionic 1,4-Bis(styryl)benzene-Based Fluorescent Probe for Mercury(II) Detection in Water via Deprotection of the Thioacetal Group" Sensors 16, no. 12: 2082. https://doi.org/10.3390/s16122082





