A Novel Wireless Wearable Volatile Organic Compound (VOC) Monitoring Device with Disposable Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Sensing Mechanism
2.2. Apparatus
2.2.1. Photo-Ionization Detector (PID)
2.2.2. Gas Chromatography/Mass Spectrometry (GC-MS)
2.2.3. Carbon Monoxide Monitor
2.3. Abbreviations Used Throughout This Work
2.4. Field Testing’s Subjects
3. Results and Discussion
3.1. Device Evaluation
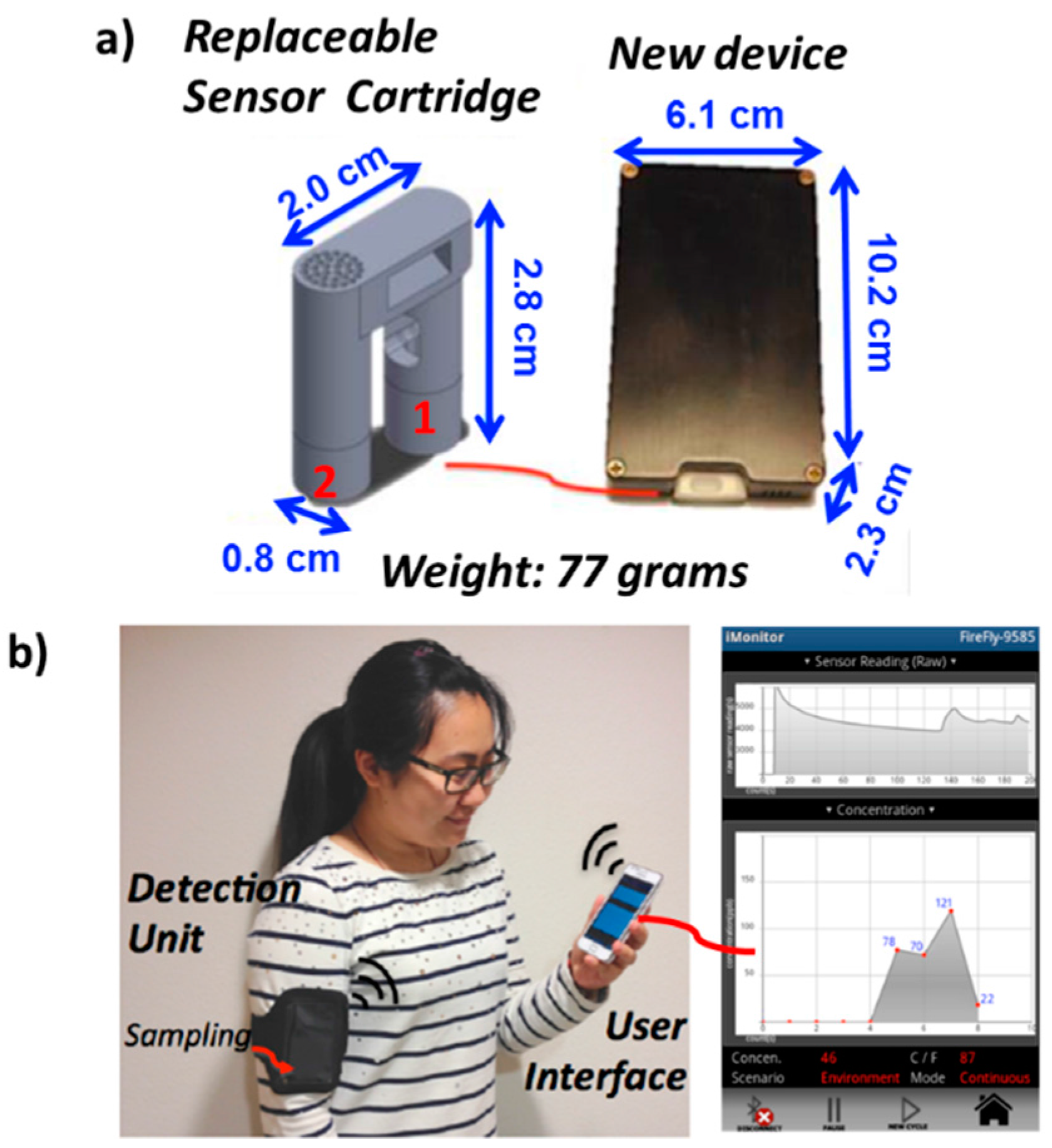
3.1.1. Device Development and Comparison
3.1.2. Device Calibration, Selectivity and Reproducibility of Sensors
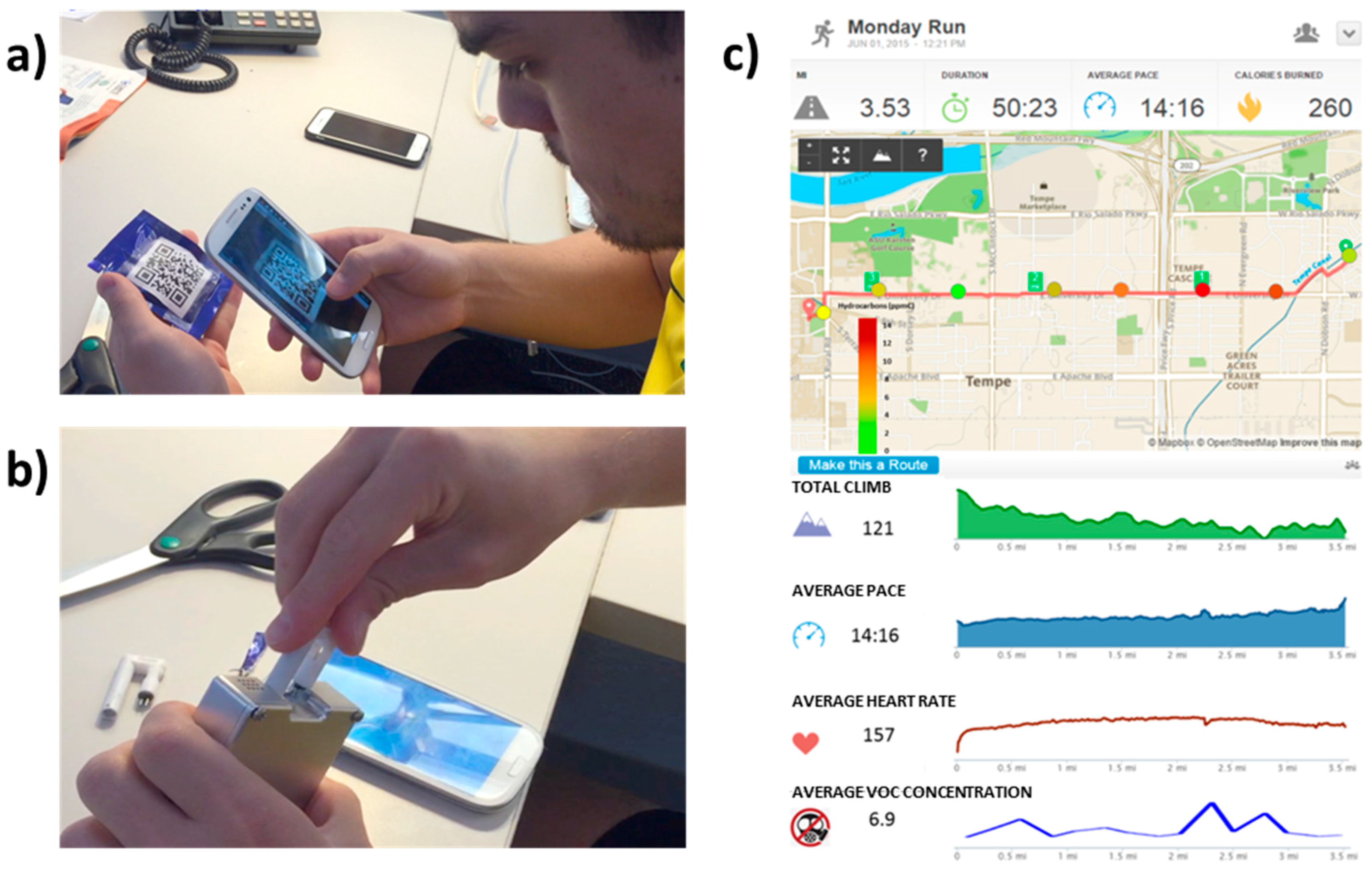
3.1.3. Personal VOCs Exposure Monitor
3.2. Device Comparison with Current Technology and Commercial Device
3.2.1. Response Comparison with Photo Ionization Detector (PID)
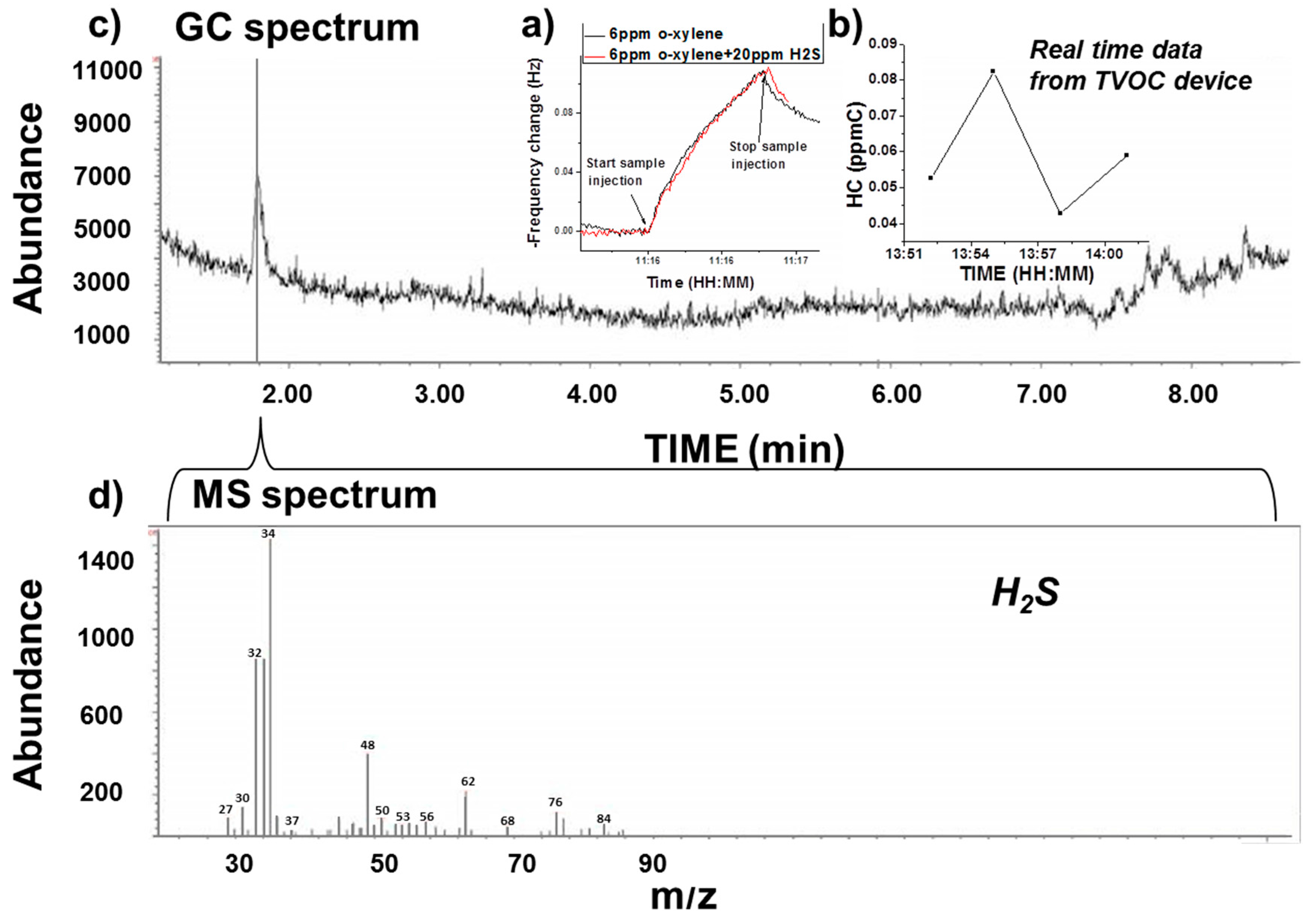
3.2.2. Selectivity Validation Example with GC-MS
3.2.3. Correlation with Carbon Monoxide Concentration
3.3. Device Validation under Different Scenarios
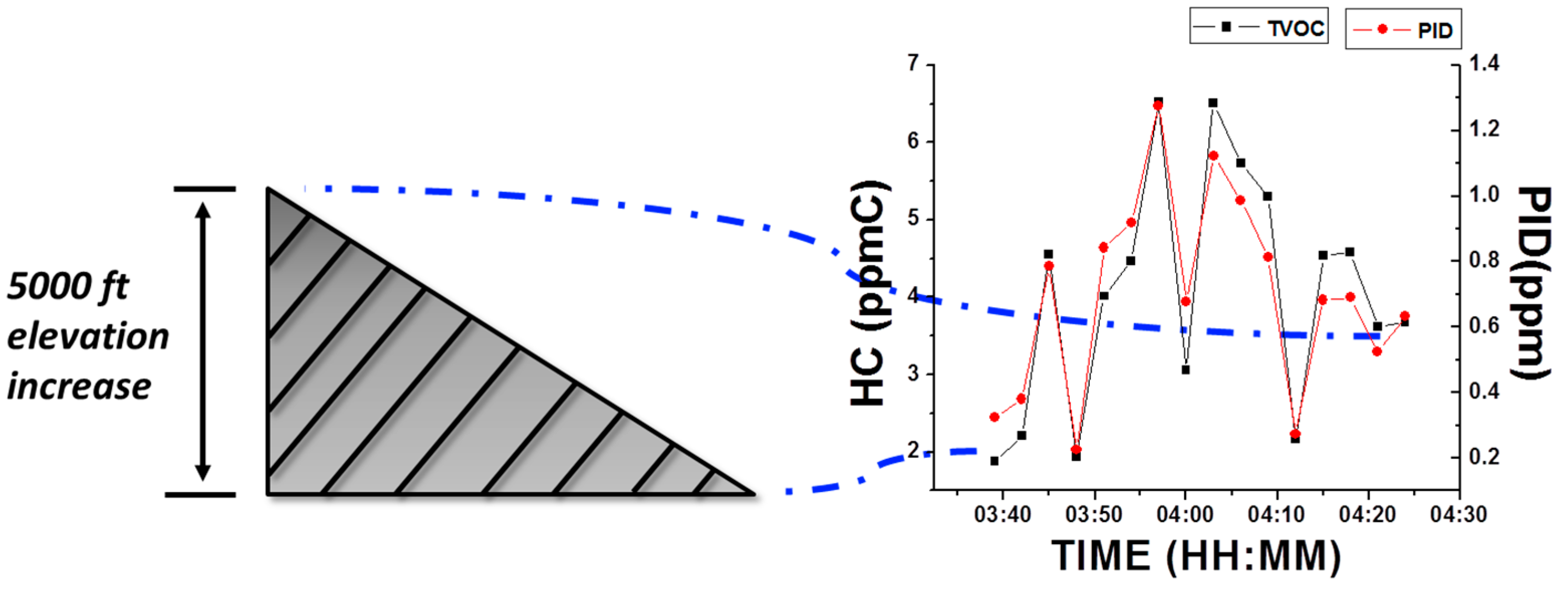
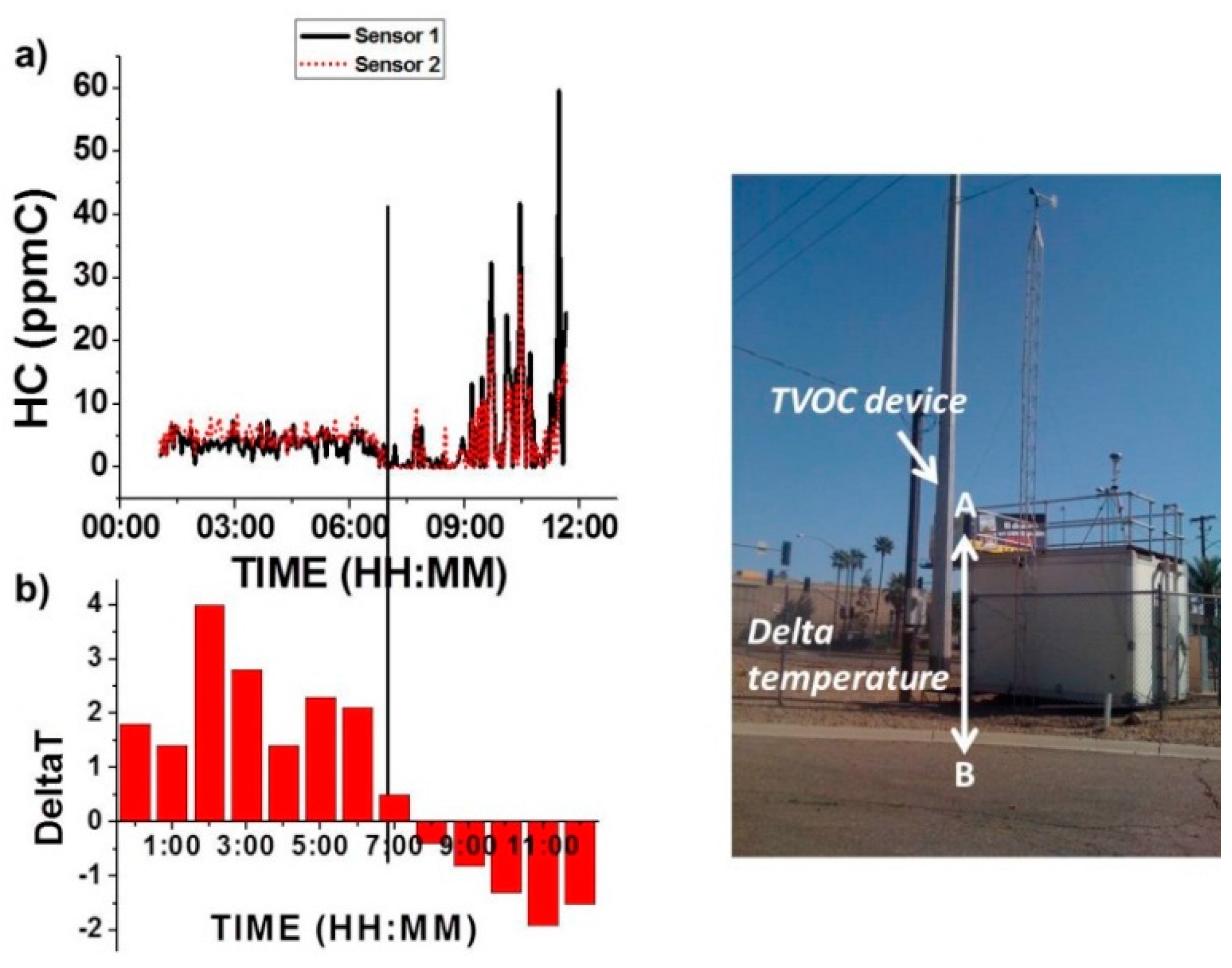
3.3.1. Barometric Effect
3.3.2. Temperature Inversion Effect
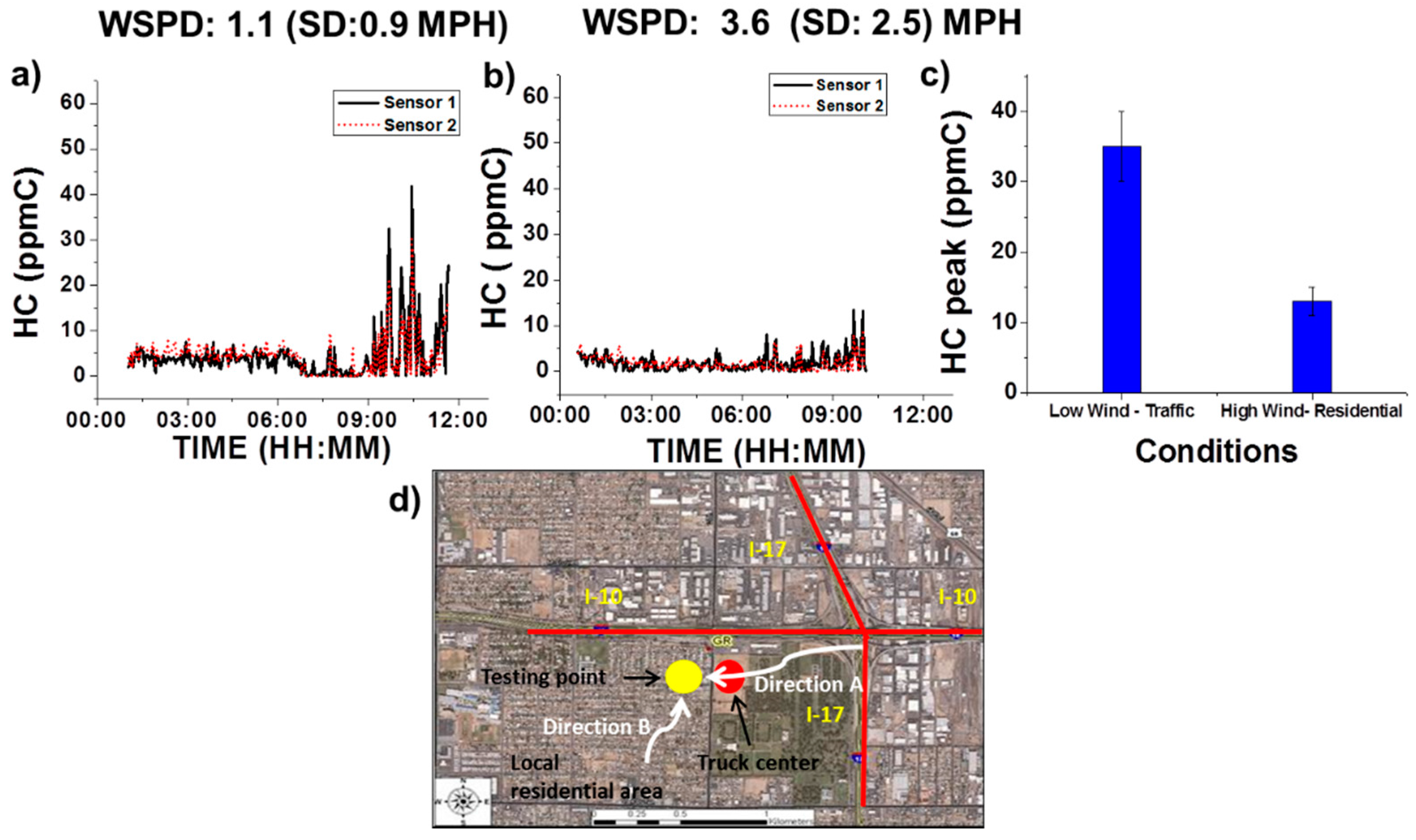
3.3.3. Wind Condition Effect
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Sim, R.; Abramson, J.; Gray, N. Concentrations of Volatile Organic Compounds in indoor air—A review. Indoor Air 1994, 4, 123–134. [Google Scholar] [CrossRef]
- Win-Shwe, T.-T.; Fujimaki, H.; Arashidani, K.; Kunugita, N. Indoor Volatile Organic Compounds and chemical sensitivity reactions. Clin. Dev. Immunol. 2013, 2013, 623812. [Google Scholar] [CrossRef] [PubMed]
- Chagger, H.K.; Jones, J.M.; Pourkashanian, M.; Williams, A.; Owen, A.; Fynes, G. Emission of volatile organic compounds from coal combustion. Fuel 1999, 78, 1527–1538. [Google Scholar] [CrossRef]
- Sofuoglu, S. C.; Aslan, G.; Inal, F.; Sofuoglu, A. An assessment of indoor air concentrations and health risks of volatile organic compounds in three primary schools. Int. J. Hyg. Environ. Health 2011, 214, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lerchner, J.; Caspary, D.; Wolf, G. Calorimetric detection of volatile organic compounds. Sens. Actuators B Chem. 2000, 70, 57–66. [Google Scholar] [CrossRef]
- Martínez-Hipatl, C.; Muñoz-Aguirre, S.; Beltrán-Pérez, G.; Castillo-Mixcóatl, J.; Rivera-De la Rosa, J. Detection of volatile organic compounds by an interferometric sensor. Sens. Actuators B Chem. 2010, 147, 37–42. [Google Scholar] [CrossRef]
- Patel, S.V.; Mlsna, T.E.; Fruhberger, B.; Klaassen, E.; Cemalovic, S.; Baselt, D.R. Chemicapacitive microsensors for volatile organic compound detection. Sens. Actuators B Chem. 2003, 96, 541–553. [Google Scholar] [CrossRef]
- Smith, P.A.; Lepage, C.J.; Harrer, K.L.; Brochu, P.J. Hand-held photoionization instruments for quantitative detection of sarin vapor and for rapid qualitative screening of contaminated objects. J. Occup. Environ. Hyg. 2007, 4, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Lingg, R.D.; Melton, R.G.; Kopfler, F.C.; Coleman, W.E.; Mitchell, D.E. Quantitative Analysis of Volatile Organic Compounds by GC-MS. J. Am. Water Works Assoc. 1977, 69, 605–612. [Google Scholar]
- Rastrello, F.; Placidi, P.; Scorzoni, A.; Cozzani, E.; Messina, M.; Elmi, I.; Zampolli, S.; Cardinali, G.C. Thermal Conductivity Detector for Gas Chromatography: Very Wide Gain Range Acquisition System and Experimental Measurements. IEEE Trans. Instrum. Meas. 2013, 62, 974–981. [Google Scholar] [CrossRef]
- Deng, C.; Yang, X.; Li, N.; Huang, Y.; Zhang, X. A Novel Miniaturized Flame Ionization Detector for Portable Gas Chromatography. J. Chromatogr. Sci. 2005, 43, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Cagan, A.; Schmidt, H.; Rodriguez, J.E.; Eiceman, G.A. Fast gas chromatography-differential mobility spectrometry of explosives from TATP to Tetryl without gas atmosphere modifiers. Int. J. Ion Mobil. Spectrom. 2010, 13, 157–165. [Google Scholar] [CrossRef]
- Su, X.; Dai, C.; Zhang, J.; O’Shea, S.J. Quartz tuning fork biosensor. Biosens. Bioelectron. 2002, 17, 111–117. [Google Scholar] [CrossRef]
- Kosterev, A.A.; Tittel, F.K.; Serebryakov, D.V.; Malinovsky, A.L.; Morozov, I.V. Applications of quartz tuning forks in spectroscopic gas sensing. Rev. Sci. Instrum. 2005, 76, 043105. [Google Scholar] [CrossRef]
- Tsow, F.; Forzani, E.; Rai, A.; Wang, R.; Tsui, R.; Mastroianni, S.; Knobbe, C.; Gandolfi, A.J.; Tao, N.J. A wearable and wireless sensor system for real-time monitoring of toxic environmental Volatile Organic Compounds. IEEE Sens. J. 2009, 9, 1734–1740. [Google Scholar] [CrossRef]
- Wang, R.; Tsow, F.; Zhang, X.; Peng, J.H.; Forzani, E.S.; Chen, Y.; Crittenden, J.C.; Destaillats, H.; Tao, N. Real-time ozone detection based on a microfabricated quartz crystal tuning fork sensor. Sensors 2009, 9, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Negi, I.; Tsow, F.; Tanwar, K.; Zhang, L.; Iglesias, R.A.; Chen, C.; Rai, A.; Forzani, E.S.; Tao, N. Novel monitor paradigm for real-time exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, C.; Qin, X.; Xian, X.; Alford, T.L.; Choi, H.W.; Tsow, F.; Forzani, E.S. Aging effect of a molecularly imprinted polymer on a quartz tuning fork sensor for detection of volatile organic compounds. Sens. Actuators B Chem. 2015, 211, 25–32. [Google Scholar] [CrossRef]
- Chen, C.; Campbell, K.D.; Negi, I.; Iglesias, R.A.; Owens, P.; Tao, N.; Tsow, F.; Forzani, E. A new sensor for the assessment of personal exposure to Volatile Organic Compounds. Atmos. Environ. 2012, 54, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Pitten, F.A.; Bremer, J.; Kramer, A. Air contamination with volatile organic compounds (VOC's) and health complaints. Dtsch. Med. Wochenschr. 2000, 125, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhao, X.; Di, J. Hydrogen sulfide removal from petroleum refinery by immobilized thiobacillus ferrooxidans in fixed-bed bioreactor. Pet. Sci. Technol. 2009, 27, 2134–2144. [Google Scholar] [CrossRef]
- Klouda, G.A.; Connolly, M.V. Radiocarbon (14C) measurements to quantify sources of atmospheric carbon monoxide in urban air. Atmos. Environ. 1995, 29, 3309–3318. [Google Scholar] [CrossRef]
- Thomson, G.W. The Antoine Equation for Vapor-pressure Data. Chem. Rev. 1946, 38, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Weisel, C.P.; Zhang, J.; Turpin, B.J.; Morandi, M.T.; Colome, S.; Stock, T.H.; Spektor, D.M.; Korn, L.; Winer, A.M.; Kwon, J.; et al. Relationships of Indoor, Outdoor, and Personal Air (RIOPA). Part I. Collection methods and descriptive analyses. Res. Rep. 2005, 130, 1–107. [Google Scholar]
- Brown, S.G.; Frankel, A.; Hafner, H.R. Source apportionment of VOCs in the Los Angeles area using positive matrix factorization. Atmos. Environ. 2007, 41, 227–237. [Google Scholar] [CrossRef]
- Grober, R.D.; Acimovic, J.; Schuck, J.; Hessman, D.; Kindlemann, P.J.; Hespanha, J.; Morse, A.S.; Karrai, K.; Tiemann, I.; Manus, S. Fundamental limits to force detection using quartz tuning forks. Rev. Sci. Instrum. 2000, 71, 2776–2780. [Google Scholar] [CrossRef]
- Friedt, J.-M.; Carry, É. Introduction to the quartz tuning fork. Am. J. Phys. 2007, 75, 415–422. [Google Scholar] [CrossRef]
- Portland State Aerospace Society. A Quick Derivation Relating Altitude to Air Pressure. Available online: http://www.psas.pdx.edu (accessed on 2 December 2016).
- Surridge, A.D. Extrapolation of the nocturnal temperature inversion from ground-based measurements. Atmos. Environ. 1986, 20, 803–806. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Chen, C.; Xian, X.; Tsow, F.; Verma, G.; McConnell, R.; Fruin, S.; Tao, N.; Forzani, E.S. A Novel Wireless Wearable Volatile Organic Compound (VOC) Monitoring Device with Disposable Sensors. Sensors 2016, 16, 2060. https://doi.org/10.3390/s16122060
Deng Y, Chen C, Xian X, Tsow F, Verma G, McConnell R, Fruin S, Tao N, Forzani ES. A Novel Wireless Wearable Volatile Organic Compound (VOC) Monitoring Device with Disposable Sensors. Sensors. 2016; 16(12):2060. https://doi.org/10.3390/s16122060
Chicago/Turabian StyleDeng, Yue, Cheng Chen, Xiaojun Xian, Francis Tsow, Gaurav Verma, Rob McConnell, Scott Fruin, Nongjian Tao, and Erica S. Forzani. 2016. "A Novel Wireless Wearable Volatile Organic Compound (VOC) Monitoring Device with Disposable Sensors" Sensors 16, no. 12: 2060. https://doi.org/10.3390/s16122060





